Antimicrobial Activity of Aztreonam-Avibactam and Other β-Lactamase Inhibitor Combinations Tested Against Enterobacterales Isolates from Pediatric Patients from United States Medical Centers (2019–2023)
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Organism Collection
4.2. Susceptibility Testing
4.3. Screening for β-Lactamases
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Gniadkowski, M. Evolution and epidemiology of extended-spectrum beta-lactamases (ESBLs) and ESBL-producing microorganisms. Clin. Microbiol. Infect. 2001, 7, 597–608. [Google Scholar] [CrossRef]
- Logan, L.K.; Braykov, N.P.; Weinstein, R.A.; Laxminarayan, R.; Centers for Disease Control; Prevention Epicenters Program. Extended-Spectrum beta-Lactamase-Producing and Third-Generation Cephalosporin-Resistant Enterobacteriaceae in Children: Trends in the United States, 1999–2011. J. Pediatr. Infect. Dis. Soc. 2014, 3, 320–328. [Google Scholar] [CrossRef]
- Logan, L.K.; Renschler, J.P.; Gandra, S.; Weinstein, R.A.; Laxminarayan, R.; Centers for Disease, C.; Prevention Epicenters, P. Carbapenem-Resistant Enterobacteriaceae in Children, United States, 1999–2012. Emerg. Infect. Dis. 2015, 21, 2014–2021. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Epidemiology of beta-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, 2. [Google Scholar] [CrossRef] [PubMed]
- Little, M.L.; Qin, X.; Zerr, D.M.; Weissman, S.J. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int. J. Antimicrob. Agents 2012, 39, 52–57. [Google Scholar] [CrossRef] [PubMed]
- CDC. Vital Signs: Carbapenem-Resistant Enterobacteriaceae. Morb. Mortal. Wkly. Rep. 2013, 62, 165–170. [Google Scholar]
- Hauck, C.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Scalera, N.M.; Doi, Y.; Kaye, K.S.; et al. Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin. Microbiol. Infect. 2016, 22, 513–519. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Abner, S.; Benin, A.L.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; et al. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 19–30. [Google Scholar] [CrossRef]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 guidance on the treatment of antimicrobial-resistant Gram-negative infections. Clin. Infect. Dis. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Sader, H.S.; Kimbrough, J.H.; Doyle, T.B.; Winkler, M.L.; Castanheira, M. Frequency, Antimicrobial susceptibility, and molecular characterization of carbapenem-resistant Enterobacterales stratified by United States census divisions: Results from the INFORM Program (2018–2022). Open Forum Infect. Dis. 2025, 12, ofaf005. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Giske, C.G.; Gramatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New beta-lactam-beta-lactamase inhibitor combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef]
- Carmeli, Y.; Cisneros, J.M.; Paul, M.; Daikos, G.L.; Wang, M.; Torre-Cisneros, J.; Singer, G.; Titov, I.; Gumenchuk, I.; Zhao, Y.; et al. Aztreonam-avibactam versus meropenem for the treatment of serious infections caused by Gram-negative bacteria (REVISIT): A descriptive, multinational, open-label, phase 3, randomised trial. Lancet Infect. Dis. 2025, 25, 218–230. [Google Scholar] [CrossRef]
- CLSI. M07Ed12; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical Laboratory Standards Institute: Berwyn, PA, USA, 2024.
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 15.0; European Committee on Antimicrobial Susceptibility Testing: Vaxjo, Sweden, 2025. [Google Scholar]
- CLSI. M100Ed35; Performance Standards for Antimicrobial Susceptibility Testing; 35the Informational Supplement. Clinical Laboratory Standards Institute: Berwyn, PA, USA, 2025.
- Miles-Jay, A.; Weissman, S.J.; Adler, A.L.; Tchesnokova, V.; Sokurenko, E.V.; Baseman, J.G.; Zerr, D.M. Epidemiology and antimicrobial resistance characteristics of the sequence type 131-H30 subclone among extraintestinal Escherichia coli collected from US children. Clin. Infect. Dis. 2018, 66, 411–419. [Google Scholar] [CrossRef]
- Medernach, R.L.; Logan, L.K. The growing threat of antibiotic resistance in children. Infect. Dis. Clin. N. Am. 2018, 32, 1–17. [Google Scholar] [CrossRef]
- Lukac, P.J.; Bonomo, R.A.; Logan, L.K. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in children: Old foe, emerging threat. Clin. Infect. Dis. 2015, 60, 1389–1397. [Google Scholar] [CrossRef]
- Logan, L.K.; Hujer, A.M.; Marshall, S.H.; Domitrovic, T.N.; Rudin, S.D.; Zheng, X.; Qureshi, N.K.; Hayden, M.K.; Scaggs, F.A.; Karadkhele, A.; et al. Analysis of beta-Lactamase resistance determinants in Enterobacteriaceae from Chicago children: A multicenter survey. Antimicrob. Agents Chemother. 2016, 60, 3462–3469. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Huband, M.D.; Duncan, L.R.; Flamm, R.K. Ceftazidime-avibactam antimicrobial activity and spectrum when tested against Gram-negative organisms from pediatric patients: Results from the INFORM Surveillance Program (United States, 2011–2015). Pediatr. Infect. Dis. J. 2018, 37, 549–554. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Jones, R.N.; Woosley, L.N.; Cattoir, V.; Castanheira, M. Application of next-generation sequencing for characterization of surveillance and clinical trial isolates: Analysis of the distribution of beta-lactamase resistance genes and lineage background in the United States. Open Forum Infect. Dis. 2019, 6, S69–S78. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
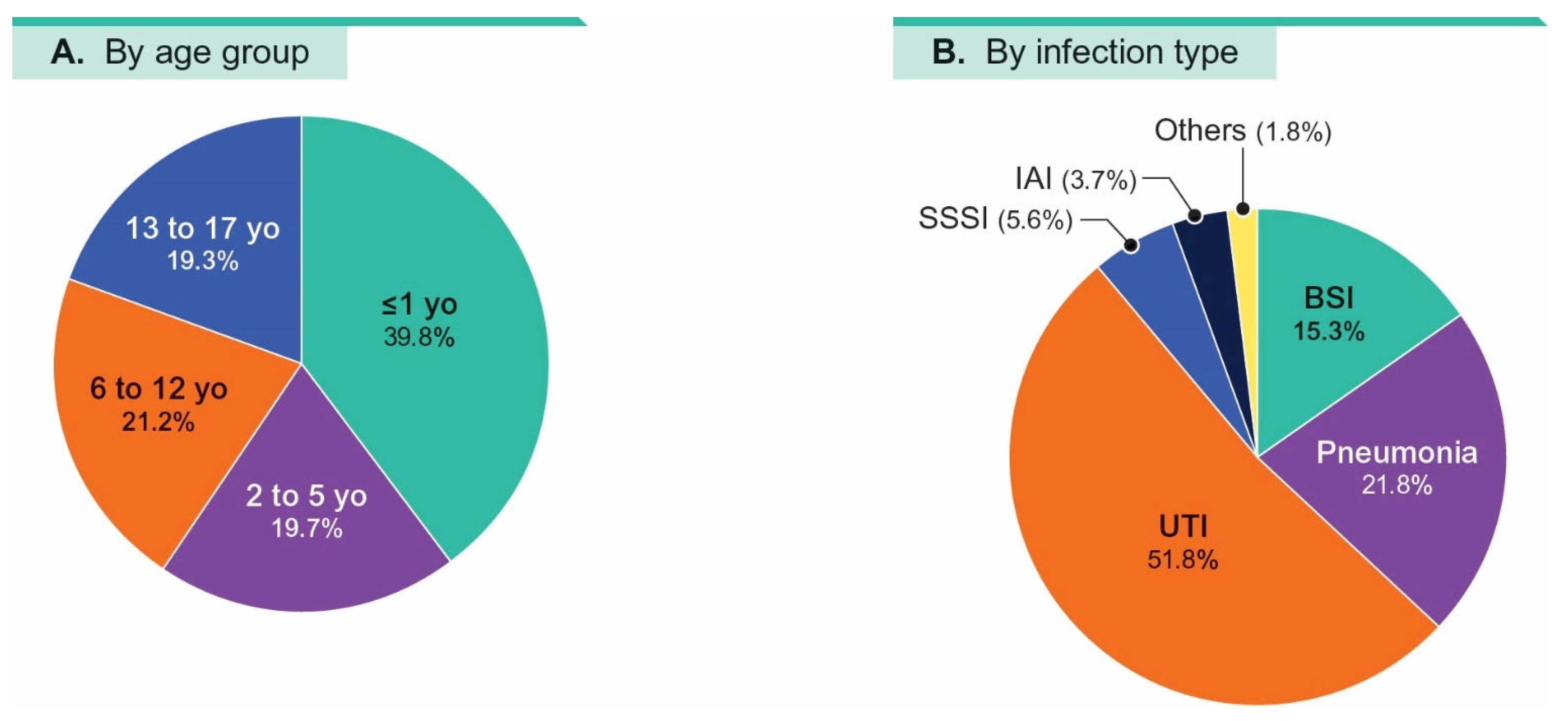


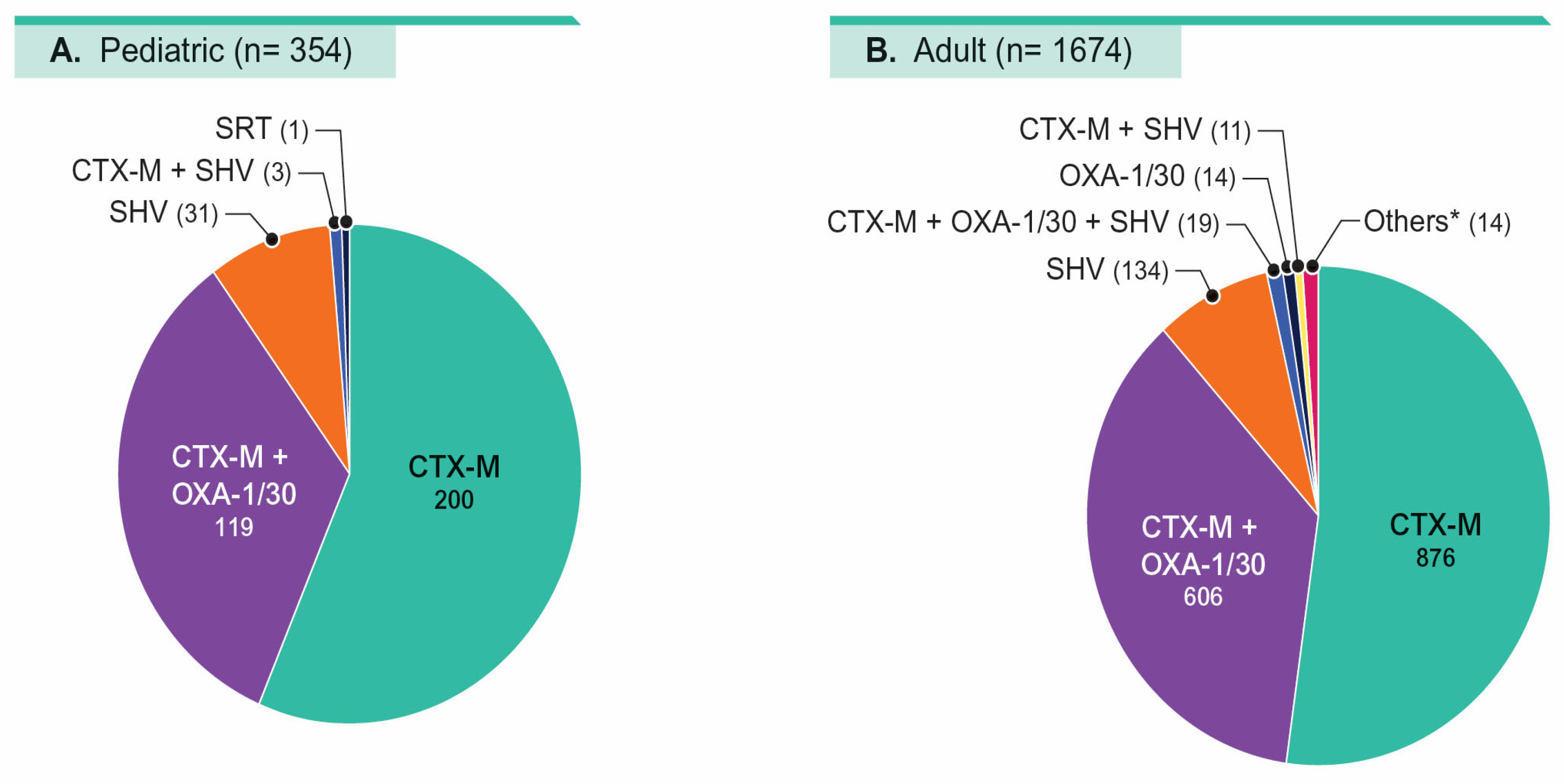
| % Susceptible by Patient Age Group a (No. of Isolates in Parenthesis): | ||||||
|---|---|---|---|---|---|---|
| Organism/Antimicrobial Agent | ≤1 yo | 2–5 yo | 6–12 yo | 13–17 yo | All Peds | Adults b |
| Enterobacterales | (2275) | (1130) | (1213) | (1105) | (5723) | (17,712) |
| Aztreonam–avibactam c | 99.9 | 100.0 | 100.0 | 99.9 | 99.9 | 99.9 |
| Ceftazidime–avibactam | 100.0 | 99.9 | 100.0 | 99.9 | >99.9 | 99.7 |
| Meropenem–vaborbactam | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 99.6 |
| Imipenem–relebactam | 98.1 | 92.7 | 95.3 | 94.6 | 95.9 | 93.1 |
| Ceftolozane–tazobactam | 96.5 | 97.7 | 96.5 | 97.6 | 96.9 | 93.9 |
| Piperacillin–tazobactam | 92.0 | 93.5 | 90.3 | 93.4 | 92.2 | 88.0 |
| Ceftriaxone | 87.6 | 89.2 | 84.5 | 89.2 | 87.6 | 81.5 |
| Cefepime | 94.0 | 93.9 | 91.5 | 93.0 | 93.3 | 88.5 |
| Meropenem | 99.8 | 99.7 | 100.0 | 99.8 | 99.8 | 98.6 |
| Levofloxacin | 94.2 | 88.8 | 87.5 | 89.4 | 90.8 | 82.1 |
| Gentamicin | 93.8 | 90.8 | 90.4 | 91.8 | 92.1 | 91.2 |
| Amikacin | 96.0 | 95.0 | 94.6 | 96.0 | 95.5 | 95.0 |
| % Susceptible by Infection Type a (No. of Isolates in Parenthesis): | ||||||
|---|---|---|---|---|---|---|
| Organism/Antimicrobial Agent | BSI | IAI | Pneumoniae | SSSI | UTI | Others |
| Enterobacterales | (874) | (214) | (1245) | (321) | (2967) | (102) |
| Aztreonam–avibactam b | 100.0 | 100.0 | 99.8 | 100.0 | >99.9 | 99.9 |
| Ceftazidime–avibactam | 99.9 | 100.0 | 100.0 | 99.7 | 100.0 | 100.0 |
| Meropenem–vaborbactam | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Imipenem–relebactam | 98.6 | 99.1 | 98.1 | 92.4 | 93.6 | 100.0 |
| Ceftolozane–tazobactam | 96.6 | 93.5 | 96.5 | 94.1 | 97.8 | 96.1 |
| Piperacillin–tazobactam | 92.3 | 88.3 | 90.2 | 89.0 | 93.6 | 95.1 |
| Ceftriaxone | 87.3 | 86.4 | 86.0 | 87.2 | 88.4 | 88.2 |
| Cefepime | 92.8 | 93.5 | 93.6 | 96.9 | 92.8 | 96.1 |
| Meropenem | 99.5 | 99.5 | 99.8 | 99.7 | >99.9 | 100.0 |
| Levofloxacin | 90.1 | 92.1 | 92.0 | 93.8 | 89.8 | 99.0 |
| Gentamicin | 93.7 | 91.1 | 92.3 | 96.0 | 91.0 | 97.1 |
| Amikacin | 95.7 | 94.9 | 96.2 | 96.6 | 95.0 | 99.0 |
| Resistant Subset (No.)/Antimicrobial Agent | MIC (mg/L) | Susceptibility per CLSI and/or US FDA) | |||
|---|---|---|---|---|---|
| 50% | 90% | % Susceptible | % Intermediate | % Resistant | |
| Ceftriaxone-NS (712) | |||||
| Aztreonam–avibactam | 0.06 | 0.5 | 99.4 | 0.3 | 0.3 |
| Ceftazidime–avibactam | 0.25 | 0.5 | 99.7 | 0.3 | |
| Meropenem–vaborbactam | 0.03 | 0.06 | 100.0 | 0.0 | 0.0 |
| Imipenem–relebactam | 0.12 | 0.5 | 97.1 b | 2.6 | 0.3 |
| Ceftolozane–tazobactam | 1 | 16 | 75.8 | 7.3 | 16.9 |
| Piperacillin–tazobactam | 8 | 128 | 53.1 | 12.8 | 34.1 |
| Ceftriaxone | >8 | >8 | 0.0 | 6.6 | 93.4 |
| Cefepime | 4 | >32 | 45.8 | 12.5 | 41.7 |
| Meropenem | 0.03 | 0.12 | 98.9 | 0.6 | 0.6 |
| Levofloxacin | 0.25 | 16 | 64.6 | 5.9 | 29.5 |
| Gentamicin | 0.5 | >16 | 70.1 | 0.7 | 29.2 |
| Amikacin | 2 | 8 | 86.5 | 7.4 | 6.0 |
| MDR (906) a | |||||
| Aztreonam–avibactam | 0.06 | 0.5 | 99.7 | 0.1 | 0.2 |
| Ceftazidime–avibactam | 0.12 | 0.5 | 99.8 | 0.2 | |
| Meropenem–vaborbactam | 0.03 | 0.03 | 100.0 | 0.0 | 0.0 |
| Imipenem–relebactam | 0.12 | 0.25 | 98.4 b | 1.3 | 0.2 |
| Ceftolozane–tazobactam | 0.5 | 8 | 80.9 | 5.8 | 13.2 |
| Piperacillin–tazobactam | 8 | 128 | 57.7 | 12.5 | 29.8 |
| Ceftriaxone | >8 | >8 | 34.0 | 2.4 | 63.6 |
| Cefepime | 1 | >32 | 61.6 | 8.6 | 29.8 |
| Meropenem | 0.03 | 0.06 | 99.0 | 0.4 | 0.6 |
| Levofloxacin | 0.5 | 16 | 61.0 | 8.1 | 30.9 |
| Gentamicin | 1 | >16 | 57.4 | 0.9 | 41.7 |
| Amikacin | 2 | 8 | 85.3 | 9.2 | 5.5 |
| ESBL producers (354) c | |||||
| Aztreonam–avibactam | 0.06 | 0.12 | 99.7 | 0.0 | 0.3 |
| Ceftazidime–avibactam | 0.12 | 0.5 | 100.0 | 0.0 | |
| Meropenem–vaborbactam | 0.03 | 0.03 | 100.0 | 0.0 | 0.0 |
| Imipenem–relebactam | 0.12 | 0.25 | 100.0 b | 0.0 | 0.0 |
| Ceftolozane–tazobactam | 0.5 | 2 | 91.8 | 2.3 | 5.9 |
| Piperacillin–tazobactam | 4 | 32 | 72.8 | 14.2 | 13.0 |
| Ceftriaxone | >8 | >8 | 0.0 | 0.3 | 99.7 |
| Cefepime | >32 | >32 | 10.2 | 13.8 | 76.0 |
| Meropenem | 0.03 | 0.06 | 99.2 | 0.6 | 0.3 |
| Levofloxacin | 1 | 16 | 41.8 | 8.2 | 50.0 |
| Gentamicin | 1 | >16 | 53.1 | 0.6 | 46.3 |
| Amikacin | 4 | 8 | 78.8 | 12.4 | 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sader, H.S.; Winkler, M.L.; Papp-Wallace, K.M.; Mendes, R.E.; Castanheira, M. Antimicrobial Activity of Aztreonam-Avibactam and Other β-Lactamase Inhibitor Combinations Tested Against Enterobacterales Isolates from Pediatric Patients from United States Medical Centers (2019–2023). Antibiotics 2025, 14, 1107. https://doi.org/10.3390/antibiotics14111107
Sader HS, Winkler ML, Papp-Wallace KM, Mendes RE, Castanheira M. Antimicrobial Activity of Aztreonam-Avibactam and Other β-Lactamase Inhibitor Combinations Tested Against Enterobacterales Isolates from Pediatric Patients from United States Medical Centers (2019–2023). Antibiotics. 2025; 14(11):1107. https://doi.org/10.3390/antibiotics14111107
Chicago/Turabian StyleSader, Helio S., Marisa L. Winkler, Krisztina M. Papp-Wallace, Rodrigo E. Mendes, and Mariana Castanheira. 2025. "Antimicrobial Activity of Aztreonam-Avibactam and Other β-Lactamase Inhibitor Combinations Tested Against Enterobacterales Isolates from Pediatric Patients from United States Medical Centers (2019–2023)" Antibiotics 14, no. 11: 1107. https://doi.org/10.3390/antibiotics14111107
APA StyleSader, H. S., Winkler, M. L., Papp-Wallace, K. M., Mendes, R. E., & Castanheira, M. (2025). Antimicrobial Activity of Aztreonam-Avibactam and Other β-Lactamase Inhibitor Combinations Tested Against Enterobacterales Isolates from Pediatric Patients from United States Medical Centers (2019–2023). Antibiotics, 14(11), 1107. https://doi.org/10.3390/antibiotics14111107







