Anti-Infective-Associated AKI: A Narrative Review of the Epidemiology, Mechanisms, Risk Factors, Biomarkers, Clinical Course, Monitoring, Prevention, and Therapeutic Strategies
Abstract
1. Introduction
2. Amphotericin B
2.1. Introduction
2.2. Epidemiology
2.3. Definition
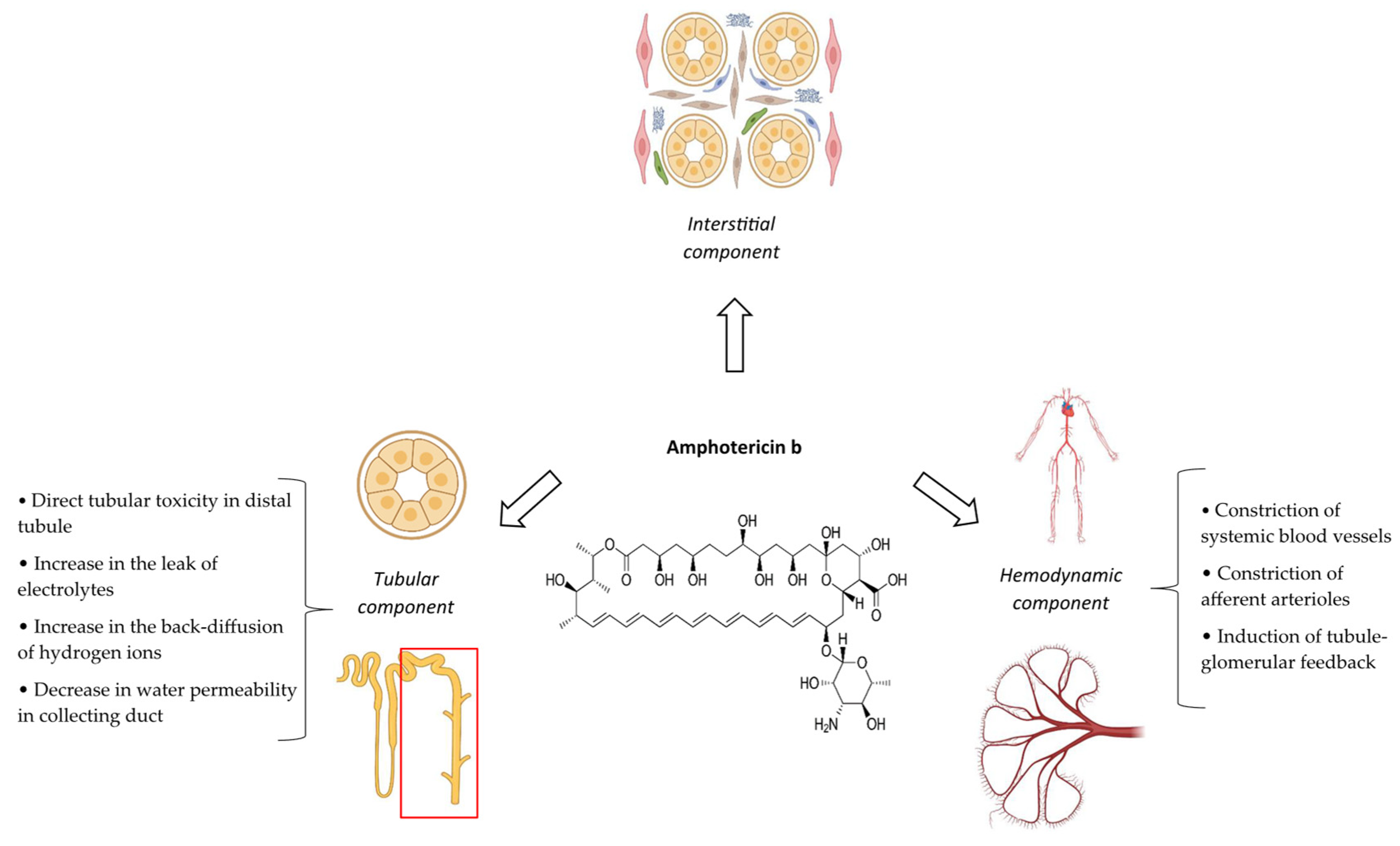
2.4. Pathophysiological Mechanisms
2.5. Risk Factors
2.6. Clinical/Paraclinical Manifestations and Outcomes
2.7. Biomarkers
2.8. Prevention
2.8.1. Salt Loading
2.8.2. Lipid-Based Formulations
2.8.3. Preparing an Intravenous Lipid Emulsion
2.8.4. Prolonging the Duration of Infusion
2.8.5. Co-Administering Diuretics
2.8.6. Co-Administering Nephroprotective Agents
2.8.7. Other Measures
| Strategy | Description |
|---|---|
| Salt loading | Administering intravenous sodium chloride at a dose of 150 mEq (1 L or 10–15 mL/kg of 0.9% sodium chloride solution) per day as a bolus or continuous infusion during the course of treatment is recommended. |
| Using lipid-based formulations | Using lipid-based formulations, including a lipid complex, colloidal dispersion, or liposomal (preferably), instead of conventional formulation is recommended. |
| Preparing in intravenous lipid emulsion | Preparing a conventional formulation in intravenous lipid emulsions such as Intralipid® 20% may be effective. |
| Prolonging the duration of infusion | Administering a conventional formulation as a continuous 24 h infusion may be effective. |
| Co-administering diuretics | Potassium-sparing diuretics including amiloride (5.0 mg orally twice a day) or spironolactone (100 mg orally twice a day) may be effective in the management of electrolyte disorders including hypokalemia and hypomagnesemia. |
| Co-administering nephroprotective agents |
|
| Using alternative antifungal therapy | Administering itraconazole, voriconazole, or caspofungin in patients with more than two risk factors of AKI or those with baseline creatinine clearance less than 25 mL/min. |
2.9. Monitoring
2.10. Treatment
3. Cidofovir
3.1. Introduction
3.2. Epidemiology
3.3. Definition
3.4. Pathophysiological Mechanisms
3.5. Risk Factors
3.6. Clinical/Paraclinical Manifestations and Outcomes
3.7. Biomarkers
3.8. Prevention
3.9. Monitoring
3.10. Treatment
4. Foscarnet
4.1. Introduction
4.2. Epidemiology
4.3. Definition
4.4. Pathophysiological Mechanisms
4.5. Risk Factors
4.6. Clinical/Paraclinical Manifestations and Outcomes
4.7. Biomarkers
4.8. Prevention
4.9. Monitoring
4.10. Treatment
5. Polymyxins
5.1. Introduction
5.2. Epidemiology
5.3. Definition
5.4. Pathophysiological Mechanisms
5.5. Risk Factors
5.6. Clinical/Paraclinical Manifestations and Outcomes
5.7. Biomarkers
5.8. Prevention
5.8.1. Selecting the Type of Polymyxin
5.8.2. Modifying Loading/Daily Dose, Administration Interval, and Infusion Duration
5.8.3. Avoiding or Reducing Modifiable Risk Factors
5.8.4. Co-Administration of Nephroprotective Agents
| Strategy | Description |
|---|---|
| Using polymyxin B instead of colistin | There are inconclusive or controversial data, and it is generally not recommended. |
| Appropriate dose selection and modification | Consider dose adjustments based on:
|
| Avoid using loading dose | It is not recommended. |
| Once-daily dosing | There are inconclusive or controversial data, and it is not recommended. |
| Performing therapeutic drug monitoring | Although there is no standard analytical method and consensus about the procedure, performing TDM, wherever possible, is recommended, especially in the early treatment period. |
| Prolonging the duration of infusion | There are no clinical data, and it is not recommended. |
| Avoiding co-administered nephrotoxic agents | Whenever possible, all agents with nephrotoxic potential should be discontinued. |
| Using alternative regimens or medications | If the risk of AKI is estimated to be high, consider using less nephrotoxic agents (e.g., ampicillin–sulbactam, tigecycline, and eravacycline ± meropenem) instead of polymyxins. |
| Correcting hypoalbuminemia | There are no clinical data, and it is not recommended. |
| Co-administration of nephroprotective agents |
|
5.9. Monitoring
5.10. Treatment
6. Vancomycin
6.1. Introduction
6.2. Epidemiology
6.3. Definition
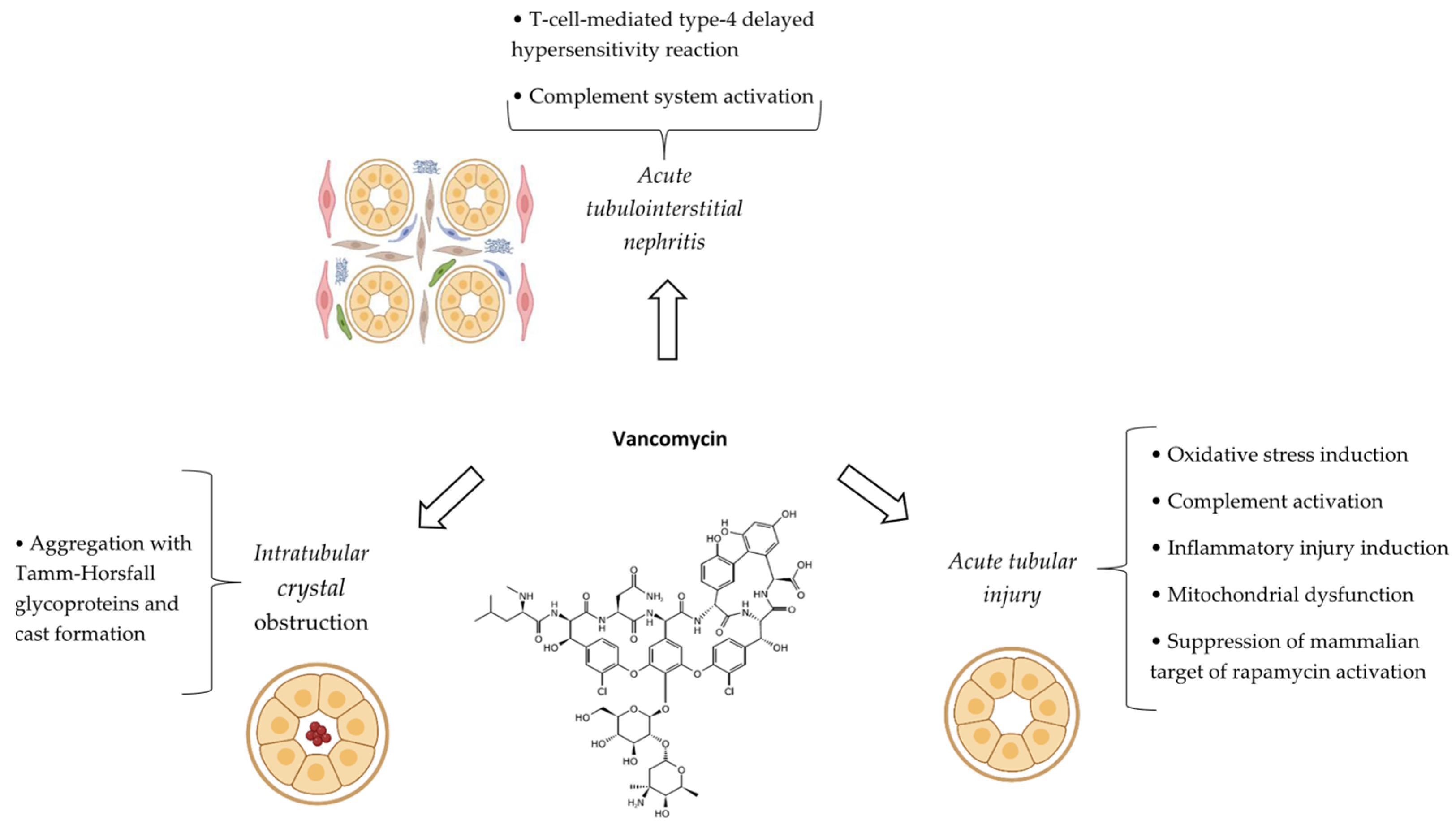
6.4. Pathophysiological Mechanisms
6.5. Risk Factors
6.6. Clinical/Paraclinical Manifestations and Outcomes
6.7. Biomarkers
6.8. Prevention
6.8.1. Risk Stratification
6.8.2. Optimizing Modifiable Risk Factors
6.8.3. Method of Administration as Continuous Infusion
6.8.4. Co-Administration of Nephroprotective Agents
| Strategy | Description |
|---|---|
| Calculating appropriate dose | The vancomycin dose should be calculated based on actual body weight (even in patients with obesity), preferably not exceeding 3600 mg/day in pediatric patients and 4500 mg/day in adults with obesity. |
| Monitoring serum level |
|
| Limiting the duration of treatment | The duration of vancomycin therapy must be limited, preferably less than 7 days. |
| Prolonging the duration of infusion | Administering vancomycin as a continuous infusion (24 h) may be beneficial in selected cases. |
| Avoiding the co-administration of nephrotoxic agents |
|
| Using less nephrotoxic alternative agents | If the risk of AKI is estimated to be high, consider using other less nephrotoxic agents instead of vancomycin such as teicoplanin (not available in the United States), linezolid, daptomycin, tigecycline, telavancin, or ceftaroline. |
| Co-administration of nephroprotective agents |
|
6.9. Monitoring
6.10. Treatment
7. Aminoglycosides
7.1. Introduction
7.2. Epidemiology
7.3. Definition
7.4. Pathophysiological Mechanisms
7.5. Risk Factors
7.6. Clinical/Paraclinical Manifestations and Outcomes
7.7. Biomarkers
7.8. Prevention
7.8.1. Choice of Aminoglycoside
7.8.2. Alternative Antibiotics
7.8.3. Modifiable Risk Factors
7.8.4. Therapeutic Drug Monitoring and Dose Calculation
7.8.5. Co-Administering Nephroprotective Agents
7.8.6. Aminoglycoside Congeners, New Derivatives, and Novel Formulations
7.8.7. Time of Dosing
| Strategy | Description |
|---|---|
| Calculating appropriate dose | Considering total, ideal, and adjusted body weights for calculating required doses for patients underweight, with normal weight/overweight, and with obesity, respectively. |
| Monitoring serum level |
|
| Using the aminoglycoside with less nephrotoxicity potential | In the case of empirical or definite treatment of infections caused by P. aeruginosa, amikacin or tobramycin appear to be relatively more appropriate aminoglycosides. |
| Using alternative antibiotics with less nephrotoxicity | If the risk of AKI is estimated to be high, fluoroquinolones and third- or fourth-generation cephalosporins can be used instead of aminoglycosides. |
| Limiting the duration of treatment |
|
| Avoiding the co-administration of nephrotoxic agents | Whenever possible, the co-administration of nephrotoxic agents (e.g., vancomycin and amphotericin B) should be avoided. |
| Administering as once-daily dosing | In certain and selected clinical conditions, aminoglycosides can be given as once-daily dosing (versus multiple-daily dosing). |
| Co-administration of nephroprotective agents |
|
| Using aminoglycoside congeners, new derivatives, and novel formulations |
|
| Administering aminoglycosides in the morning | Administering aminoglycosides in the morning may be associated with less nephrotoxicity compared to that given in the evening. |
7.9. Monitoring
7.10. Treatment
8. Conclusions
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weisbord, S.D.; Palevsky, P.M. Prevention and management of acute kidney injury. In Brenner and Rector’s the Kidney, 11th ed.; Chertow, G., Luyckx, V., Marsden, P., Skorecki, K., Taal, M., Alan, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Dasta, J.F.; Kane-Gill, S. Review of the Literature on the Costs Associated With Acute Kidney Injury. J. Pharm. Pract. 2019, 32, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Kane-Gill, S.L.; Smithburger, P.L.; Kashani, K.; Kellum, J.A.; Frazee, E. Clinical Relevance and Predictive Value of Damage Biomarkers of Drug-Induced Kidney Injury. Drug Saf. 2017, 40, 1049–1074. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Awdishu, L.; Davenport, A.; Murray, P.T.; Macedo, E.; Cerda, J.; Chakaravarthi, R.; Holden, A.L.; Goldstein, S.L. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015, 88, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Dennen, P.; Douglas, I.S.; Anderson, R. Acute kidney injury in the intensive care unit: An update and primer for the intensivist. Crit. Care Med. 2010, 38, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Stottlemyer, B.A.; Tran, T.; Suh, K.; Kane-Gill, S.L. A Systematic Review of the Costs of Drug-Associated Acute Kidney Injury and Potential Cost Savings With Nephrotoxin Stewardship Prevention Strategies. Clin. Pharmacol. Ther. 2025, 117, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.P.; Barreto, E.F.; Schreier, D.J.; Kellum, J.A.; Suh, K.; Kashani, K.B.; Rule, A.D.; Kane-Gill, S.L. Consensus Obtained for the Nephrotoxic Potential of 167 Drugs in Adult Critically Ill Patients Using a Modified Delphi Method. Drug Saf. 2022, 45, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Goswami, E.; Ogden, R.K.; Bennett, W.E.; Goldstein, S.L.; Hackbarth, R.; Somers, M.J.G.; Yonekawa, K.; Misurac, J. Evidence-based development of a nephrotoxic medication list to screen for acute kidney injury risk in hospitalized children. Am. J. Health Syst. Pharm. 2019, 76, 1869–1874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Llaneza, D.; Vos, R.M.P.; Lieverse, J.E.; Gosselt, H.R.; Kane-Gill, S.L.; van Gelder, T.; Klopotowska, J.E.; LEAPfROG Consortium. An Integrated Approach for Representing Knowledge on the Potential of Drugs to Cause Acute Kidney Injury. Drug Saf. 2025, 48, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drew, R.H. Pharmacology of Amphotericin B. UpToDate 2025. Available online: https://www.uptodate.com/contents/pharmacology-of-amphotericin-b (accessed on 5 March 2025).
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Farsaei, S.; Khalili, H.; Dashti-Khavidaki, S. Are salt loading and prolonging infusion period effective in prevention of amphotericin B-induced nephrotoxicity? Expert Opin. Drug Saf. 2012, 11, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Nolin, T.D.; Perazella, M.A. Drug-induced kidney disease. In Pharmacotherapy: A Pathophysiologic Approach, 12th ed.; DiPiro, J.T., Yee, G.C., Posey, L.M., Haines, S.T., Nolin, T.D., Ellingrod, V., Eds.; McGraw Hill: New York, NY, USA, 2023. [Google Scholar]
- Karimzadeh, I.; Khalili, H.; Farsaei, S.; Dashti-Khavidaki, S.; Sagheb, M.M. Role of diuretics and lipid formulations in the prevention of amphotericin B-induced nephrotoxicity. Eur. J. Clin. Pharmacol. 2013, 69, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Mistro, S.; Maciel, I.M.; de Menezes, R.G.; Maia, Z.P.; Schooley, R.T.; Badaró, R. Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta-analysis. Clin. Infect. Dis. 2012, 54, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L. Infectious Diseases II. In Updates in Therapeutics®: Pharmacotherapy Preparatory Review and Recertification Course, 2021 ed.; Bondi, D.S., Bridgeman, M.M., Burke, J.M., Eds.; American College of Clinical Pharmacy: Lenexa, KS, USA, 2021. [Google Scholar]
- Harbarth, S.; Pestotnik, S.L.; Lloyd, J.F.; Burke, J.P.; Samore, M.H. The epidemiology of nephrotoxicity associated with conventional amphotericin B therapy. Am. J. Med. 2001, 111, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Koren, G. Amphotericin B nephrotoxicity in children. J. Pediatr. Hematol. Oncol. 2004, 26, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Awdishu, L.; Mehta, R.L. The 6R’s of drug induced nephrotoxicity. BMC Nephrol. 2017, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafez, Y.; Siaj, H.; Janajri, M.; Abu-Baker, Y.; Nazzal, Z.; Hamdan, Z.; Adwan, R.; Aiesh, B.M.; Anaya, A.I. Tolerability and epidemiology of nephrotoxicity associated with conventional amphotericin B therapy: A retrospective study in tertiary care centers in Palestine. BMC Nephrol. 2022, 23, 132. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Amphotericin B Nephrotoxicity. UpToDate. 2025. Available online: https://www.uptodate.com/contents/amphotericin-b-nephrotoxicity (accessed on 6 April 2025).
- Downes, K.J.; Hayes, M.; Fitzgerald, J.C.; Pais, G.M.; Liu, J.; Zane, N.R.; Goldstein, S.L.; Scheetz, M.H.; Zuppa, A.F. Mechanisms of antimicrobial-induced nephrotoxicity in children. J. Antimicrob. Chemother. 2020, 75, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Smogorzewski, M.J.; Rude, R.K.; Yu, A.S.L. Disorders of calcium, magnesium, and phosphate balance. In Brenner and Rector’s the Kidney, 11th ed.; Chertow, G., Luyckx, V., Marsden, P., Skorecki, K., Taal, M., Alan, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- DuBose, T.D., Jr. Disorders of acid-base balance. In Brenner and Rector’s the Kidney, 11th ed.; Chertow, G., Luyckx, V., Marsden, P., Skorecki, K., Taal, M., Alan, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- John, J.; Loo, A.; Mazur, S.; Walsh, T.J. Therapeutic drug monitoring of systemic antifungal agents: A pragmatic approach for adult and pediatric patients. Expert Opin. Drug Metab. Toxicol. 2019, 15, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Takazono, T.; Tashiro, M.; Ota, Y.; Obata, Y.; Wakamura, T.; Miyazaki, T.; Nishino, T.; Izumikawa, K. Factor analysis of acute kidney injury in patients administered liposomal amphotericin B in a real-world clinical setting in Japan. Sci. Rep. 2020, 10, 15033. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Guglielmo, B.J. Principles of Infectious Diseases. In Applied Therapeutics: The Clinical Use of Drugs, 12th ed.; Zeind, C.S., Carvalho, M.G., Eds.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2023. [Google Scholar]
- Gursoy, V.; Ozkalemkas, F.; Ozkocaman, V.; Serenli Yegen, Z.; Ethem Pinar, I.; Ener, B.; Akalın, H.; Kazak, E.; Ali, R.; Ersoy, A. Conventional Amphotericin B Associated Nephrotoxicity in Patients With Hematologic Malignancies. Cureus 2021, 13, e16445. [Google Scholar] [CrossRef] [PubMed]
- Personett, H.A.; Kayhart, B.M.; Barreto, E.F.; Tosh, P.; Dierkhising, R.; Mara, K.; Leung, N. Renal Recovery following Liposomal Amphotericin B-Induced Nephrotoxicity. Int. J. Nephrol. 2019, 2019, 8629891. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J. Nephrotoxicity in the setting of invasive fungal diseases. Mycoses 2008, 51, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Aoki, F.Y. Antivirals against herpesviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Izzedine, H.; Launay-Vacher, V.; Deray, G. Antiviral drug-induced nephrotoxicity. Am. J. Kidney Dis. 2005, 45, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Vistide (Cidofovir) Package Insert; Gilead Sciences: Forest City, CA, USA, 2000.
- Rodriguez, M.; Zachary, K.C. Foscarnet: An Overview. UpToDate. 2025. Available online: https://www.uptodate.com/contents/foscarnet-an-overview (accessed on 16 April 2025).
- Pike, M.; Saltiel, E. Colistin- and polymyxin-induced nephrotoxicity: Focus on literature utilizing the RIFLE classification scheme of acute kidney injury. J. Pharm. Pract. 2014, 27, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.E.; Arias, C.A.; Nannini, E.C. Glycopeptides (Vancomycin and Teicoplanin) and Lipoglycopeptides (Telavancin, Oritavancin, and Dalbavancin). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Wazny, L.D.; Brophy, D.F. Amiloride for the prevention of amphotericin B-induced hypokalemia and hypomagnesemia. Ann. Pharmacother. 2000, 34, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Santos, F.; Hand, M. Fluid, electrolyte, and acid-base disorders in children. In Brenner and Rector’s the Kidney, 11th ed.; Chertow, G., Luyckx, V., Marsden, P., Skorecki, K., Taal, M., Alan, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Ferguson, M.A.; Vaidya, V.S.; Bonventre, J.V. Biomarkers of nephrotoxic acute kidney injury. Toxicology 2008, 245, 182–193. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, J.E.; Lee, S.; Ma, J.Y.; Chen, Y.; Snook, S. Acute biomarker panel changes associated with amphotericin B nephrotoxicity in female Sprague-Dawley rats. J. Toxicol. Sci. 2016, 41, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.R.; Faubel, S.; Edelstein, C.L. Biomarkers of Drug-Induced Kidney Toxicity. Ther. Drug Monit. 2019, 41, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Da, Y.; Akalya, K.; Murali, T.; Vathsala, A.; Tan, C.S.; Low, S.; Lim, H.N.; Teo, B.W.; Lau, T.; Ong, L.; et al. Serial Quantification of Urinary Protein Biomarkers to Predict Drug-induced Acute Kidney Injury. Curr. Drug Metab. 2019, 20, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Heydari, M.; Ramzi, M.; Sagheb, M.M.; Zomorodian, K. Urinary Neutrophil Gelatinase-associated Lipocalin as a Biomarker of Kidney Injury in Hematologic-Oncologic Patients Receiving Amphotericin B. Iran. J. Kidney Dis. 2017, 11, 201–208. [Google Scholar] [PubMed]
- Rocha, P.N.; Macedo, M.N.; Kobayashi, C.D.; Moreno, L.; Guimarães, L.H.; Machado, P.R.; Badaró, R.; Carvalho, E.M.; Glesby, M.J. Role of urine neutrophil gelatinase-associated lipocalin in the early diagnosis of amphotericin B-induced acute kidney injury. Antimicrob. Agents. Chemother. 2015, 59, 6913–6921. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A. Antifungal agents: Amphotericin B. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Armstrong-James, D.; Koh, M.; Ostermann, M.; Cockwell, P. Optimal management of acute kidney injury in critically ill patients with invasive fungal infections being treated with liposomal amphotericin B. BMJ Case Rep. 2020, 13, e233072. [Google Scholar] [CrossRef] [PubMed]
- Tonin, F.S.; Steimbach, L.M.; Borba, H.H.; Sanches, A.C.; Wiens, A.; Pontarolo, R.; Fernandez-Llimos, F. Efficacy and safety of amphotericin B formulations: A network meta-analysis and a multicriteria decision analysis. J. Pharm. Pharmacol. 2017, 69, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, U.; Seifert, B.; Schaffner, A. Comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours: Randomized controlled trial. BMJ 2001, 322, 579. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Karageorgopoulos, D.E.; Tansarli, G.S. Continuous versus conventional infusion of amphotericin B deoxycholate: A meta-analysis. PLoS ONE 2013, 8, e77075. [Google Scholar] [CrossRef] [PubMed]
- Geersing, T.H.; Franssen, E.J.F.; Spronk, P.E.; van Kan, H.J.M.; den Reijer, M.; van der Voort, P.H.J. Nephrotoxicity of continuous amphotericin B in critically ill patients with abdominal sepsis: A retrospective analysis with propensity score matching. J. Antimicrob. Chemother. 2021, 77, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Olivero, J.J.; Lozano-Mendez, J.; Ghafary, E.M.; Eknoyan, G.; Suki, W.N. Mitigation of amphotericin B nephrotoxicity by mannitol. Br. Med. J. 1975, 1, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Bullock, W.E.; Luke, R.G.; Nuttall, C.E.; Bhathena, D. Can mannitol reduce amphotericin B nephrotoxicity? Double-blind study and description of a new vascular lesion in kidneys. Antimicrob. Agents. Chemother. 1976, 10, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Galloway, M.J.; Reilly, J.T.; Davies, J.M. Amiloride prevents amphotericin B related hypokalaemia in neutropenic patients. J. Clin. Pathol. 1988, 41, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Ural, A.U.; Avcu, F.; Cetin, T.; Beyan, C.; Kaptan, K.; Nazaroglu, N.K.; Yalcin, A. Spironolactone: Is it a novel drug for the prevention of amphotericin B-related hypokalemia in cancer patients? Eur. J. Clin. Pharmacol. 2002, 57, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Camp, M.J.; Wingard, J.R.; Gilmore, C.E.; Lin, L.S.; Dix, S.P.; Davidson, T.G.; Geller, R.B. Efficacy of low-dose dopamine in preventing amphotericin B nephrotoxicity in bone marrow transplant patients and leukemia patients. Antimicrob. Agents Chemother. 1998, 42, 3103–3106. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Sepehr-Sobhani, A.; Khoshnoud, M.J.; Sagheb, M.M.; Vejdani, R.; Jalali, A.; Mahi-Birjand, M. Comparison of intravenous sodium bicarbonate and sodium chloride combination versus intravenous sodium chloride hydration alone in reducing amphotericin B nephrotoxicity: A randomized clinical trial. Res. Pharm. Sci. 2020, 15, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Panahi-Shokouh, M.; Moghaddas, A.; Badri, S.; Jabalameli, S.; Momenzadeh, M.; Mehrzad, V.; Ashrafi, F. Pentoxifylline in Prevention of Amphotericin B-induced Nephrotoxicity and Electrolyte Abnormalities. J. Res. Pharm. Pract. 2020, 9, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Khalili, H.; Sagheb, M.M.; Farsaei, S. A double-blinded, placebo-controlled, multicenter clinical trial of N-acetylcysteine for preventing amphotericin B-induced nephrotoxicity. Expert Opin. Drug. Metab. Toxicol. 2015, 11, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.D.; Lewis, R.E. Fungal Infections. In Applied Therapeutics: The Clinical Use of Drugs, 12th ed.; Zeind, C.S., Carvalho, M.G., Eds.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2023. [Google Scholar]
- Carver, P.L.; Eschenauer, G.A. Invasive Fungal Infections. In Pharmacotherapy: A Pathophysiologic Approach, 12th ed.; DiPiro, J.T., Yee, G.C., Posey, L.M., Haines, S.T., Nolin, T.D., Ellingrod, V., Eds.; McGraw Hill: New York, NY, USA, 2023. [Google Scholar]
- Londeree, J.; Winterberg, P.D.; Garro, R.; George, R.P.; Shin, S.; Liverman, R.; Serluco, A.; Romero, R.; Yildirim, I. Brincidofovir for the treatment of human adenovirus infection in pediatric solid organ transplant recipients: A case series. Pediatr. Transplant. 2020, 24, e13769. [Google Scholar] [CrossRef] [PubMed]
- Heil, E.L.; Corbett, A.H. Opportunistic Infections in patients living with HIV. In Applied Therapeutics: The Clinical Use of Drugs, 12th ed.; Zeind, C.S., Carvalho, M.G., Eds.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2023. [Google Scholar]
- Foscarnet: Drug Information. UpToDate 2025. Available online: https://www.uptodateonline.ir/contents/mobipreview.htm?28/56/29575?source=see_link (accessed on 21 April 2025).
- UCSF Medical Center: Foscarnet Dosing and Monitoring. Available online: https://idmp.ucsf.edu/sites/g/files/tkssra4251/f/wysiwyg/UCSF%20Medical%20Center_%20Foscarnet%20Dosing%20and%20Monitoring.pdf (accessed on 20 April 2025).
- Foscarnet (Foscavir®). Available online: https://globalrph.com/dilution/foscarnet-foscavir/ (accessed on 21 April 2025).
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [PubMed]
- Giacobbe, D.R.; Karaiskos, I.; Bassetti, M. How do we optimize the prescribing of intravenous polymyxins to increase their longevity and efficacy in critically ill patients? Expert Opin. Pharmacother. 2022, 23, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Falagas, M.E. The safety of polymyxin antibiotics. Expert Opin. Drug Saf. 2015, 14, 1687–1701. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.M.; Kane-Gill, S.L.; Murray, P.T. A ray of hope in the discord: Is adding piperacillin-tazobactam to vancomycin truly more nephrotoxic? Intensive Care Med. 2022, 48, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.; Côté, J.M.; Cotter, A.; Lynch, B.; Redahan, L.; Murray, P.T. Nephrotoxicity from Vancomycin Combined with Piperacillin-Tazobactam: A Comprehensive Review. Am. J. Nephrol. 2021, 52, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.H.; Sakoulas, G. Vancomycin: Parenteral Dosing, Monitoring, and Adverse Effects in Adults. Uptodate. 2025. Available online: https://www.uptodate.com/contents/vancomycin-parenteral-dosing-monitoring-and-adverse-effects-in-adults (accessed on 30 April 2025).
- He, N.; Su, S.; Ye, Z.; Du, G.; He, B.; Li, D.; Liu, Y.; Yang, K.; Zhang, X.; Zhang, Y.; et al. Evidence-based Guideline for Therapeutic Drug Monitoring of Vancomycin: 2020 Update by the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Clin. Infect. Dis. 2020, 71, S363–S371. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, B.Y.; Song, J.Y.; Seo, K.H.; Lee, S.H.; Choi, S.; Rhew, K. Effects of AUC-Based Vancomycin Therapeutic Drug Monitoring on AKI Incidence and Drug Utilization: A Propensity Score-Weighted Analysis. J. Clin. Med. 2025, 14, 1863. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaviria, R.; Norman, S.J.; Elgendi, S.H.; Chou, J.; Ramdeen, S. Incidence of Acute Kidney Injury in Trough and AUC/MIC Vancomycin Dosing Strategies in a Large Tertiary Care Center: A Retrospective Cohort. J. Clin. Pharmacol. 2025, 65, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zamoner, W.; Eid, K.Z.C.; de Almeida, L.M.B.; Pierri, I.G.; Santos, A.D.; Balbi, A.L.; Ponce, D. The Serum Concentration of Vancomycin as a Diagnostic Predictor of Nephrotoxic Acute Kidney Injury in Critically Ill Patients. Antibiotics 2022, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.H. Dosing and Administration of Parenteral Aminoglycosides. Uptodate. 2025. Available online: https://www.uptodate.com/contents/dosing-and-administration-of-parenteral-aminoglycosides (accessed on 1 May 2025).
- Destache, C.J. Aminoglycoside-induced nephrotoxicity--a focus on monitoring: A review of literature. J. Pharm. Pract. 2014, 27, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.P.; Cipullo, J.P.; Burdmann, E.A. Aminoglycoside nephrotoxicity. Braz. J. Cardiovasc. Surg. 2006, 21, 444–452. [Google Scholar] [CrossRef]
- Tashiro, M.; Obata, Y.; Takazono, T.; Ota, Y.; Wakamura, T.; Shiozawa, Y.; Tsuyuki, A.; Miyazaki, T.; Nishino, T.; Izumikawa, K. Association between fluid infusions and the recovery from acute kidney injury in patients administered liposomal amphotericin B: A nationwide observational study. Ren. Fail. 2022, 44, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Zachary, K.C. Cidofovir: An Overview. UpToDate. 2025. Available online: https://www.uptodate.com/contents/cidofovir-an-overview (accessed on 11 April 2025).
- Ortiz, A.; Justo, P.; Sanz, A.; Melero, R.; Caramelo, C.; Guerrero, M.F.; Strutz, F.; Müller, G.; Barat, A.; Egido, J. Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir. Ther. 2005, 10, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Caruso Brown, A.E.; Cohen, M.N.; Tong, S.; Braverman, R.S.; Rooney, J.F.; Giller, R.; Levin, M.J. Pharmacokinetics and safety of intravenous cidofovir for life-threatening viral infections in pediatric hematopoietic stem cell transplant recipients. Antimicrob. Agents. Chemother. 2015, 59, 3718–3725. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.B.; Brothers, A.W.; Englund, J.A. Renal Toxicity in Pediatric Patients Receiving Cidofovir for the Treatment of Adenovirus Infection. J. Pediatric. Infect. Dis. Soc. 2017, 6, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.; Alonso, C.D.; Garcia-Vidal, C.; Cardozo, C.; Slavin, M.; Yong, M.K.; Ho, S.A.; Mehta Steinke, S.; Avery, R.K.; Koehler, P.; et al. Safety and efficacy of intravenously administered cidofovir in adult haematopoietic cell transplant recipients: A retrospective multicentre cohort study. J. Antimicrob. Chemother. 2021, 76, 3020–3028. [Google Scholar] [CrossRef] [PubMed]
- Leowattana, W. Antiviral Drugs and Acute Kidney Injury (AKI). Infect. Disord. Drug Targets. 2019, 19, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Sharfuddin, A.A.; Weisbord, S.D.; Palevsky, P.M.; Molitoris, B.A. Acute Kidney Injury. In Brenner and Rector’s the Kidney, 11th ed.; Chertow, G., Luyckx, V., Marsden, P., Skorecki, K., Taal, M., Alan, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Tang, Z.; Li, T.; Dai, H.; Feng, C.; Xie, X.; Peng, F.; Lan, G.; Yu, S.; Wang, Y.; Fang, C.; et al. Drug-induced Fanconi syndrome in patients with kidney allograft transplantation. Front. Immunol. 2022, 13, 979983. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Bass, P.; Unwin, R.J. Drug-induced renal Fanconi syndrome. QJM 2014, 107, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Taber, D.J.; Dupuis, R.E.; Pilch, N.A.; Szempruch, K. Kidney and liver transplantation. In Applied Therapeutics: The Clinical Use of Drugs, 12th ed.; Zeind, C.S., Carvalho, M.G., Eds.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2023. [Google Scholar]
- Grimley, M.S.; Chemaly, R.F.; Englund, J.A.; Kurtzberg, J.; Chittick, G.; Brundage, T.M.; Bae, A.; Morrison, M.E.; Prasad, V.K. Brincidofovir for Asymptomatic Adenovirus Viremia in Pediatric and Adult Allogeneic Hematopoietic Cell Transplant Recipients: A Randomized Placebo-Controlled Phase II Trial. Biol. Blood Marrow Transplant. 2017, 23, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, T.; Sifontis, N. Drug interactions and toxicities associated with the antiviral management of cytomegalovirus infection. Am. J. Health Syst. Pharm. 2010, 67, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Beaufils, H.; Deray, G.; Katlama, C.; Dohin, E.; Henin, D.; Sazdovitch, V.; Jouanneau, C. Foscarnet and crystals in glomerular capillary lumens. Lancet 1990, 336, 755. [Google Scholar] [CrossRef] [PubMed]
- Zavras, P.; Su, Y.; Fang, J.; Stern, A.; Gupta, N.; Tang, Y.; Raval, A.; Giralt, S.; Perales, M.A.; Jakubowski, A.A.; et al. Impact of Preemptive Therapy for Cytomegalovirus on Toxicities after Allogeneic Hematopoietic Cell Transplantation in Clinical Practice: A Retrospective Single-Center Cohort Study. Biol. Blood Marrow Transplant. 2020, 26, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Arav-Boger, R.; Marr, K.A.; Kraus, E.; Shoham, S.; Lees, L.; Trollinger, B.; Shah, P.; Ambinder, R.; Neofytos, D.; et al. Outcomes in Transplant Recipients Treated With Foscarnet for Ganciclovir-Resistant or Refractory Cytomegalovirus Infection. Transplantation 2016, 100, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Minces, L.R.; Nguyen, M.H.; Mitsani, D.; Shields, R.K.; Kwak, E.J.; Silveira, F.P.; Abdel-Massih, R.; Pilewski, J.M.; Crespo, M.M.; Bermudez, C.; et al. Ganciclovir-resistant cytomegalovirus infections among lung transplant recipients are associated with poor outcomes despite treatment with foscarnet-containing regimens. Antimicrob. Agents Chemother. 2014, 58, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Reusser, P.; Einsele, H.; Lee, J.; Volin, L.; Rovira, M.; Engelhard, D.; Finke, J.; Cordonnier, C.; Link, H.; Ljungman, P.; et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood 2002, 99, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Domingo, W.; Nguyen, I.T.; Johnsrud, J.J.; Brown, J.W. Continuous-Infusion Foscarnet Facilitates Administration in Hematopoietic Stem Cell Transplantation Patients. Transplant. Cell. Ther. 2021, 27, 622.e1–622.e5. [Google Scholar] [CrossRef] [PubMed]
- Frochot, V.; Bazin, D.; Letavernier, E.; Jouanneau, C.; Haymann, J.P.; Daudon, M. Nephrotoxicity induced by drugs: The case of foscarnet and atazanavir—A SEM and μFTIR investigation. Comptes Rendus Chim. 2016, 19, 1565–1572. [Google Scholar] [CrossRef]
- Deffert, C.; Stoermann, C.; Ernandez, T.; Nabergoj, M.; Chalandon, Y.; Jaeger, P. Phosphonoformate Crystalluria, A Warning Signal of Foscarnet-Induced Kidney Injury. Kidney Int. Rep. 2020, 5, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.C.; Burgess, D.S. Antimicrobial regimen selection. In Pharmacotherapy: A Pathophysiologic Approach, 12th ed.; DiPiro, J.T., Yee, G.C., Posey, L.M., Haines, S.T., Nolin, T.D., Ellingrod, V., Eds.; McGraw Hill: New York, NY, USA, 2023. [Google Scholar]
- Bacigalupo, A.; Boyd, A.; Slipper, J.; Curtis, J.; Clissold, S. Foscarnet in the management of cytomegalovirus infections in hematopoietic stem cell transplant patients. Expert Rev. Anti. Infect. Ther. 2012, 10, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.G.; Grant, M.J.; Thomas, S.M.; Cameron, B.; Raiff, D.; Corbet, K.; Loitsch, G.; Ferreri, C.; Horwitz, M. Treatment with Foscarnet after Allogeneic Hematopoietic Cell Transplant (Allo-HCT) Is Associated with Long-Term Loss of Renal Function. Biol. Blood Marrow Transplant. 2020, 26, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Crystalline-Induced Acute Kidney Injury. UpToDate. 2025. Available online: https://www.uptodate.com/contents/crystalline-induced-acute-kidney-injury (accessed on 20 April 2025).
- Refardt, J. Diagnosis and differential diagnosis of diabetes insipidus: Update. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101398. [Google Scholar] [CrossRef] [PubMed]
- Akalya, K.; Murali, T.M.; Vathsala, A.; Teo, B.W.; Low, S.; Dharmasegaran, D.; Koh, L.P.; Bonney, G.K.; Hong, W.Z.; Da, Y.; et al. Elevated Urinary Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor Binding Protein-7 Predict Drug-Induced Acute Kidney Injury. Curr. Drug Metab. 2022, 23, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Deray, G.; Martinez, F.; Katlama, C.; Levaltier, B.; Beaufils, H.; Danis, M.; Rozenheim, M.; Baumelou, A.; Dohin, E.; Gentilini, M.; et al. Foscarnet nephrotoxicity: Mechanism, incidence and prevention. Am. J. Nephrol. 1989, 9, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.W.; Jayaweera, D.T.; Pearce, D.; Benson, P.; Nahass, R.; Olson, C.; Wool, G.M. Safety of oral versus intravenous hydration during induction therapy with intravenous foscarnet in AIDS patients with cytomegalovirus infections. Int. J. STD AIDS 2000, 11, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Chang, A.; Lee-Lam, F.; Tan, S.K.; Shashidhar, S. Tolerability of foscarnet as a continuous infusion for treatment of herpesvirus infections. Biol. Blood Marrow Transplant. 2014, 20, S184–S210. [Google Scholar] [CrossRef]
- Kaye, K.S.; Pogue, J.M.; Kaye, D. Polymyxins (Polymyxin Band Colistin). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of Infections Due to MDR Gram-Negative Bacteria. Front. Med. 2019, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A Review of the Clinical Pharmacokinetics of Polymyxin B. Antibiotics 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Elyasi, S. Prevention of colistin induced nephrotoxicity: A review of preclinical and clinical data. Expert Rev. Clin. Pharmacol. 2021, 14, 1113–1131. [Google Scholar] [CrossRef] [PubMed]
- Oliota, A.F.; Penteado, S.T.; Tonin, F.S.; Fernandez-Llimos, F.; Sanches, A.C. Nephrotoxicity prevalence in patients treated with polymyxins: A systematic review with meta-analysis of observational studies. Diagn. Microbiol. Infect. Dis. 2019, 94, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Eljaaly, K.; Bidell, M.R.; Gandhi, R.G.; Alshehri, S.; Enani, M.A.; Al-Jedai, A.; Lee, T.C. Colistin Nephrotoxicity: Meta-Analysis of Randomized Controlled Trials. Open Forum Infect. Dis. 2021, 8, ofab026. [Google Scholar] [CrossRef] [PubMed]
- Sisay, M.; Hagos, B.; Edessa, D.; Tadiwos, Y.; Mekuria, A.N. Polymyxin-induced nephrotoxicity and its predictors: A systematic review and meta-analysis of studies conducted using RIFLE criteria of acute kidney injury. Pharmacol. Res. 2021, 163, 105328. [Google Scholar] [CrossRef] [PubMed]
- Nakwan, N.; Chokephaibulkit, K.; Imberti, R. The Use of Colistin for the Treatment of Multidrug-resistant Gram-negative Infections in Neonates and Infants: A Review of the Literature. Pediatr. Infect. Dis. J. 2019, 38, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Karageorgos, S.A.; Bassiri, H.; Siakallis, G.; Miligkos, M.; Tsioutis, C. Intravenous colistin use for infections due to MDR Gram-negative bacilli in critically ill paediatric patients: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2019, 74, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Dewan, A. Comparison of nephrotoxicity of Colistin with Polymyxin B administered in currently recommended doses: A prospective study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Ballı, F.N.; Ekinci, P.B.; Kurtaran, M.; Kara, E.; Dizman, G.T.; Sönmezer, M.Ç.; Hayran, M.; Demirkan, K.; Metan, G. Battle of polymyxin induced nephrotoxicity: Polymyxin B versus colistin. Int. J. Antimicrob. Agents 2024, 63, 107035. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xiang, B.X.; Song, X.L.; Que, R.M.; Zuo, X.C.; Xie, Y.L. Prevalence of polymyxin-induced nephrotoxicity and its predictors in critically ill adult patients: A meta-analysis. World J. Clin. Cases 2022, 10, 11466–11485. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.; Lucenteforte, E.; Pea, F.; Soriano, A.; Tavoschi, L.; Steele, V.R.; Henriksen, A.S.; Longshaw, C.; Manissero, D.; Pecini, R.; et al. Systematic review on estimated rates of nephrotoxicity and neurotoxicity in patients treated with polymyxins. Clin. Microbiol. Infect. 2021, 27, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Ordooei Javan, A.; Shokouhi, S.; Sahraei, Z. A review on colistin nephrotoxicity. Eur. J. Clin. Pharmacol. 2015, 71, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Y.; Lee, Y.T.; Pan, S.W.; Yang, K.Y.; Chen, Y.M.; Yen, D.H.; Li, S.Y.; Wang, F.D. Comparison of colistin-induced nephrotoxicity between two different formulations of colistin in critically ill patients: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.B.; Zhang, X.S.; Wang, Y.X.; Wang, Y.Z.; Zhou, H.M.; Xu, F.M.; Yu, J.H.; Zhang, L.W.; Dai, Y.; Zhou, Z.Y.; et al. Population Pharmacokinetics of Colistin Sulfate in Critically Ill Patients: Exposure and Clinical Efficacy. Front. Pharmacol. 2022, 13, 915958. [Google Scholar] [CrossRef] [PubMed]
- Ozel, A.S.; Ergönül, Ö.; Korten, V. Colistin nephrotoxicity in critically ill patients after implementation of a new dosing strategy. J. Infect. Dev. Ctries. 2019, 13, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Rigatto, M.H.P.; Falci, D.R.; Zavascki, A.P. Polymyxin Acute Kidney Injury: Dosing and Other Strategies to Reduce Toxicity. Antibiotics 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef] [PubMed]
- Beirne, G.J.; Hansing, C.E.; Octaviano, G.N.; Burns, R.O. Acute renal failure caused by hypersensitivity to polymyxin B sulfate. JAMA 1967, 202, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Kallel, H.; Hamida, C.B.; Ksibi, H.; Bahloul, M.; Hergafi, L.; Chaari, A.; Chelly, H.; Bouaziz, M. Suspected acute interstitial nephritis induced by colistin. J. Nephrol. 2005, 18, 323–326. [Google Scholar] [PubMed]
- Zavascki, A.P.; Nation, R.L. Nephrotoxicity of Polymyxins: Is There Any Difference between Colistimethate and Polymyxin B? Antimicrob. Agents Chemother. 2017, 61, e02319-16. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Lee, J.; Marchaim, D.; Yee, V.; Zhao, J.J.; Chopra, T.; Lephart, P.; Kaye, K.S. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin. Infect. Dis. 2011, 53, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Sorlí, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Álvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Mount, K.; Murphy, C. Nephrotoxicity associated with intravenous colistin in critically ill patients. Pharmacotherapy 2011, 31, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Fan, W.; Griffith, D.C.; Dudley, M.N.; Sulham, K.A. A Retrospective Cohort Analysis Shows that Coadministration of Minocycline with Colistin in Critically Ill Patients Is Associated with Reduced Frequency of Acute Renal Failure. Antimicrob. Agents Chemother. 2017, 62, e01165-17. [Google Scholar] [CrossRef] [PubMed]
- Heybeli, C.; Canaslan, K.; Oktan, M.A.; Yıldız, S.; Arda, H.Ü.; Çavdar, C.; Çelik, A.; Gökmen, N.; Cömert, B. Acute kidney injury following colistin treatment in critically-ill patients: May glucocorticoids protect? J. Chemother. 2021, 33, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Niu, H.; Wang, R.; Cai, Y. Safety and efficacy of colistin alone or in combination in adults with Acinetobacter baumannii infection: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2019, 53, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.J.; Wang, F.; Tang, L.; Bakker, J.; Liu, J.C. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2014, 44, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Y.; Fang, Y.; Wang, X.; Chen, Y.; Qi, Q.; Huang, F.; Xiao, X. Meta-analysis of colistin for the treatment of Acinetobacter baumannii infection. Sci. Rep. 2015, 5, 17091. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Wang, H.; Zhao, J.; Yang, X.; Wu, B.; Sun, W.; Huang, M.; Cheng, Z.; Chen, H.; Song, Y.; et al. Risk factors for polymyxin B-associated acute kidney injury. Int. J. Infect. Dis. 2022, 117, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.A.; Lee, J.E.; Huh, W.; Peck, K.R.; Kim, Y.G.; Kim, D.J.; Oh, H.Y. Predictors of acute kidney injury associated with intravenous colistin treatment. Int. J. Antimicrob. Agents 2010, 35, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Deryke, C.A.; Crawford, A.J.; Uddin, N.; Wallace, M.R. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrob. Agents Chemother. 2010, 54, 4503–4505. [Google Scholar] [CrossRef] [PubMed]
- Justo, J.A.; Bosso, J.A. Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy 2015, 35, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.H.; Yoo, S.; Pai, H. Clinical characteristics and risk factors of colistin-induced nephrotoxicity. Int. J. Antimicrob. Agents 2009, 34, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; di Masi, A.; Leboffe, L.; Del Bono, V.; Rossi, M.; Cappiello, D.; Coppo, E.; Marchese, A.; Casulli, A.; Signori, A.; et al. Hypoalbuminemia as a predictor of acute kidney injury during colistin treatment. Sci. Rep. 2018, 8, 11968. [Google Scholar] [CrossRef] [PubMed]
- Özkarakaş, H.; Köse, I.; Zincircioğlu, Ç.; Ersan, S.; Ersan, G.; Şenoğlu, N.; Köse, Ş.; Erbay, R.H. Risk factors for colistin-associated nephrotoxicity and mortality in critically ill patients. Turk. J. Med. Sci. 2017, 47, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Spapen, H.; Jacobs, R.; Van Gorp, V.; Troubleyn, J.; Honoré, P.M. Renal and neurological side effects of colistin in critically ill patients. Ann. Intensive Care 2011, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Li, J.; Silveira, F.P.; Nation, R.L. Pharmacokinetic/Toxicodynamic Analysis of Colistin-Associated Acute Kidney Injury in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e01367-17. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, J.; Garzón, V.; Pacheco, T.; Avila, M.P.; Garcia, J.C.; Jaimes, D.; Torres, A.; Bustos, R.H.; Escobar-Perez, J.; Abril, D. Estimation of the Difference in Colistin Plasma Levels in Critically Ill Patients with Favorable or Unfavorable Clinical Outcomes. Pharmaceutics 2021, 13, 1630. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Gu, N.; Kwack, W.G.; Kang, Y.; Park, S.Y.; Yoon, Y.S. Prospective observational study of the impact of plasma colistin levels in patients with carbapenem-resistant Acinetobacter baumannii pneumonia. J. Glob. Antimicrob. Resist. 2021, 27, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, S.; Lu, J.; Sun, T.; Wang, P.; Zhang, X. An area under the concentration-time curve threshold as a predictor of efficacy and nephrotoxicity for individualizing polymyxin B dosing in patients with carbapenem-resistant gram-negative bacteria. Crit. Care 2022, 26, 320. [Google Scholar] [CrossRef] [PubMed]
- Shabani-Borujeni, M.; Farvadi, F.; Abolmaali, S.S.; Firouzabadi, D.; Rasaei, N.; Karimzadeh, I. The frequency, risk factors, onset time, and outcome of acute kidney injury induced by vancomycin, colistin, and liposomal amphotericin B in hospitalized patients. J. Renal Inj. Prev. 2025, 14, e37314. [Google Scholar] [CrossRef]
- Ko, H.j.; Jeon, M.h.; Choo, E.j.; Lee, E.j.; Kim, T.h.; Jun, J.B.; Gil, H.W. Early acute kidney injury is a risk factor that predicts mortality in patients treated with colistin. Nephron Clin. Pract. 2011, 117, c284–c288. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, G.; Spelman, D. Polymyxins: An Overview. UpToDate. 2025. Available online: https://www.uptodate.com/contents/polymyxins-an-overview (accessed on 25 April 2025).
- Falagas, M.E.; Rizos, M.; Bliziotis, I.A.; Rellos, K.; Kasiakou, S.K.; Michalopoulos, A. Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect. Dis. 2005, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Okusa, M.D.; Knicely, D.H. Etiology and Diagnosis of Prerenal Disease and Acute Tubular Necrosis in Acute Kidney Injury in Adults. UpToDate. 2025. Available online: https://www.uptodate.com/contents/etiology-and-diagnosis-of-prerenal-disease-and-acute-tubular-necrosis-in-acute-kidney-injury-in-adults (accessed on 25 April 2025).
- Moledina, D.G.; Perazella, M.A. Drug-Induced Acute Interstitial Nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Vazin, A.; Malek, M.; Karimzadeh, I. Evaluation of colistin nephrotoxicity and urinary level of kidney injury molecule-1 in hospitalized adult ICU patients. J. Renal Inj. Prev. 2020, 9, e13. [Google Scholar] [CrossRef]
- Ciftci, A.; Izdes, S.; Altintas, N.D. Factors Determining Nephrotoxicity and Mortality in Critical Care Patients Receiving Colistin. J. Infect. Dev. Ctries. 2018, 11, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.J.; Kazarov, C.L.; Wong, A.; Kane-Gill, S.L. Kidney Damage and Stress Biomarkers for Early Identification of Drug-Induced Kidney Injury: A Systematic Review. Drug Saf. 2022, 45, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Babic, J.T.; Manchandani, P.; Ledesma, K.R.; Tam, V.H. Evaluation of Urinary KIM-1 for Prediction of Polymyxin B-Induced Nephrotoxicity. Antimicrob. Agents Chemother. 2017, 61, e01735-17. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Eom, J.S.; Lee, J.S.; Ju, Y.S.; Park, J.Y. Neutrophil Gelatinase-associated Lipocalin as a Predictor of Acute Kidney Injury in Patients during Treatment with Colistimethate Sodium. Infect. Chemother. 2018, 50, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Thammathiwat, T.; Tiranathanagul, K.; Srisawat, N.; Susantitaphong, P.; Praditpornsilpa, K.; Eiam-Ong, S. Clinical and subclinical acute kidney injury in multidrug-resistant septic patients treated with colistimethate sodium: Incidence and clinical outcomes. Nephrology 2020, 25, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Lakota, E.A.; Landersdorfer, C.B.; Nation, R.L.; Li, J.; Kaye, K.S.; Rao, G.G.; Forrest, A. Personalizing Polymyxin B Dosing Using an Adaptive Feedback Control Algorithm. Antimicrob. Agents Chemother. 2018, 62, e00483-18. [Google Scholar] [CrossRef] [PubMed]
- Abdelraouf, K.; Braggs, K.H.; Yin, T.; Truong, L.D.; Hu, M.; Tam, V.H. Characterization of polymyxin B-induced nephrotoxicity: Implications for dosing regimen design. Antimicrob. Agents Chemother. 2012, 56, 4625–4629. [Google Scholar] [CrossRef] [PubMed]
- Okoduwa, A.; Ahmed, N.; Guo, Y.; Scipione, M.R.; Papadopoulos, J.; Eiras, D.P.; Dubrovskaya, Y. Nephrotoxicity Associated with Intravenous Polymyxin B Once- versus Twice-Daily Dosing Regimen. Antimicrob. Agents Chemother. 2018, 62, e00025-18. [Google Scholar] [CrossRef] [PubMed]
- Alvarado Reyes, Y.; Cruz, R.; Gonzalez, J.; Perez, Y.; Wolowich, W.R. Incidence of Acute Kidney Injury in Intermittent Versus Continuous Infusion of Polymyxin B in Hospitalized Patients. Ann. Pharmacother. 2019, 53, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Shojaei, L.; Mohammadi, M.; Beigmohammadi, M.T.; Abdollahi, A.; Doomanlou, M. Meropenem/colistin versus meropenem/ampicillin-sulbactam in the treatment of carbapenem-resistant pneumonia. J. Comp. Eff. Res. 2018, 7, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Zalts, R.; Neuberger, A.; Hussein, K.; Raz-Pasteur, A.; Geffen, Y.; Mashiach, T.; Finkelstein, R. Treatment of Carbapenem-Resistant Acinetobacter baumannii Ventilator-Associated Pneumonia: Retrospective Comparison Between Intravenous Colistin and Intravenous Ampicillin-Sulbactam. Am. J. Ther. 2016, 23, e78–e85. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Garau, J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J. Antimicrob. Chemother. 2021, 76, iv23–iv37. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Mirzaei, E.; Vazin, A. Pharmacological agents for the prevention of colistin-induced nephrotoxicity. Eur. J. Med. Res. 2022, 27, 64. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, I.; Sharma, A.; Esen, S. Colistin-induced nephrotoxicity and the role of N-acetylcysteine: A retrospective cohort study. J. Infect. Dev. Ctries. 2017, 11, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Timuroğlu, A.; Muslu, S.; Menteş, S.; Ünver, S. Effect of N-Acetyl Cysteine on acute kidney injury in patients with colistin used in intensive care; retrospective study. Acta Oncol. Turc. 2018, 51, 390–398. [Google Scholar] [CrossRef]
- Mosayebi, S.; Soltani, R.; Shafiee, F.; Assarzadeh, S.; Hakamifard, A. Evaluation of the Effectiveness of N-Acetylcysteine in the Prevention of Colistin-Induced Nephrotoxicity: A Randomized Controlled Clinical Trial. J. Res. Pharm. Pract. 2022, 10, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sirijatuphat, R.; Limmahakhun, S.; Sirivatanauksorn, V.; Nation, R.L.; Li, J.; Thamlikitkul, V. Preliminary clinical study of the effect of ascorbic acid on colistin-associated nephrotoxicity. Antimicrob. Agents Chemother. 2015, 59, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Dalfino, L.; Puntillo, F.; Ondok, M.J.; Mosca, A.; Monno, R.; Coppolecchia, S.; Spada, M.L.; Bruno, F.; Brienza, N. Colistin-associated Acute Kidney Injury in Severely Ill Patients: A Step Toward a Better Renal Care? A Prospective Cohort Study. Clin. Infect. Dis. 2015, 61, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Samsami, M.; Shabani, M.; Hajiesmaeili, M.; Tavakoli-Ardakani, M.; Ardehali, S.H.; Fatemi, A.; Barati, S.; Moradi, O.; Sahraei, Z. The effects of vitamin E on colistin-induced nephrotoxicity in treatment of drug-resistant gram-negative bacterial infections: A randomized clinical trial. J. Infect. Chemother. 2021, 27, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Torabi, M.; Sistanizad, M.; Kouchek, M.; Miri, M.M.; Salarian, S.; Ansar, P. Oral melatonin for colistin-induced nephrotoxicity reduction in intensive care unit: A randomized placebo controlled clinical trial. Nephro-Urol. Mon. 2023, 15, e135436. [Google Scholar] [CrossRef]
- Shields, R.K.; Paterson, D.L.; Tamma, P.D. Navigating Available Treatment Options for Carbapenem-Resistant Acinetobacter baumannii-calcoaceticus Complex Infections. Clin. Infect. Dis. 2023, 76, S179–S193. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [PubMed]
- Zasowski, E.J.; Blackford, M. Lower respiratory tract infections. In Pharmacotherapy: A Pathophysiologic Approach, 12th ed.; DiPiro, J.T., Yee, G.C., Posey, L.M., Haines, S.T., Nolin, T.D., Ellingrod, V., Eds.; McGraw Hill: New York, NY, USA, 2023. [Google Scholar]
- Roberts, J.A.; Taccone, F.S.; Udy, A.A.; Vincent, J.L.; Jacobs, F.; Lipman, J. Vancomycin dosing in critically ill patients: Robust methods for improved continuous-infusion regimens. Antimicrob. Agents Chemother. 2011, 55, 2704–2709. [Google Scholar] [CrossRef] [PubMed]
- Kan, W.C.; Chen, Y.C.; Wu, V.C.; Shiao, C.C. Vancomycin-Associated Acute Kidney Injury: A Narrative Review from Pathophysiology to Clinical Application. Int. J. Mol. Sci. 2022, 23, 2052. [Google Scholar] [CrossRef] [PubMed]
- Chotiprasitsakul, D.; Tamma, P.D.; Gadala, A.; Cosgrove, S.E. The Role of Negative Methicillin-Resistant Staphylococcus aureus Nasal Surveillance Swabs in Predicting the Need for Empiric Vancomycin Therapy in Intensive Care Unit Patients. Infect. Control Hosp. Epidemiol. 2018, 39, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, F.C.S.; Libório, A.B. Attributable nephrotoxicity of vancomycin in critically ill patients: A marginal structural model study. J. Antimicrob. Chemother. 2020, 75, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, D.Y.; Kane-Gill, S.; Goldstein, S.L.; Priyanka, P.; Kellum, J.A. Acute kidney injury epidemiology, risk factors, and outcomes in critically ill patients 16-25 years of age treated in an adult intensive care unit. Ann. Intensive Care 2018, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.; João, P.R.D.; Sylvestre, L.C. Impact of the use of nephrotoxic drugs in critically ill pediatric patients. Rev. Bras. Ter. Intensiv. 2020, 32, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Hays, W.B.; Tillman, E. Vancomycin-Associated Acute Kidney Injury in Critically Ill Adolescent and Young Adult Patients. J. Pharm. Pract. 2020, 33, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Sinha Ray, A.; Haikal, A.; Hammoud, K.A.; Yu, A.S. Vancomycin and the Risk of AKI: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Bailie, G.R.; Neal, D. Vancomycin ototoxicity and nephrotoxicity. A review. Med. Toxicol. Advers. Drug Exp. 1988, 3, 376–386. [Google Scholar]
- Buckley, M.S.; Komerdelj, I.A.; D’Alessio, P.A.; Rangan, P.; Agarwal, S.K.; Tinta, N.C.; Martinez, B.K.; Ziadat, D.S.; Yerondopoulos, M.J.; Kobic, E.; et al. Vancomycin with concomitant piperacillin/tazobactam vs. cefepime or meropenem associated acute kidney injury in the critically ill: A multicenter propensity score-matched study. J. Crit. Care 2022, 67, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tantranont, N.; Luque, Y.; Hsiao, M.; Haute, C.; Gaber, L.; Barrios, R.; Adrogue, H.E.; Niasse, A.; Truong, L.D. Vancomycin-Associated Tubular Casts and Vancomycin Nephrotoxicity. Kidney Int. Rep. 2021, 6, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Pergialiotis, V.; Perrea, D.N. Kidney biopsy findings in vancomycin-induced acute kidney injury: A pooled analysis. Int. Urol. Nephrol. 2022, 54, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Nachiappa Ganesh, R.; Edwards, A.; El Zaatari, Z.; Gaber, L.; Barrios, R.; Truong, L.D. Vancomycin nephrotoxicity: A comprehensive clinico-pathological study. PLoS ONE 2024, 19, e0295136. [Google Scholar] [CrossRef] [PubMed]
- Elyasi, S.; Khalili, H.; Dashti-Khavidaki, S.; Mohammadpour, A. Vancomycin-induced nephrotoxicity: Mechanism, incidence, risk factors and special populations. A literature review. Eur. J. Clin. Pharmacol. 2012, 68, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E.; Domański, L.; Dziedziejko, V.; Kajdy, A.; Stefańska, K.; Kwiatkowski, S. The Mechanism of Drug Nephrotoxicity and the Methods for Preventing Kidney Damage. Int. J. Mol. Sci. 2021, 22, 6109. [Google Scholar] [CrossRef] [PubMed]
- Kannan, L.; Raj, R. Case Report: Vancomycin-Associated Tubulointerstitial Nephritis in Clinical Practice-Case Report and Review of Literature. Front. Med. 2022, 9, 899886. [Google Scholar] [CrossRef] [PubMed]
- Michail, S.; Vaiopoulos, G.; Nakopoulou, L.; Revenas, C.; Aroni, K.; Karam, P.; Stathakis, C.; Thosios, T. Henoch-Schoenlein purpura and acute interstitial nephritis after intravenous vancomycin administration in a patient with a staphylococcal infection. Scand. J. Rheumatol. 1998, 27, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Luque, Y.; Louis, K.; Jouanneau, C.; Placier, S.; Esteve, E.; Bazin, D.; Rondeau, E.; Letavernier, E.; Wolfromm, A.; Gosset, C.; et al. Vancomycin-Associated Cast Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.B.; Stevens, J.S. Vancomycin-Associated Cast Nephropathy: Reality or Fantasy? Kidney360 2021, 3, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Van Driest, S.L.; McGregor, T.L.; Velez Edwards, D.R.; Saville, B.R.; Kitchner, T.E.; Hebbring, S.J.; Brilliant, M.; Jouni, H.; Kullo, I.J.; Creech, C.B.; et al. Genome-Wide Association Study of Serum Creatinine Levels during Vancomycin Therapy. PLoS ONE 2015, 10, e0127791. [Google Scholar] [CrossRef] [PubMed]
- Luther, M.K.; Timbrook, T.T.; Caffrey, A.R.; Dosa, D.; Lodise, T.P.; LaPlante, K.L. Vancomycin Plus Piperacillin-Tazobactam and Acute Kidney Injury in Adults: A Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Karageorgiou, V.; Pergialiotis, V.; Perrea, D.N. Acute kidney injury following the concurrent administration of antipseudomonal β-lactams and vancomycin: A network meta-analysis. Clin. Microbiol. Infect. 2020, 26, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Blears, E.E.; Morris, J.; Popp, D.; Lee, J.O.; Norbury, W.B. Kidney Injury in Critically Ill Patients Treated with Vancomycin and Zosyn or an Alternative: A Systematic Review and Meta-Analysis. Surg. Infect. (Larchmt) 2022, 23, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Li, R.; Li, Y.; Ding, X.; Li, X.; Lv, Q. Vancomycin combined with piperacillin/tazobactam increases the risk of acute kidney injury compared with vancomycin plus other anti-pseudomonal beta-lactams: A systematic review and network meta-analysis. J. Antimicrob. Chemother. 2025, 80, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.T.; Akova, M. Piperacillin-Tazobactam Plus Vancomycin-Associated Acute Kidney Injury in Adults: Can Teicoplanin or Other Antipseudomonal Beta-Lactams Be Remedies? Healthcare 2022, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Miano, T.A.; Hennessy, S.; Yang, W.; Dunn, T.G.; Weisman, A.R.; Oniyide, O.; Agyekum, R.S.; Turner, A.P.; Ittner, C.A.G.; Anderson, B.J.; et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: A prospective cohort study. Intensive Care Med. 2022, 48, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ren, J.; Li, J.; Jin, X.; Gao, Y.; Li, R.; Zhang, J.; Wang, X.; Li, X.; Wang, G. Relationship Between Mean Vancomycin Trough Concentration and Mortality in Critically Ill Patients: A Multicenter Retrospective Study. Front. Pharmacol. 2021, 12, 690157. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Vazin, A.; Zand, F.; Haem, E.; Karimzadeh, I.; Azadi, A.; Masjedi, M.; Sabetian, G.; Nikandish, R.; Mohammadi-Samani, S. Pharmacokinetic assessment of vancomycin in critically ill patients and nephrotoxicity prediction using individualized pharmacokinetic parameters. Front. Pharmacol. 2022, 13, 912202. [Google Scholar] [CrossRef] [PubMed]
- Aljefri, D.M.; Avedissian, S.N.; Rhodes, N.J.; Postelnick, M.J.; Nguyen, K.; Scheetz, M.H. Vancomycin Area Under the Curve and Acute Kidney Injury: A Meta-analysis. Clin. Infect. Dis. 2019, 69, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Selby, P.R.; Reuter, S.E. Determination of a vancomycin nephrotoxicity threshold and assessment of target attainment in hematology patients. Pharmacol. Res. Perspect. 2024, 12, e1231. [Google Scholar] [CrossRef] [PubMed]
- Tangvichitrerk, P.; Changpradub, D.; Hemapanpairoa, J.; Juntanawiwat, P.; Santimaleeworagun, W. Impact of vancomycin area under the curve in early or later phase on efficacy and nephrotoxicity in patients with enterococcal bloodstream infections: A multicenter study. BMC Infect. Dis. 2025, 25, 133. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Alqurashi, M.K.; Althaqafi, A.S.; Alsharif, J.M.; Faidah, H.S.; Bushyah, M.; Alotaibi, A.F.; Elrggal, M.E.; Mahrous, A.J.; Abuhussain, S.S.A.; et al. A Systematic Review on Clinical Safety and Efficacy of Vancomycin Loading Dose in Critically Ill Patients. Antibiotics 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Wang, J.; Che, H.; Wang, R.; Cai, Y. The clinical efficacy and safety of vancomycin loading dose: A systematic review and meta-analysis. Medicine 2019, 98, e17639. [Google Scholar] [CrossRef] [PubMed]
- Wong-Beringer, A.; Joo, J.; Tse, E.; Beringer, P. Vancomycin-associated nephrotoxicity: A critical appraisal of risk with high-dose therapy. Int. J. Antimicrob. Agents 2011, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Awdishu, L.; Le, A.; Amato, J.; Jani, V.; Bal, S.; Mills, R.H.; Carrillo-Terrazas, M.; Gonzalez, D.J.; Tolwani, A.; Acharya, A.; et al. Urinary Exosomes Identify Inflammatory Pathways in Vancomycin Associated Acute Kidney Injury. Int. J. Mol. Sci. 2021, 22, 2784. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, N.; Wei, W.; Jiang, C. Outcomes and Nephrotoxicity Associated with Vancomycin Treatment in Patients 80 Years and Older. Clin. Interv. Aging 2021, 16, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.C.J.; Murray, K.P.; Lagnf, A.M.; Melvin, S.; Bhatia, S.; Shamim, M.D.; Smith, J.R.; Brade, K.D.; Simon, S.P.; Nagel, J.; et al. A Multicenter Evaluation of Vancomycin-Associated Acute Kidney Injury in Hospitalized Patients with Acute Bacterial Skin and Skin Structure Infections. Infect. Dis. Ther. 2020, 9, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Cano, E.L.; Haque, N.Z.; Welch, V.L.; Cely, C.M.; Peyrani, P.; Scerpella, E.G.; Ford, K.D.; Zervos, M.J.; Ramirez, J.A.; Kett, D.H. Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Study Group. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: Retrospective analysis of the IMPACT-HAP Database. Clin. Ther. 2012, 34, 149–157. [Google Scholar] [PubMed]
- Kane-Gill, S.L.; Ostermann, M.; Shi, J.; Joyce, E.L.; Kellum, J.A. Evaluating Renal Stress Using Pharmacokinetic Urinary Biomarker Data in Critically Ill Patients Receiving Vancomycin and/or Piperacillin-Tazobactam: A Secondary Analysis of the Multicenter Sapphire Study. Drug Saf. 2019, 42, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.M.; Qin, X.L.; Liu, T.T.; Wei, W.X.; Cheng, D.H.; Lu, H.; Guo, Q.; Jing, L. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as early biomarkers for predicting vancomycin-associated acute kidney injury: A prospective study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4203–4213. [Google Scholar] [PubMed]
- Imai, S.; Yamada, T.; Kasashi, K.; Kobayashi, M.; Iseki, K. Usefulness of a decision tree model for the analysis of adverse drug reactions: Evaluation of a risk prediction model of vancomycin-associated nephrotoxicity constructed using a data mining procedure. J. Eval. Clin. Pract. 2017, 23, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Yamada, T.; Kasashi, K.; Niinuma, Y.; Kobayashi, M.; Iseki, K. Construction of a risk prediction model of vancomycin-associated nephrotoxicity to be used at the time of initial therapeutic drug monitoring: A data mining analysis using a decision tree model. J. Eval. Clin. Pract. 2019, 25, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Miyai, T.; Imai, S.; Kashiwagi, H.; Sato, Y.; Kadomura, S.; Yoshida, K.; Yoshimura, E.; Teraya, T.; Tsujimoto, T.; Kawamoto, Y.; et al. A Risk Prediction Flowchart of Vancomycin-Induced Acute Kidney Injury to Use When Starting Vancomycin Administration: A Multicenter Retrospective Study. Antibiotics 2020, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Sun, Y.; Qiu, P.; Li, X. Development and validation of a nomogram to predict the risk of vancomycin-related acute kidney injury in critical care patients. Front. Pharmacol. 2024, 15, 1389140. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Su, S.; Yan, Y.; Liu, W.; Zhai, S. The Benefit of Individualized Vancomycin Dosing Via Pharmacokinetic Tools: A Systematic Review and Meta-analysis. Ann. Pharmacother. 2020, 54, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Executive Summary: Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2020, 40, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Flannery, A.H.; Bissell, B.D.; Bastin, M.T.; Morris, P.E.; Neyra, J.A. Continuous Versus Intermittent Infusion of Vancomycin and the Risk of Acute Kidney Injury in Critically Ill Adults: A Systematic Review and Meta-Analysis. Crit. Care Med. 2020, 48, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Elyasi, S.; Khalili, H.; Hatamkhani, S.; Dashti-Khavidaki, S. Prevention of vancomycin induced nephrotoxicity: A review of preclinical data. Eur. J. Clin. Pharmacol. 2013, 69, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, H.; Khalili, H. Prevention of Vancomycin-Induced Nephrotoxicity; An Updated Review of Clinical and Preclinical Studies. Infect. Disord. Drug Targets 2022, 22, e310321192584. [Google Scholar] [CrossRef] [PubMed]
- Bamgbola, O. Review of vancomycin-induced renal toxicity: An update. Ther. Adv. Endocrinol. Metab. 2016, 7, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Akundi, S.; Lee, Y.R.; Perry, G.K.; Fike, D.S.; Mnjoyan, S. Nephrotoxicity in Recipients of Vancomycin vs. Vancomycin with Vitamin C. Int. J. Med. Pharm. 2015, 3, 1–15. [Google Scholar] [CrossRef]
- He, J.; Mao, E.; Xu, W.; Zhao, B.; Jing, F.; Bian, X.; Chen, E. High dose vitamin C significantly reduces the nephrotoxicity of vancomycin in critically ill patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 468–472. [Google Scholar] [PubMed]
- Badri, S.; Soltani, R.; Sayadi, M.; Khorvash, F.; Meidani, M.; Taheri, S. Effect of N-acetylcysteine against Vancomycin-Induced Nephrotoxicity: A Randomized Controlled Clinical Trial. Arch. Iran. Med. 2020, 23, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Khorvash, F.; Meidani, M.; Badri, S.; Alaei, S.; Taheri, S. Vitamin E in the prevention of vancomycin-induced nephrotoxicity. Res. Pharm. Sci. 2020, 15, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.S.; Briscese, K.; Yuan, M.; Deshpande, K.; Aleksunes, L.M.; Brunetti, L. Renoprotective Effects of Melatonin against Vancomycin-Related Acute Kidney Injury in Hospitalized Patients: A Retrospective Cohort Study. Antimicrob. Agents Chemother. 2021, 65, e0046221. [Google Scholar] [CrossRef] [PubMed]
- Karimian, A.; Karimzadeh, I.; Shafiekhani, M.; Heidari, R.; Masjedi, F.; Izadi, F.; Barshan-Tashnizi, N.; Kane-Gill, S.L.; Mahmoudi, L. Protective effects of silymarin on preventing vancomycin nephrotoxicity in infectious patients: A randomized, double-blinded, placebo-controlled, pilot clinical trial. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 2945–2960. [Google Scholar] [CrossRef] [PubMed]
- Vancomycin-Induced Acute Interstitial Nephritis. J. Hosp. Med. 2011, 6 (Suppl. 2), Abstract 419, Abstract Published at Hospital Medicine 10–13 May 2011, Dallas, TX, USA. Available online: https://shmabstracts.org/abstract/vancomycininduced-acute-interstitial-nephritis/ (accessed on 1 May 2025).
- Sawada, A.; Kawanishi, K.; Morikawa, S.; Nakano, T.; Kodama, M.; Mitobe, M.; Taneda, S.; Koike, J.; Ohara, M.; Nagashima, Y.; et al. Biopsy-proven vancomycin-induced acute kidney injury: A case report and literature review. BMC Nephrol. 2018, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Strader, M.; Kane-Gill, S.L.; Murray, P.T. Prevention and management of antibiotic associated acute kidney injury in critically ill patients: New insights. Curr. Opin. Crit. Care 2023, 29, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Pagkalis, S.; Mantadakis, E.; Mavros, M.N.; Ammari, C.; Falagas, M.E. Pharmacological considerations for the proper clinical use of aminoglycosides. Drugs 2011, 71, 2277–2294. [Google Scholar] [CrossRef] [PubMed]
- Leggett, J.E. Aminoglycosides. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Wargo, K.A.; Edwards, J.D. Aminoglycoside-induced nephrotoxicity. J. Pharm. Pract. 2014, 27, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Duong, A.; Simard, C.; Wang, Y.L.; Williamson, D.; Marsot, A. Aminoglycosides in the Intensive Care Unit: What Is New in Population PK Modeling? Antibiotics 2021, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.; Silva, C.A.; Barbieri, C.D.; Oliveira, G.M.; Zanetta, D.M.; Burdmann, E.A. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob. Agents Chemother. 2009, 53, 2887–2891. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, B.A. Manifestations of and Risk Factors for Aminoglycoside Nephrotoxicity. Uptodate, 2025. Available online: https://www.uptodate.com/contents/manifestations-of-and-risk-factors-for-aminoglycoside-nephrotoxicity (accessed on 1 May 2025).
- Ehrmann, S.; Helms, J.; Joret, A.; Martin-Lefevre, L.; Quenot, J.P.; Herbrecht, J.E.; Benzekri-Lefevre, D.; Robert, R.; Desachy, A.; Bellec, F.; et al. Nephrotoxic drug burden among 1001 critically ill patients: Impact on acute kidney injury. Ann. Intensive Care 2019, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.B.; Gruneir, A.; Rochon, P.A.; Howard, A.W.; Koren, G.; Parshuram, C.S. Identifying High-Risk Medications Associated with Acute Kidney Injury in Critically Ill Patients: A Pharmacoepidemiologic Evaluation. Paediatr. Drugs 2017, 19, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, B.A. Pathogenesis and Prevention of Aminoglycoside Nephrotoxicity and Ototoxicity. Uptodate, 2025. Available online: https://www.uptodate.com/contents/pathogenesis-and-prevention-of-aminoglycoside-nephrotoxicity-and-ototoxicity (accessed on 1 May 2025).
- Buring, J.E.; Evans, D.A.; Mayrent, S.L.; Rosner, B.; Colton, T.; Hennekens, C.H. Randomized trials of aminoglycoside antibiotics: Quantitative overview. Rev. Infect Dis. 1988, 10, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Duszynska, W.; Taccone, F.S.; Hurkacz, M.; Kowalska-Krochmal, B.; Wiela-Hojeńska, A.; Kübler, A. Therapeutic drug monitoring of amikacin in septic patients. Crit. Care 2013, 17, R165. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L.; Ambrose, P.G.; Bhavnani, S.M.; Bertino, J.S.; Nafziger, A.N.; Louie, A. Back to the future: Using aminoglycosides again and how to dose them optimally. Clin. Infect. Dis. 2007, 45, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, S.; Yousefichaijan, P.; Rezagholizamenjany, M.; Rafie, Y.; Kahbazi, M.; Arjmand, A. Nephrotoxic effect of gentamicin and amikacin in neonates with infection. Nephro-Urol. Mon. 2018, 10, e58580. [Google Scholar] [CrossRef]
- Gerlach, A.T.; Stawicki, S.P.; Cook, C.H.; Murphy, C. Risk factors for aminoglycoside-associated nephrotoxicity in surgical intensive care unit patients. Int. J. Crit. Illn. Inj. Sci. 2011, 1, 17–21. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, S.J.; Antoine, D.J.; Sabbisetti, V.; Turner, M.A.; Farragher, T.; Bonventre, J.V.; Park, B.K.; Smyth, R.L.; Pirmohamed, M. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: A proof-of-concept study. PLoS ONE 2012, 7, e43809. [Google Scholar] [CrossRef] [PubMed]
- Shihana, F.; Barron, M.L.; Mohamed, F.; Seth, D.; Buckley, N.A. MicroRNAs in toxic acute kidney injury: Systematic scoping review of the current status. Pharmacol. Res. Perspect. 2021, 9, e00695. [Google Scholar] [CrossRef] [PubMed]
- Klementa, V.; Petejova, N.; Horak, P.; Kurasova, E.; Zadrazil, J. Acute kidney injury due to gentamicin nephrotoxicity and specific miRNAs as biomarkers. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2025, 169, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saikumar, J.; Hoffmann, D.; Kim, T.M.; Gonzalez, V.R.; Zhang, Q.; Goering, P.L.; Brown, R.P.; Bijol, V.; Park, P.J.; Waikar, S.S.; et al. Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol. Sci. 2012, 129, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Mensa, J.; Barberán, J.; Soriano, A.; Llinares, P.; Marco, F.; Cantón, R.; Bou, G.; González Del Castillo, J.; Maseda, E.; Azanza, J.R.; et al. Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa: Guidelines by the Spanish Society of Chemotherapy. Rev. Española Quimioter. 2018, 31, 78–100. [Google Scholar]
- Karimzadeh, I.; Abdollahpour-Alitappeh, M.; Ghaffari, S.; Mahi-Birjand, M.; Barkhordari, A.; Alemzadeh, E. Aminoglycosides: Single- or Multiple-daily Dosing? An Updated Qualitative Systematic Review of Randomized Trials on Toxicity and Efficacy. Curr. Mol. Med. 2024, 24, 1358–1373. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, S.J.; Antoine, D.J.; Smyth, R.L.; Pirmohamed, M. Aminoglycoside-induced nephrotoxicity in children. Pediatr. Nephrol. 2017, 32, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.P.; Huang, H.B.; Zhou, H.; Zhu, Y.; Xu, Y.; Du, B. Amikacin nebulization for the adjunctive therapy of gram-negative pneumonia in mechanically ventilated patients: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2021, 11, 6969. [Google Scholar] [CrossRef] [PubMed]
- Velissaris, D.; Karamouzos, V.; Marangos, M.; Pierrakos, C.; Karanikolas, M. Pharmacokinetic changes and dosing modification of aminoglycosides in critically ill obese patients: A literature review. J. Clin. Med. Res. 2014, 6, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.M.; Chawla, L.S.; Kane-Gill, S.L.; Hsu, R.K.; Kramer, A.A.; Goldstein, S.L.; Kellum, J.A.; Ronco, C.; Bagshaw, S.M. Utilizing electronic health records to predict acute kidney injury risk and outcomes: Workgroup statements from the 15th ADQI consensus conference. Can. J. Kidney Health Dis. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feng, T.; Thapa-Chhetry, B.; Cho, B.G.; Shum, T.; Inwald, D.P.; Newth, C.J.L.; Vaidya, V.U. Machine learning model for early prediction of acute kidney injury (AKI) in pediatric critical care. Crit. Care 2021, 25, 288. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.R.; Mudireddy, A.; Horne, B.D.; Chonchol, M.; Goldstein, S.L.; Goto, M.; Matheny, M.E.; Street, W.N.; Vaughan-Sarrazin, M.; Jalal, D.I.; et al. Predicting Nephrotoxic Acute Kidney Injury in Hospitalized Adults: A Machine Learning Algorithm. Kidney Med. 2024, 6, 100918. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Vicente, L.; Casanova, A.G.; Hernández-Sánchez, M.T.; Pescador, M.; López-Hernández, F.J.; Morales, A.I. A systematic meta-analysis on the efficacy of pre-clinically tested nephroprotectants at preventing aminoglycoside nephrotoxicity. Toxicology 2017, 377, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Mahi-Birjand, M.; Yaghoubi, S.; Abdollahpour-Alitappeh, M.; Keshtkaran, Z.; Bagheri, N.; Pirouzi, A.; Khatami, M.; Sineh Sepehr, K.; Peymani, P.; Karimzadeh, I. Protective effects of pharmacological agents against aminoglycoside-induced nephrotoxicity: A systematic review. Expert Opin. Drug Saf. 2020, 19, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Vlasic-Matas, J.; Rumboldt, Z.; Karelovic, D. Renoprotective role of nifedipine during gentamicin therapy: Randomized controlled trial. Croat. Med. J. 2000, 41, 417–422. [Google Scholar] [PubMed]
- Heydari, B.; Khalili, H.; Dashti-Khavidaki, S.; Beig-Mohammadi, M.T.; Mohammadi, M. Atorvastatin for Prevention of Amikacin-induced Electrolytes Imbalances; a Randomized Clinical Trial. Iran. J. Pharm. Res. 2016, 15, 627–634. [Google Scholar] [PubMed]
- Heydari, B.; Khalili, H.; Beigmohammadi, M.T.; Abdollahi, A.; Karimzadeh, I. Effects of atorvastatin on biomarkers of acute kidney injury in amikacin recipients: A pilot, randomized, placebo-controlled, clinical trial. J. Res. Med. Sci. 2017, 22, 39. [Google Scholar] [PubMed]
- McWilliam, S.J.; Rosala-Hallas, A.; Jones, A.P.; Shaw, V.; Greenhalf, W.; Jaki, T.; Smyth, A.R.; Smyth, R.L.; Pirmohamed, M. A randomised controlled trial of rosuvastatin for the prevention of aminoglycoside-induced kidney toxicity in children with cystic fibrosis. Sci. Rep. 2020, 10, 1796. [Google Scholar] [PubMed]
- Mahi-Birjand, M.; Karimzadeh, I.; Zarban, A.; Abdollahpour-Alitappeh, M.; Seyed Alireza Saadatjoo, S.A.; Ziaee, M. Protective effects of silymarin on gentamicin-induced nephrotoxicity in infectious patients: A randomized double blinded placebo-controlled clinical trial. Pharm. Sci. 2020, 26, 287–295. [Google Scholar] [CrossRef]
- Mousavinasab, S.R.; Akhoundi-Meybodi, Z.; Mahmoudi, L.; Karimzadeh, I. A randomized double-blinded placebo-controlled clinical trial on protective effects of pentoxifylline on gentamicin nephrotoxicity in infectious patients. Clin. Exp. Nephrol. 2021, 25, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Bulman, Z.P.; Cirz, R.; Hildebrandt, D.; Kane, T.; Rosario, Z.; Wlasichuk, K.; Park, M.; Andrews, L.D. Unraveling the Gentamicin Drug Product Complexity Reveals Variation in Microbiological Activities and Nephrotoxicity. Antimicrob. Agents Chemother. 2020, 64, e00533-20. [Google Scholar] [CrossRef] [PubMed]
- Jospe-Kaufman, M.; Siomin, L.; Fridman, M. The relationship between the structure and toxicity of aminoglycoside antibiotics. Bioorg. Med. Chem. Lett. 2020, 30, 127218. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.J.; Lai, C.C. Plazomicin-associated Nephrotoxicity. Clin. Infect. Dis. 2020, 71, 1130–1131. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Mondal, D. Antibacterial Efficacies of Nanostructured Aminoglycosides. ACS. Omega 2022, 7, 4724–4734. [Google Scholar] [CrossRef] [PubMed]
- Elfaky, M.A.; Thabit, A.K.; Sirwi, A.; Fahmy, U.A.; Bahabri, R.M.; Al-Awad, E.A.; Basaeed, L.F. Development of a Novel Pharmaceutical Formula of Nanoparticle Lipid Carriers of Gentamicin/α-Tocopherol and In Vivo Assessment of the Antioxidant Protective Effect of α-Tocopherol in Gentamicin-Induced Nephrotoxicity. Antibiotics 2019, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, N.; Ooda, A.; Nabil, A.; Nasser, A.; Ahmed, E.; Ali, F.; Mohamed, F.; Faried, H.; Badran, M.; Ahmed, M.; et al. The renoprotective activity of amikacin-gamma-amino butyric acid-chitosan nanoparticles: A comparative study. Inflammopharmacology 2024, 32, 2629–2645. [Google Scholar] [CrossRef] [PubMed]
- Prayle, A.P.; Jain, K.; Touw, D.J.; Koch, B.C.; Knox, A.J.; Watson, A.; Smyth, A.R. The pharmacokinetics and toxicity of morning vs. evening tobramycin dosing for pulmonary exacerbations of cystic fibrosis: A randomised comparison. J. Cyst. Fibros. 2016, 15, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.M.; Weverling, G.J.; van Ketel, R.J.; Speelman, P. Circadian variations in serum levels and the renal toxicity of aminoglycosides in patients. Clin. Pharmacol. Ther. 1997, 62, 106–111. [Google Scholar] [CrossRef] [PubMed]
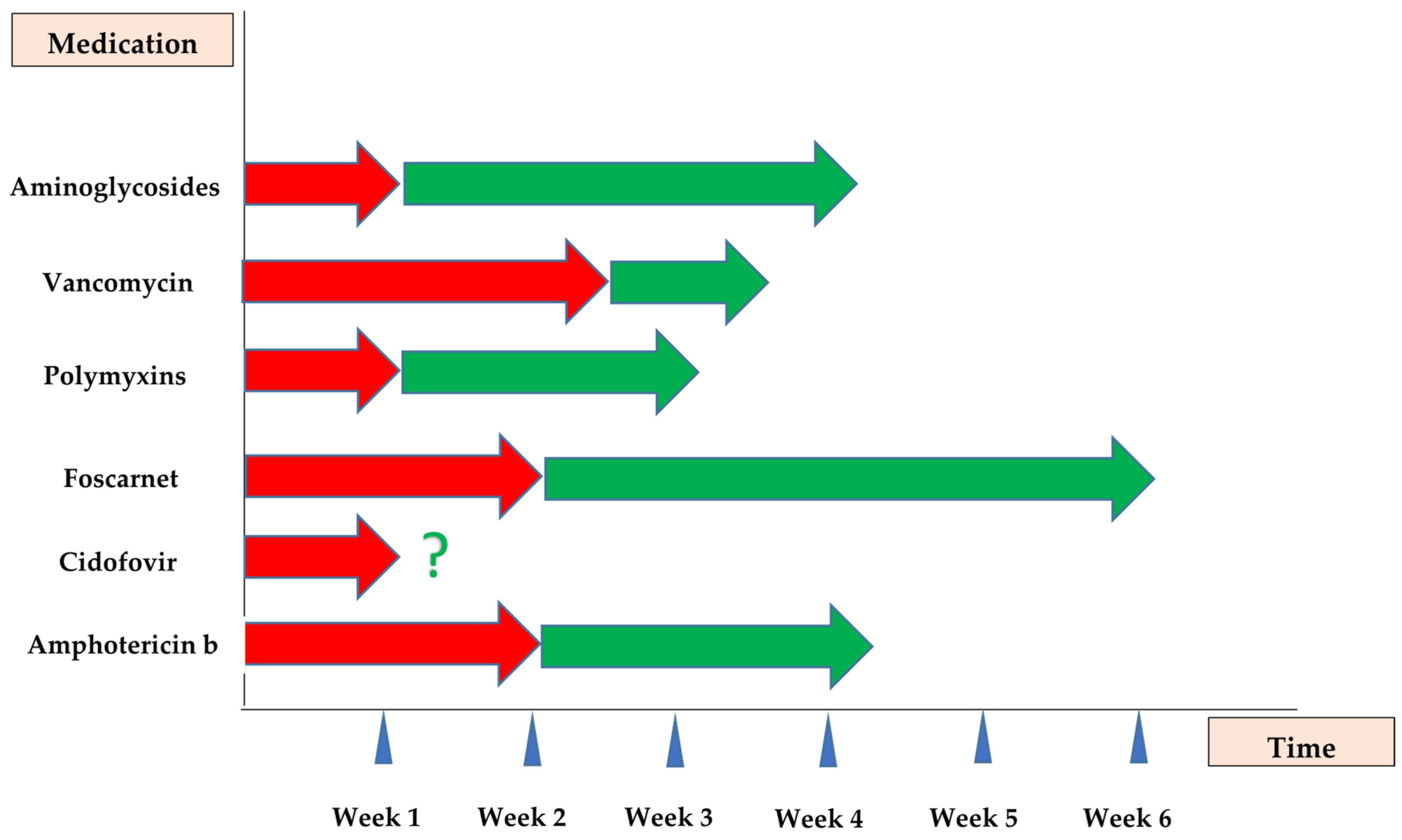

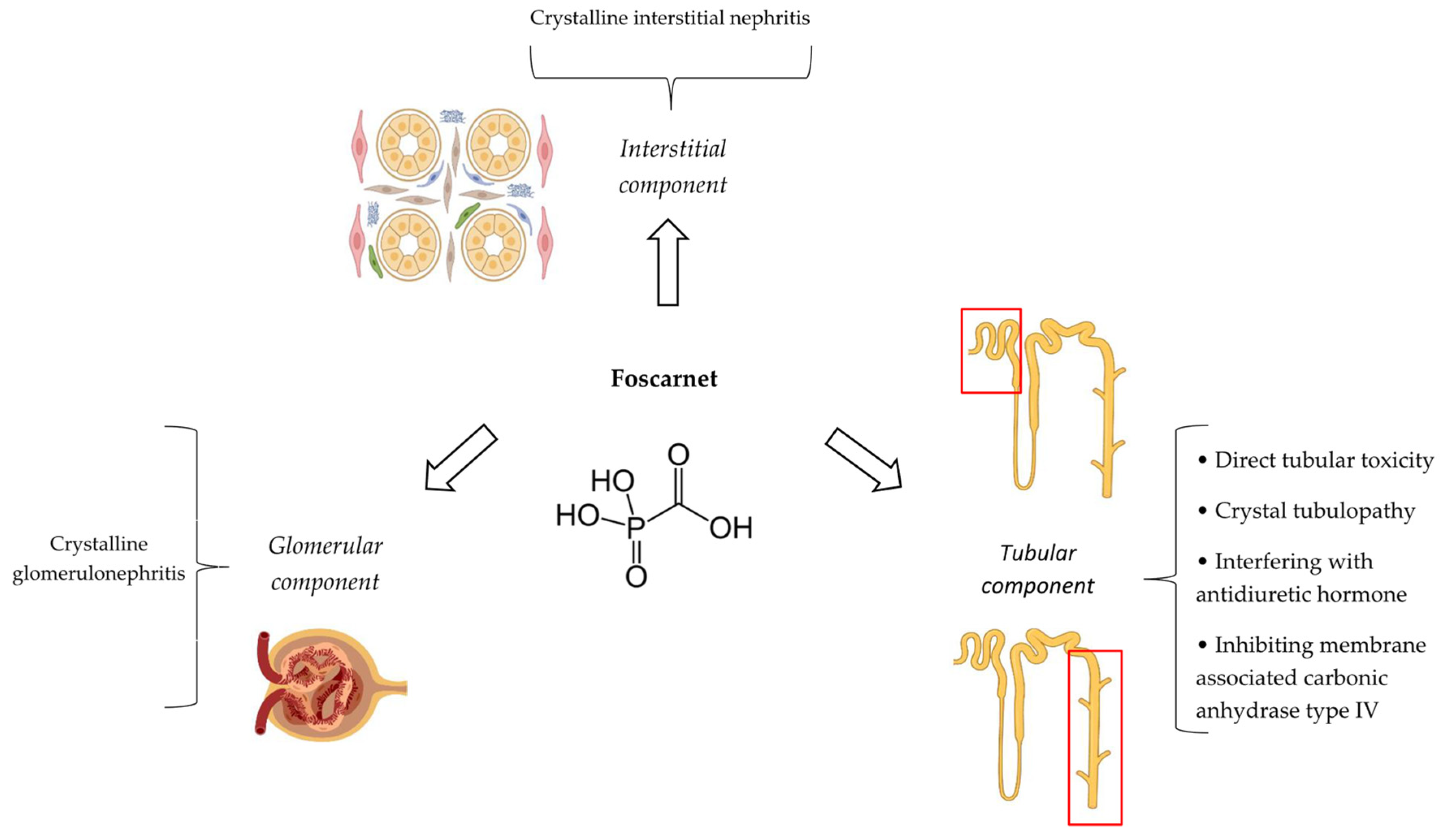
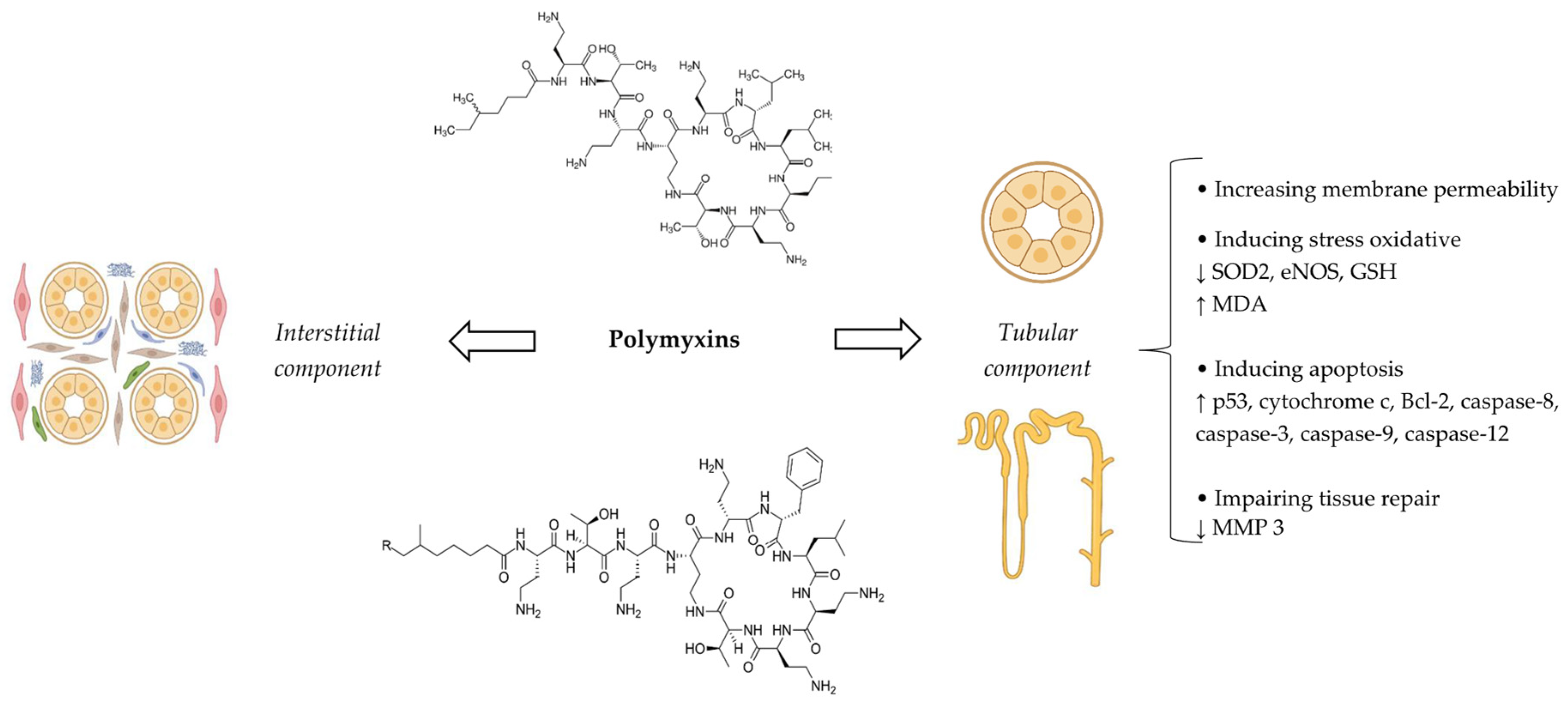
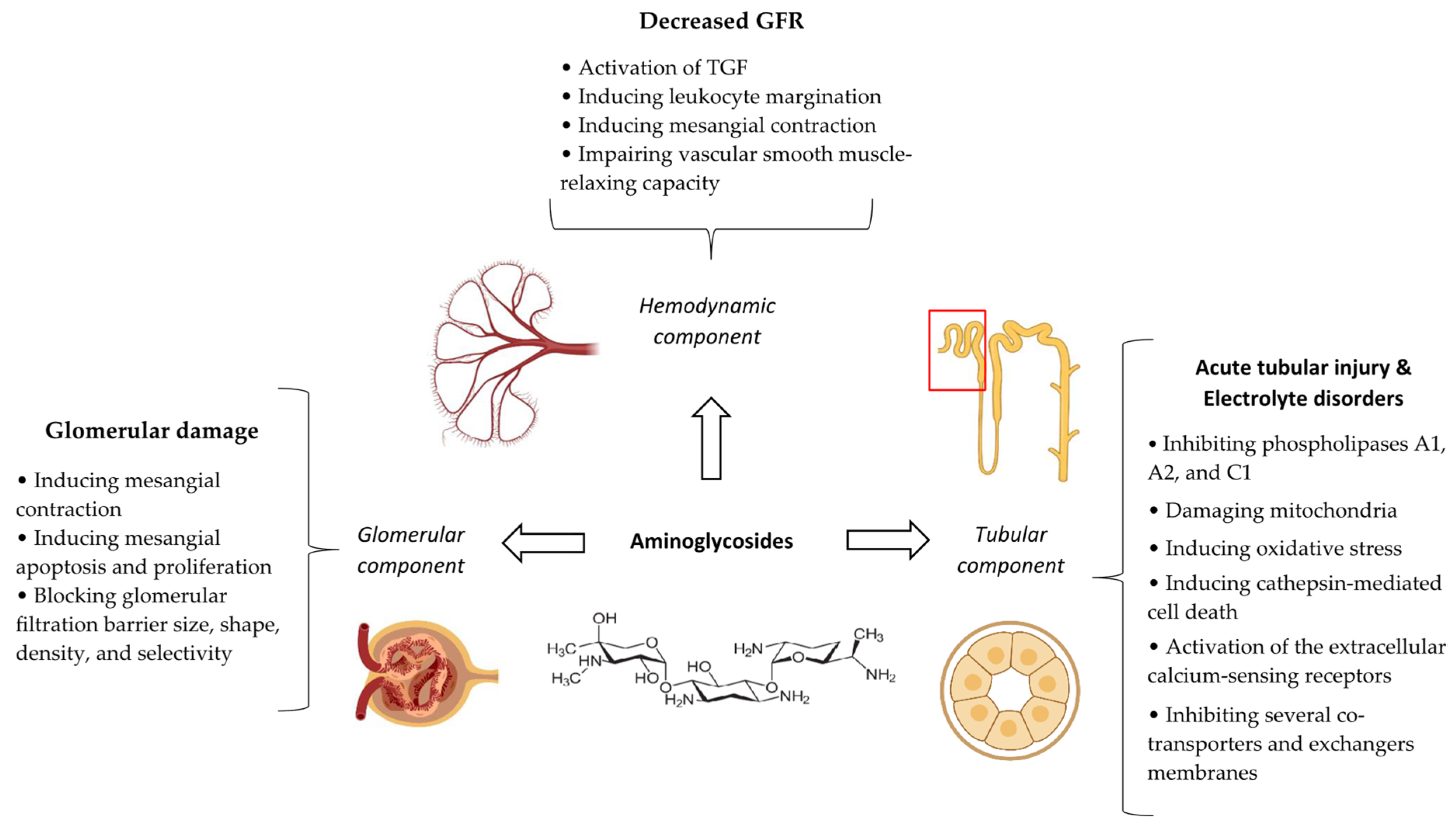
| Patient Related | Medication Related | Co-Administered Medications |
|---|---|---|
| Male gender | High average daily dose (e.g., more than 60 mg) | Cyclosporine |
| Older age | High cumulative dose (e.g., more than 600 mg or 2–5 g) | Foscarnet |
| Weight equal or more than 90 kg | High serum levels (e.g., more than 1.0 mg/L) | Cidofovir |
| Baseline kidney dysfunction | Rapid infusion | Pentamidine |
| Ganciclovir/Valganciclovir | ||
| Dehydration | Furosemide | |
| Mechanical ventilation | ||
| ICU admission | Angiotensin-converting enzyme inhibitors/Angiotensin receptor blockers | |
| Vasopressors | ||
| Vancomycin | ||
| Aminoglycosides | ||
| Carbapenems | ||
| Cisplatin | ||
| Ifosfamide |
| Medication | Monitoring Parameters/Suggestions |
|---|---|
| Amphotericin B | |
| Cidofovir | Urine protein levels along with serum creatinine should be measured within 24 to 48 h prior to the administration of each dose in induction and maintenance phases [62]. |
| Foscarnet |
|
| Polymyxins |
|
| Vancomycin |
|
| Aminoglycosides |
|
| Strategy | Description |
|---|---|
| Hydration | One liter of normal saline 1 h before infusing each dose of cidofovir; if the patient tolerates this, a second liter will also be given. |
| Co-administration of probenecid | 2 g two hours before and 1 g two hours and also eight hours after each infusion for a total of 4 g. |
| Alternative formulations of cidofovir | Using brincidofovir with less nephrotoxicity potential instead of cidofovir may be effective. |
| Avoiding the co-administration of nephrotoxic agents | Discontinuing NSAIDs, contrast media, aminoglycosides, AmB, foscarnet, and pentamidine at least 7 days before starting cidofovir. |
| Avoiding cidofovir administration in patients with underlying kidney disease | Not starting cidofovir in patients with:
|
| Strategy | Description |
|---|---|
| Intravenous hydration |
|
| Oral hydration | Sixteen ounces (450 mL) of any type of oral fluids (preferably non-alcohol and non-caffeine) to achieve a urine output of 200 mL. |
| Continuous infusion | Administering foscarnet as a continuous infusion (24 h) appears to have no superiority over intermittent infusion (at least 1 to 2 h). |
| Avoiding in patients with moderate-to-severe underlying kidney disease | Not starting foscarnet in patients with:
|
| Patient Related | Medication Related | Co-Administered Medications |
|---|---|---|
| Higher age | High daily dose (e.g., more than 5.0 mg/kg/day for colistin and equal or more than 200 mg for polymyxin B) | Loop diuretics |
| Higher weight | High trough level (e.g., more than 4 μg/mL for colistin) | Calcineurin inhibitors |
| Chronic comorbid conditions (e.g., diabetes and pre-existing kidney disease) | High area under the concentration–time curve 24 h at the steady state (e.g., more than 100 mg h/L for polymyxin B) | Nonsteroidal anti-inflammatory drugs |
| Severity of illness (e.g., higher APACHE-II scores) | Intravenous contrast media | |
| Septic shock | Glycopeptides | |
| Hypoalbuminemia | Aminoglycosides | |
| Hyperbilirubinemia | Vasopressors | |
| Proton pump inhibitor | ||
| Rifampin (possibly) |
| Patient Related | Medication Related | Co-Administered Medications |
|---|---|---|
| Older age | Serum trough level greater than 20 mg/L | Aminoglycosides |
| Female gender | Serum peak level | Piperacillin–tazobactam |
| Obesity (e.g., total body weight > 91 kg or 101.4 kg) | AUC equal or more than 650 mg·h/L | Loop diuretics |
Pre-existing comorbidities:
| Daily doses more than 4 g | Amphotericin b |
Severity of current illness:
| Treatment duration more than 1 week | Acyclovir |
| Race | Intermittent intravenous infusion (versus continuous infusion) | Calcineurin inhibitors |
| Pharmacogenetics | Intravenous contrast media | |
| Vasopressors | ||
| Renin–angiotensin system blockers |
| Patient Related | Medication Related | Co-Administered Medications |
|---|---|---|
| Older age | Type of aminoglycoside: Neomycin > gentamicin > tobramycin > amikacin > netilmicin > streptomycin | Vancomycin |
| Pregnancy | ||
| Pre-existing kidney disease | Serum concentrations more than 2 mg/mL (gentamicin and tobramycin) or more than 10 mg/mL (amikacin) | Amphotericin B |
| Foscarnet | ||
| Pre-existing liver disease | Multiple-daily dosing | Cephalotin |
| Obstructive jaundice | Prolonged duration of treatment (e.g., >10 days) | Piperacillin |
| Hypoalbuminemia | Clindamycin | |
| Diabetes | Cyclosporine and tacrolimus | |
| Leukemia | Loop diuretics | |
| Hypothyroidism | Iodinated radiographic contrast agents | |
| Volume depletion and hypotension | Cisplatin | |
| Shock | NSAIDs | |
| Sepsis | Loop diuretics | |
| Metabolic acidosis | ||
| Potassium or magnesium deficiencies | ||
| ICU hospitalization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimzadeh, I.; Kane-Gill, S.L.; Ma, B. Anti-Infective-Associated AKI: A Narrative Review of the Epidemiology, Mechanisms, Risk Factors, Biomarkers, Clinical Course, Monitoring, Prevention, and Therapeutic Strategies. Antibiotics 2025, 14, 1138. https://doi.org/10.3390/antibiotics14111138
Karimzadeh I, Kane-Gill SL, Ma B. Anti-Infective-Associated AKI: A Narrative Review of the Epidemiology, Mechanisms, Risk Factors, Biomarkers, Clinical Course, Monitoring, Prevention, and Therapeutic Strategies. Antibiotics. 2025; 14(11):1138. https://doi.org/10.3390/antibiotics14111138
Chicago/Turabian StyleKarimzadeh, Iman, Sandra L. Kane-Gill, and Binglei Ma. 2025. "Anti-Infective-Associated AKI: A Narrative Review of the Epidemiology, Mechanisms, Risk Factors, Biomarkers, Clinical Course, Monitoring, Prevention, and Therapeutic Strategies" Antibiotics 14, no. 11: 1138. https://doi.org/10.3390/antibiotics14111138
APA StyleKarimzadeh, I., Kane-Gill, S. L., & Ma, B. (2025). Anti-Infective-Associated AKI: A Narrative Review of the Epidemiology, Mechanisms, Risk Factors, Biomarkers, Clinical Course, Monitoring, Prevention, and Therapeutic Strategies. Antibiotics, 14(11), 1138. https://doi.org/10.3390/antibiotics14111138