Treatment Duration in Bacterial Prosthetic Joint Infections: A Narrative Review of Current Evidence
Abstract
1. Introduction
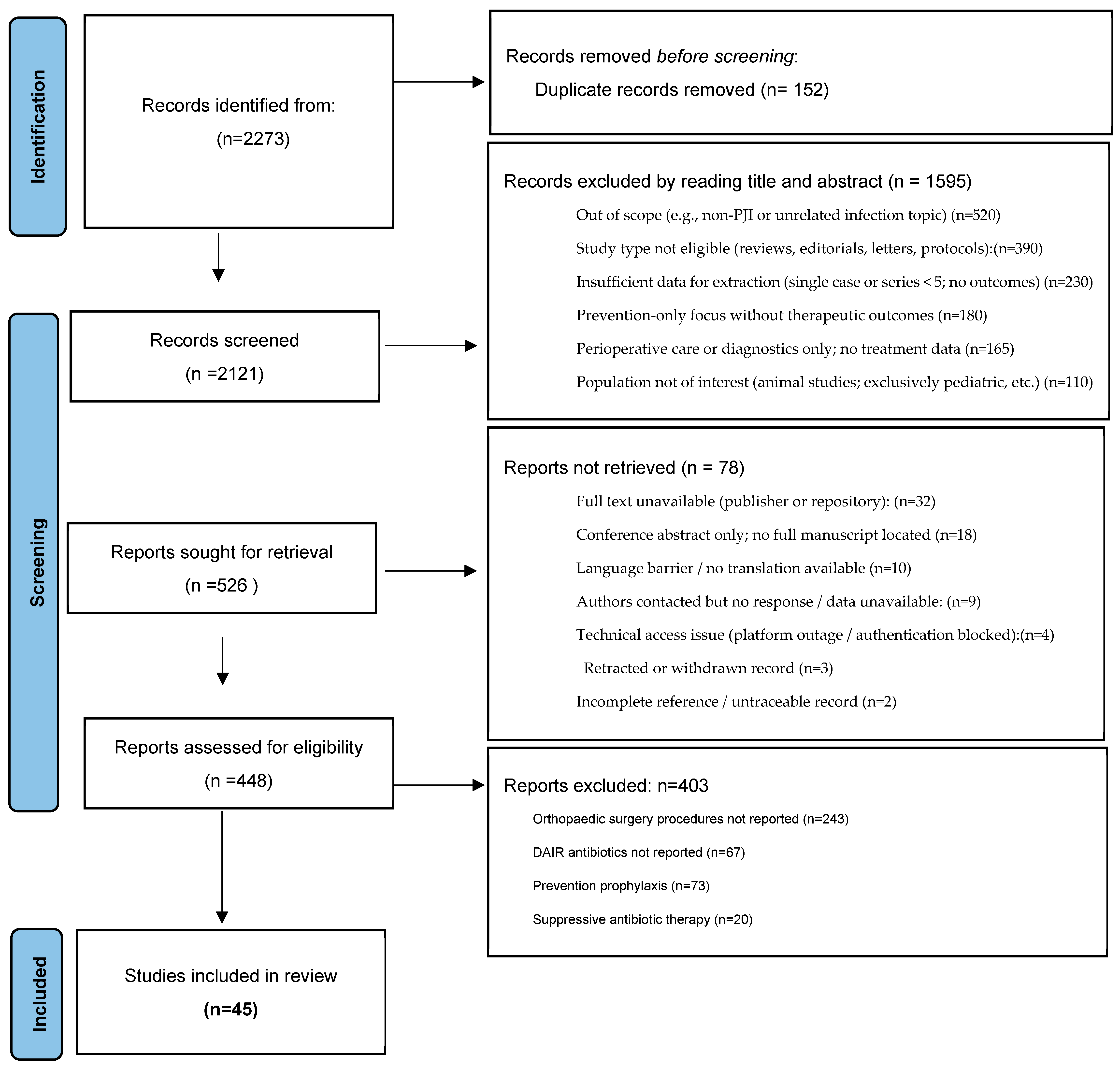
2. Methods/Search Strategy
3. General Considerations
4. Antibiotic Duration of PJI According to the Surgery Options
4.1. Prosthetic Joint Infection Treated with DAIR (Table 1 and Table 2)
| Study and Year | Design and Sample Size | Dominant Organisms | Therapeutic Approach (Systemic) | Treatment Exposure (Mean) | Follow-Up (Months) | Clinical Success (%) |
|---|---|---|---|---|---|---|
| Berdal 2005 [14] | Prospective; n = 29 | Staphylococcus aureus | Rifampicin + ciprofloxacin | ≈3 months (total) | 22.5 | 83 |
| Soriano 2006 [15] | Prospective; n = 39 | Gram-positive cocci | Levofloxacin + rifampicin | 2.7 ± 1 months (total) | 24 | 76.6 |
| Martinez-Pastor 2009 [16] | Prospective; n = 47 | Enterobacteriaceae | IV β-lactam → oral fluoroquinolone | IV 14 days; oral 2.6 months | 15.4 | 74.5 |
| Cobo 2011 [17] | Prospective; n = 117 | Gram-negative strains; Gram-positive cocci | Not specified | ≈2.5 months (total) | 25 | 57.3 |
| Vilchez 2011 [18] | Prospective; n = 53 | Staphylococcus aureus | IV → oral sequence (details in text) | IV 11 ± 7 days; oral 88 ± 46 days | 24 | 75.5 |
| Tornero 2016 [19] | Prospective; n = 143 | Gram-negative; Gram-positive cocci | Fluoroquinolone-based; rifampicin combinations | IV 8 days; oral 69 days | 48 | 88.2 |
| Study and Year (Ref.) | Design and Setting | Population/Arms | Dominant Organisms | Principal Regimens | Exposure Window | Outcomes and Conclusion |
|---|---|---|---|---|---|---|
| Bernard 2010 [3] | Prospective, observational, single-center | n = 144 episodes; 6-week arm (n = 70) vs. 12-week arm (n = 74) | Staphylococci (~66%) | Rifampicin-based combinations common; also ciprofloxacin, vancomycin, amoxicillin–clavulanate | 6 weeks vs. 12 weeks (systemic) | Overall cure 80% (115/144); by arm: 90% with 6 weeks vs. 55% with 12 weeks; authors suggest 6 weeks may suffice; RCTs needed. |
| Puhto 2012 [20] | Retrospective, pre–post, single-center | ITT: long n = 60, short n = 72; PP: long n = 38, short n = 48 | Staphylococcus aureus (~42%) | Gram-positive regimens mainly rifampicin + fluoroquinolone | Short 2–3 mo vs. long 3–6 mo | Non-inferiority of short therapy: ITT cure 57% vs. 58% (p = 0.85); PP 89% vs. 87% (p = 0.78). Short course appears acceptable; randomized data urged. |
| Lora-Tamayo 2013 [21] | Retrospective, multicenter | Total n = 231 stratified by duration: <61 d (n = 52), 61–90 d (n = 52), >90 d (n = 127) | Staphylococcus aureus; rifampicin use > 75% | Predominantly rifampicin-based combinations | Three strata: <61 d; 61–90 d; >90 d | Cure rates similar across strata: 75%, 77%, 77% (p = 0.434). Longer exposure did not improve outcomes. |
| Lora-Tamayo 2016 [22] | Randomized, open-label, multicenter clinical trial | n = 63; ITT long n = 33 vs. short n = 30; PP long n = 20 vs. short n = 24 | Staphylococci | Levofloxacin + rifampicin (L + R) | Short 8 weeks vs. longer standard (≈3 mo hip; 6 mo knee) | Non-inferiority signal: ITT cure 58% long vs. 73% short (Δ −15.7%, 95% CI −39.2 to +7.8); PP 95% vs. 92% (Δ +3.3%, 95% CI −11.7 to +18.3). Eight weeks L + R may be adequate in DAIR-managed acute staphylococcal PJI. |
| Chaussade 2017 [23] | Retrospective, multicenter | n = 87; 6-week arm n = 44; 12-week arm n = 43 | Staphylococci (~40%) | Rifampicin-based for Gram-positive; fluoroquinolones frequently used | 6 weeks vs. 12 weeks | Cure: 70.5% (short) vs. 67.4% (long); adjusted OR 0.76 (95% CI 0.27–2.10). No advantage for 12 weeks; prospective RCTs recommended. |
| Bernard 2021 (DATIPO) [4] | Randomized, open-label, multicenter | n = 151; 6-week n = 75; 12-week n = 76 | Staphylococcus aureus ~30–40% | Rifampicin-based combinations; fluoroquinolones commonly paired | 6 weeks vs. 12 weeks | Failure: 30.7% (6 weeks) vs. 14.5% (12 weeks); difference 16.2% (95% CI 2.9–29.5). Non-inferiority of 6 weeks not demonstrated. |
4.2. PJI Treated with One-Step Exchange Procedure (Table 3)
| Study and Year (Ref.) | n/Design | Systemic Regimens (Major) | Systemic Exposure | Local/Suppression | Mean Follow-Up (Years) | Key Outcomes | Conclusion |
|---|---|---|---|---|---|---|---|
| Whiteside 2011 [32] | n = 18; retrospective cohort | Not reported | IV 2–4 weeks | Intra-articular vancomycin | 5.1 | Recurrence rate ~5.5%; KSS ~78 at 1 y → ~84–85 up to 6–8 y | Single-stage TKA for MRSA with 6 weeks intra-articular vancomycin controlled infection in most cases. |
| Singer 2012 [26] | n = 57; retrospective | Rifampicin + fluoroquinolone combos (~51%) | Total 6 weeks (2 w IV post-op → 4 w oral) | Local gentamicin | 3 | Recurrence ~15%; KSS ~72; function score ~71; Oxford-12 ~27 | One-stage knee revision achieved high infection control when pathogen identified; outcomes worse with hinged prostheses and MRSA/MRSE. |
| Jenny 2013 [24] | n = 47; prospective observational cohort | IV vancomycin/teicoplanin; oral rifampicin + levofloxacin | IV 3.5 w (1–16); oral 12 w (3–16) | Long-term suppression: not reported | 3 | Recurrence ~12%; median pre-op KSS function 42; 56% had KSS > 150 post-op | Single-stage exchange is a viable alternative in chronic infected TKA, potentially reducing hospital burden and costs. |
| Baker 2013 [37] | n = 33; prospective | Not reported | Not reported | Not reported | 0.6 (7 months) | Recurrence ~21%; OKS improved from 15 (95% CI 13–18) to 25 (95% CI 21–29) | No clear difference vs. two-stage; functional gains observed at short follow-up. |
| Shanmugasundaram 2014 [30] | n = 5; retrospective | Not reported | Not reported | Antibiotic spacers | 2 | Recurrence ~17%; other FO not reported | Initial success: hip PJI 1-stage 60% vs. 2-stage 70%; knee PJI 1-stage 80% vs. 2-stage 75%; better diagnostics needed. |
| Tibrewal 2014 [33] | n = 50; prospective | Not reported | IV 2 w → oral 3 mo | Antibiotic-impregnated cement | 10 | Recurrence ~2%; OKS from 14.5 → 34.5 at 1 y (Δ ≈ +20; p < 0.001) | Single-stage may match two-stage outcomes with lower cost and morbidity. |
| Cury R de PL 2015 [34] | n = 6; retrospective | Not reported | IV 2–4 w → oral 6 mo | Suppressive therapy in 4/6 | 3 | Recurrence ~16.7%; WOMAC ~49.5 | Reported success: DAIR 75%, one-stage 83%, two-stage 100% in small series. |
| Haddad 2015 [29] | n = 28; retrospective | Not reported | 6 weeks (IV and/or oral) | Antibiotic-loaded cement (gentamicin, vancomycin) | 2 | Recurrence 0%; KSS higher in 1-stage vs. 2-stage (88 vs. 76; p < 0.001); pre-op KSS ~32 | One-stage can be an alternative for selected chronic TKA infections; RCTs needed. |
| Zahar 2016 [36] | n = 46; retrospective | Not reported | IV 14.2 days (10–17) | Antibiotic-loaded cement (gentamicin, clindamycin, vancomycin) | 10 | Recurrence ~7%; HSS improved from 35 to 69.6 | Overall infection control ~93% with favorable clinical recovery; further research warranted. |
| Cochran 2016 [35] | n = 3069; retrospective database | Not reported | Not reported | Not reported | 6 | Recurrence 24.6% at 1 y; 38.25% at 6 y | Two-stage reimplantation showed highest success despite ~19% recurrence; higher than single-stage and DAIR. |
| Jenny 2016 [25] | Intervention n = 54; control n = 77; retrospective case–control | Not reported | 3 months | Not reported | 2 | Recurrence: 15% (intervention) vs. 22% (control); ~80% KSS > 160; no significant group difference | Patient selection did not markedly influence outcomes for single-stage exchange. |
| Massin 2016 [27] | n = 108; retrospective | Not reported | Not reported | Not reported | 2 | Recurrence 24%; IKS 88.6 ± 9.4 | One-stage may be reasonable (e.g., in women) without increased recurrence; supports broader use in selected TKR. |
| Li 2018 [38] | n = 22; retrospective | Vancomycin | 4–6 weeks | Not reported | 5 | Recurrence 9.1%; other FO not reported | No significant difference between 1- and 2-stage in satisfaction and infection control. |
| Castellani 2017 [28] | n = 14; retrospective | Not reported | Not reported | Not reported | 1 | Recurrence ~7.2%; FO not reported | Superiority of one- vs. two-stage and role of antibiotic-free intervals remain unclear; larger prospective RCTs needed. |
4.3. PJI Treated with Two-Step Exchange Procedure (Table 4)
| Study and Year (Ref.) | n/Site | Design | Dominant Organisms | Systemic Antibiotics (Major) | Systemic Exposure | Local Antibiotics | Mean Follow-Up (mo) | Key Outcomes (Additional Debridement/Reimplant Cultures+/Persistence–Relapse) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Taggart 2002 [40] | n = 33; hip and knee | Prospective observational; single center; non-comparative | 93% Gram-positives; 71% staphylococci | Not reported | 5 days | Vancomycin (local) | 67 | Add. debridement 0%; cultures+ at reimplant 9%; persistence/relapse 3% | Short systemic exposure with local vancomycin yielded low relapse but some positive reimplant cultures. |
| Hoad-Reddick 2005 [41] | n = 52; knee | Prospective observational; single center; non-comparative | 63% staphylococci | None beyond prophylaxis (cefuroxime) | 1 day | Various local agents | 56 | Add. debridement 12%; cultures+ 16%; persistence/relapse 9% | Minimal systemic therapy with local measures showed moderate culture positivity and relapse rates. |
| Hart and Jones 2006 [43] | n = 48; knee | Prospective observational; single center; non-comparative | 96% Gram-positives; 76% staphylococci | Vancomycin | 14 days | Vancomycin + gentamicin (local) | 49 | Add. debridement 13%; cultures+ 23%; persistence/relapse 13% | Two-stage with short systemic vancomycin and local antibiotics achieved acceptable but non-negligible failure. |
| Stockley 2008 [39] | n = 114; hip | Prospective observational; single center; non-comparative | 61% staphylococci | None (cephalosporin prophylaxis) | 1 day | Various local agents | 74 | Add. debridement 4%; cultures+ 16%; persistence/relapse 12% | Local strategies with minimal systemic therapy produced low reoperation but notable positive cultures. |
| Whittaker 2009 [42] | n = 44; hip | Prospective observational; single center; non-comparative | All Gram-positives; 72% staphylococci | Vancomycin | 14 days | Vancomycin + gentamicin (local) | 49 | Add. debridement 7%; cultures+ 2%; persistence/relapse 7% | Short systemic vancomycin plus local therapy yielded low culture positivity and relapse. |
| McKenna 2009 [44] | n = 31; hip | Retrospective observational; single center; non-comparative | All Gram-positives; 77% staphylococci | Vancomycin | 5 days | Various local agents | 35 | Add. debridement 0%; cultures+ 0%; persistence/relapse 0% | Very favorable outcomes reported despite brief systemic exposure. |
| Mittal 2007 [45] | n = 37; knee | Retrospective observational; multicenter; comparative (short vs. long IV) | MR staphylococci | Not reported | ≥6 w IV vs. <6 w IV | Various local agents | 51 | Cultures+ 0%; persistence/relapse: short 13% (2/15) vs. long 9% (2/22); p = 0.07 | Longer IV tended toward lower relapse, not statistically significant in small sample. |
| Hsieh 2009 [46] | n = 99; knee | Retrospective observational; single center; comparative | 67% Gram-positives; 53% staphylococci | 1st-gen cephalosporin + gentamicin | 4–6 w vs. 7 d | Various local agents | 43 | Additional debridement: long 2/46 (4%) vs. short 1/53 (2%); persistence/relapse: long 4% vs. short 6% | No clear advantage of longer systemic therapy in this cohort. |
| El Helou 2011 [47] | n = 208; hip and knee | Retrospective observational; single center; comparative; propensity-adjusted | Mainly Gram-positives; 62% staphylococci | Not reported | 4 w ± 7 d vs. 6 w ± 7 d | Vancomycin ± tobramycin (local) | 60 | Cultures+: short 6.1% vs. long 8.7%; persistence/relapse: short 16% vs. long 27% | Shorter systemic duration did not worsen outcomes after adjustment. |
| Benka-bouche 2019 [48] | n = 39; hip and knee | Single-center, open-label randomized clinical trial | Various | Vancomycin IV; oral fluoroquinolone | 6 w (39–45 d) vs. 4 w (27–30 d) | Local tobramycin in 2 cases (5%) | 26 | No significant difference in PJI subgroup | Short (4 w) non-inferior to 6 w in small RCT subgroup. |
| Ma 2020 [49] | n = 64; knee | Retrospective observational; single center; comparative | 69% staphylococci | Not reported | 4–6 w vs. ≤7 d | Vancomycin ± aminoglycosides (local) | 75 | Need for salvage antimicrobials/surgery: long 26% (11/43) vs. short 14% (3/21) | Longer courses associated with fewer salvage events numerically. |
| Bernard 2021 [4] | n = 81; hip and knee | Multicenter, open-label randomized clinical trial | 40% S. aureus | Rifampicin + fluoroquinolones (common) | 6 w vs. 12 w | Not reported | ≥24 | Failure: 15% (6/40) vs. 5% (2/41); difference 10.1% (95% CI −0.9 to 22.2) | Signal favoring 12 w; authors recommend longer duration. |
4.4. PJI Treated with Total Removal Without Implantation
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Domain | Minimum Specification (REQUIRED) | Preferred/Extended Specification | Notes and Quality Assurance |
|---|---|---|---|
| Population and Setting | Adults (≥18 y) with suspected or confirmed hip/knee PJI, managed by DAIR, one-stage, or two-stage revision; consent obtained. | Include pragmatic spectrum (acute hematogenous and chronic). Pre-register site capabilities and case-mix to ensure balance. | Use ICM 2018/MSIS criteria for screening; record referral pathway and symptom duration. |
| Case Definition | Uniform diagnostic criteria (ICM 2018). Require ≥2 concordant cultures OR sinus tract OR a major criterion. | Adjudication panel confirms case status blinded to allocation. | Provide pocket card/SOP; site initiation training; periodic source-data verification. |
| Randomization and Stratification | Central concealed allocation 1:1, stratified by center, joint (hip/knee), surgical strategy (DAIR/1-stage/2-stage), and chronicity. | Use permuted blocks with variable sizes; web-based IWRS. | Document concealment; monitor strata counts. |
| Interventions of Interest | A) Antibiotic duration strategy (e.g., 6–8 w vs. 12 w) OR B) antimicrobial-coated vs. standard revision implants. | Protocolized agent choices per organism; mandate rifampicin-based combos for staphylococci unless contraindicated. | Pre-specify dosing, IV-to-oral switch rules, interactions, adherence tracking. |
| Primary Endpoint | Infection-related treatment failure at 24 months (composite: persistent/recurrent PJI, unplanned reoperation for infection, infection-related death). | Time-to-event analysis with competing risks; blinded endpoint committee. | Endpoints charter; dual review with arbitration. |
| Key Secondary Endpoints | Function (Oxford Hip/Knee Score), QoL (EQ-5D-5L), safety (CTCAE), health-economics (LOS, readmissions, costs), resistance emergence (MDR colonization/infection; C. difficile). | PROMs at baseline, 3, 6, 12, 24 months; cost-utility (QALYs); microbiological cure at reimplantation for staged procedures. | Central training for PROMs; standardized AE coding; harmonized HE CRFs. |
| Follow-up Schedule | Discharge, 6 ± 2 w, 3, 6, 12, 24 months; phone backup allowed. | Extended 36–60 months registry add-on. | Missed visit policy; vital status via national registries where available. |
| Pre-analytical Specimen Handling | At surgery obtain ≥5 separate periprosthetic tissue samples; label site; sterile dry containers; deliver to lab ≤ 2 h (≤24 h if 4 °C). | Sonication of explanted components where available; inoculate one sample into blood-culture bottles at bedside. | Chain-of-custody forms; temperature/time stamps; deviation log. |
| Microbiological Culture (Analytical) | Aerobic/anaerobic media; tissues incubated ≥7 days; extend to 14 days for low-virulence organisms (e.g., Cutibacterium). | Quantitative sonicate-fluid cultures; standardized media panel across sites. | Inter-lab proficiency testing; document negative culture workflow. |
| Molecular and Biomarker Tests | PCR/16S optional but protocolized; synovial WBC and PMN% when feasible; CRP/ESR mandatory at baseline. | Central mNGS for discordant/negative cases (optional). | Report platform/version; validation required for non-standard assays. |
| Histopathology | PMN count thresholds documented a priori; frozen/permanent sections per local standard. | Central review of 10% random sample. | Standardized report template; slide digitization where feasible. |
| Susceptibility Testing | EUCAST (preferred) or CLSI breakpoints; method documented. | Central re-testing of sentinel isolates; synergy testing for rifampicin/fluoroquinolone when indicated. | Annual QC with reference strains; discrepancy reconciliation. |
| Data Elements (Minimum) | Demographics, comorbidities (CCI), prior antibiotics, symptom duration, joint, strategy, organism(s), MICs, implant details, spacer type, local antibiotics, IV/oral days, adherence, AEs, reoperations, PROMs, costs. | FAIR-mapped core outcome set; partial EHR import. | Locked data dictionary; SI units. |
| Analysis Plan | Mixed-effects ITT (center random intercept); multiplicity-controlled secondaries; predefined PP and as-treated analyses. | Bayesian hierarchical subgroup borrowing (organism, strategy). | SAP finalized before DB lock; independent statistical oversight. |
| International Harmonization | Translated SOPs; alignment with EU/Non-EU regulations. | Central kit provision (media, labels); remote monitoring. | Maintain a regulatory/ethics concordance matrix. |
References
- Patel, R. Periprosthetic Joint Infection. N. Engl. J. Med. 2023, 388, 251–262. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Mancheño-Losa, M.; Meléndez-Carmona, M.Á.; Hernández-Jiménez, P.; Benito, N.; Murillo, O. Appropriate Duration of Antimicrobial Treatment for Prosthetic Joint Infections: A Narrative Review. Antibiotics 2024, 13, 293. [Google Scholar] [CrossRef]
- Bernard, L.; Legout, L.; Zürcher-Pfund, L.; Stern, R.; Rohner, P.; Peter, R.; Assal, M.; Lew, D.; Hoffmeyer, P.; Uçkay, I. Six weeks of antibiotic treatment is sufficient following surgery for septic arthroplasty. J. Infect. 2010, 61, 125–132. [Google Scholar] [CrossRef]
- Bernard, L.; Arvieux, C.; Brunschweiler, B.; Touchais, S.; Ansart, S.; Bru, J.-P.; Oziol, E.; Boeri, C.; Gras, G.; Druon, J.; et al. Antibiotic Therapy for 6 or 12 Weeks for Prosthetic Joint Infection. N. Engl. J. Med. 2021, 384, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef]
- Ometti, M.; Delmastro, E.; Salini, V. Management of prosthetic joint infections: A guidelines comparison. Musculoskelet. Surg. 2022, 106, 219–226. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; D’anglejan, E.; Leliepvre, H.; Bouchand, F.; Marmouset, D.; Dournon, N.; Mascitti, H.; Genet, F.; Herrmann, J.-L.; Chaussard, H.; et al. Short Antibiotic Treatment Duration for Osteomyelitis Complicating Pressure Ulcers: A Quasi-experimental Study. Open Forum Infect. Dis. 2023, 10, ofad088. [Google Scholar] [CrossRef]
- Tsukayama, D.T.; Estrada, R.; Gustilo, R.B. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Jt. Surg. Am. 1996, 78, 512–523. [Google Scholar] [CrossRef]
- Coventry, M.B. Treatment of infections occurring in total hip surgery. Orthop. Clin. N. Am. 1975, 6, 991–1003. [Google Scholar] [CrossRef]
- Gomez-Urena, E.O.; Tande, A.J.; Osmon, D.R.; Berbari, E.F. Diagnosis of Prosthetic Joint Infection: Cultures, Biomarker and Criteria. Infect. Dis. Clin. N. Am. 2017, 31, 219–235. [Google Scholar] [CrossRef]
- Berdal, J.-E.; Skråmm, I.; Mowinckel, P.; Gulbrandsen, P.; Bjørnholt, J.V. Use of rifampicin and ciprofloxacin combination therapy after surgical debridement in the treatment of early manifestation prosthetic joint infections. Clin. Microbiol. Infect. 2005, 11, 843–845. [Google Scholar] [CrossRef][Green Version]
- Soriano, A.; García, S.; Bori, G.; Almela, M.; Gallart, X.; Macule, F.; Sierra, J.; Martínez, J.A.; Suso, S.; Mensa, J. Treatment of acute post-surgical infection of joint arthroplasty. Clin. Microbiol. Infect. 2006, 12, 930–933. [Google Scholar] [CrossRef]
- Martínez-Pastor, J.C.; Muñoz-Mahamud, E.; Vilchez, F.; García-Ramiro, S.; Bori, G.; Sierra, J.; Martínez, J.A.; Font, L.; Mensa, J.; Soriano, A. Outcome of acute prosthetic joint infections due to gram-negative bacilli treated with open debridement and retention of the prosthesis. Antimicrob. Agents Chemother. 2009, 53, 4772–4777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cobo, J.; Miguel, L.G.S.; Euba, G.; Rodríguez, D.; García-Lechuz, J.M.; Riera, M.; Falgueras, L.; Palomino, J.; Benito, N.; del Toro, M.D.; et al. Early prosthetic joint infection: Outcomes with debridement and implant retention followed by antibiotic therapy. Clin. Microbiol. Infect. 2011, 17, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, F.; Martínez-Pastor, J.C.; García-Ramiro, S.; Bori, G.; Maculé, F.; Sierra, J.; Font, L.; Mensa, J.; Soriano, A. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin. Microbiol. Infect. 2011, 17, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Tornero, E.; Morata, L.; Martínez-Pastor, J.C.; Angulo, S.; Combalia, A.; Bori, G.; García-Ramiro, S.; Bosch, J.; Mensa, J.; Soriano, A. Importance of selection and duration of antibiotic regimen in prosthetic joint infections treated with debridement and implant retention. J. Antimicrob. Chemother. 2016, 71, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Puhto, A.-P.; Puhto, T.; Syrjala, H. Short-course antibiotics for prosthetic joint infections treated with prosthesis retention. Clin. Microbiol. Infect. 2012, 18, 1143–1148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lora-Tamayo, J.; Murillo, O.; Iribarren, J.A.; Soriano, A.; Sánchez-Somolinos, M.; Baraia-Etxaburu, J.M.; Rico, A.; Palomino, J.; Rodríguez-Pardo, D.; Horcajada, J.P.; et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin. Infect. Dis. 2013, 56, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Lora-Tamayo, J.; Euba, G.; Cobo, J.; Horcajada, J.P.; Soriano, A.; Sandoval, E.; Pigrau, C.; Benito, N.; Falgueras, L.; Palomino, J.; et al. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: A randomised clinical trial. Int. J. Antimicrob. Agents 2016, 48, 310–316. [Google Scholar] [CrossRef]
- Chaussade, H.; Uçkay, I.; Vuagnat, A.; Druon, J.; Gras, G.; Rosset, P.; Lipsky, B.A.; Bernard, L. Antibiotic therapy duration for prosthetic joint infections treated by Debridement and Implant Retention (DAIR): Similar long-term remission for 6 weeks as compared to 12 weeks. Int. J. Infect. Dis. 2017, 63, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Jenny, J.Y.; Barbe, B.; Gaudias, J.; Boeri, C.; Argenson, J.N. High infection control rate and function after routine one-stage exchange for chronically infected TKA. Clin. Orthop. Relat. Res. 2013, 471, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Jenny, J.Y.; Barbe, B.; Cazenave, A.; Roche, O.; Massin, P. Patient selection does not improve the success rate of infected TKA one stage exchange. Knee 2016, 23, 1012–1015. [Google Scholar] [CrossRef]
- Singer, J.; Merz, A.; Frommelt, L.; Fink, B. High rate of infection control with one-stage revision of septic knee prostheses excluding MRSA and MRSE. Clin. Orthop. Relat. Res. 2012, 470, 1461–1471. [Google Scholar] [CrossRef]
- Massin, P.; Delory, T.; Lhotellier, L.; Pasquier, G.; Roche, O.; Cazenave, A.; Estellat, C.; Jenny, J.Y. Infection recurrence factors in one- and two-stage total knee prosthesis exchanges. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3131–3139. [Google Scholar] [CrossRef]
- Castellani, L.; Daneman, N.; Mubareka, S.; Jenkinson, R. Factors Associated with Choice and Success of One Versus Two-Stage Revision Arthroplasty for Infected Hip and Knee Prostheses. HSS J. 2017, 13, 224–231. [Google Scholar] [CrossRef]
- Haddad, F.S.; Sukeik, M.; Alazzawi, S. Is Single-stage Revision According to a Strict Protocol Effective in Treatment of Chronic Knee Arthroplasty Infections? Clin. Orthop. Relat. Res. 2015, 473, 8–14. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Ricciardi, B.F.; Briggs, T.W.R.; Sussmann, P.S.; Bostrom, M.P. Evaluation and Management of Periprosthetic Joint Infection-an International, Multicenter Study. HSS J. 2014, 10, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D.; Team, I. Patient-Related Risk Factors for Periprosthetic Joint Infection after Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150866. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, L.A.; Peppers, M.; Nayfeh, T.A.; Roy, M.E. Methicillin-resistant Staphylococcus aureus in TKA treated with revision and direct intra-articular antibiotic infusion. Clin. Orthop. Relat. Res. 2011, 469, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tibrewal, S.; Malagelada, F.; Jeyaseelan, L.; Posch, F.; Scott, G. Single-stage revision for the infected total knee replacement: Results from a single centre. Bone Jt. J. 2014, 96, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.d.P.L.; Cinagawa, E.H.T.; Camargo, O.P.A.; Honda, E.K.; Klautau, G.B.; Salles, M.J.C. Treatment of Infection After total Knee Arthroplasty. Acta Ortop. Bras. 2015, 23, 239–243. [Google Scholar] [CrossRef]
- Cochran, A.R.; Ong, K.L.; Lau, E.; Mont, M.A.; Malkani, A.L. Risk of Reinfection After Treatment of Infected Total Knee Arthroplasty. J. Arthroplast. 2016, 31, 156–161. [Google Scholar] [CrossRef]
- Zahar, A.; Kendoff, D.O.; Klatte, T.O.; Gehrke, T.A. Can Good Infection Control Be Obtained in One-stage Exchange of the Infected TKA to a Rotating Hinge Design? 10-year Results. Clin. Orthop. Relat. Res. 2016, 474, 81–87. [Google Scholar] [CrossRef]
- Baker, P.; Petheram, T.G.; Kurtz, S.; Konttinen, Y.T.; Gregg, P.; Deehan, D. Patient reported outcome measures after revision of the infected TKR: Comparison of single versus two-stage revision. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2713–2720. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Stockley, I.; Mockford, B.J.; Hoad-Reddick, A.; Norman, P. The use of two-stage exchange arthroplasty with depot antibiotics in the absence of long-term antibiotic therapy in infected total hip replacement. J. Bone Jt. Surg. Br. 2008, 90, 145–148. [Google Scholar] [CrossRef]
- Taggart, T.; Kerry, R.M.; Norman, P.; Stockley, I. The use of vancomycin-impregnated cement beads in the management of infection of prosthetic joints. J. Bone Jt. Surg. Br. 2002, 84, 70–72. [Google Scholar] [CrossRef][Green Version]
- Hoad-Reddick, D.A.; Evans, C.R.; Norman, P.; Stockley, I. Is there a role for extended antibiotic therapy in a two-stage revision of the infected knee arthroplasty? J. Bone Jt. Surg. Br. 2005, 87, 171–174. [Google Scholar] [CrossRef]
- Whittaker, J.P.; Warren, R.E.; Jones, R.S.; Gregson, P.A. Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chronic Gram-positive infection? J. Bone Jt. Surg. Br. 2009, 91, 44–51. [Google Scholar] [CrossRef]
- Hart, W.J.; Jones, R.S. Two-stage revision of infected total knee replacements using articulating cement spacers and short-term antibiotic therapy. J. Bone Jt. Surg. Br. 2006, 88, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- McKenna, P.B.; O’Shea, K.; Masterson, E.L. Two-stage revision of infected hip arthroplasty using a shortened post-operative course of antibiotics. Arch. Orthop. Trauma. Surg. 2009, 129, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Mittal, Y.; Fehring, T.K.; Hanssen, A.; Marculescu, C.; Odum, S.M.; Osmon, D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J. Bone Jt. Surg. Am. 2007, 89, 1227–1231. [Google Scholar] [CrossRef]
- Hsieh, P.-H.; Huang, K.-C.; Lee, P.-C.; Lee, M.S. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: Retrospective comparison between short-term and prolonged antibiotic therapy. J. Antimicrob. Chemother. 2009, 64, 392–397. [Google Scholar] [CrossRef]
- El Helou, O.C.; Berbari, E.F.; Lahr, B.D.; Marculescu, C.E.; Razonable, R.R.; Steckelberg, J.M.; Hanssen, A.D.; Osmon, D.R. Management of prosthetic joint infection treated with two-stage exchange: The impact of antimicrobial therapy duration. Curr. Orthop. Pract. 2011, 22, 333–338. [Google Scholar] [CrossRef]
- Benka-bouche, M.; Racloz, G.; Spechbach, H.; Lipsky, B.A.; Gaspoz, J.-M.; Uçkay, I. Four versus six weeks of antibiotic therapy for osteoarticular infections after implant removal: A randomized trial. J. Antimicrob. Chemother. 2019, 74, 2394–2399. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-H.; Chou, T.-F.A.; Tsai, S.-W.; Chen, C.-F.; Wu, P.-K.; Chen, C.-M.; Chen, W.-M. Is short-course systemic antibiotic therapy using an antibiotic-loaded cement spacer safe after resection for infected total knee arthroplasty? A comparative study. J. Formos. Med. Assoc. 2020, 119, 1070–1079. [Google Scholar] [CrossRef]
- Falahee, M.H.; Matthews, L.S.; Kaufer, H. Resection arthroplasty as a salvage procedure for a knee with infection after a total arthroplasty. J. Bone Jt. Surg. Am. 1987, 69, 1013–1021. [Google Scholar] [CrossRef]
- Uçkay, I.; Jugun, K.; Gamulin, A.; Wagener, J.; Hoffmeyer, P.; Lew, D. Chronic osteomyelitis. Curr. Infect. Dis. Rep. 2012, 14, 566–575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wasielewski, R.C.; Barden, R.M.; Rosenberg, A.G. Results of different surgical procedures on total knee arthroplasty infections. J. Arthroplast. 1996, 11, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Kutscha-Lissberg, F.; Hebler, U.; Esenwein, S.A.; Muhr, G.; Wick, M. Fusion of the septic knee with external hybrid fixator. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 968–974. [Google Scholar] [CrossRef]
- Spina, M.; Gualdrini, G.; Fosco, M.; Giunti, A. Knee arthrodesis with the Ilizarov external fixator as treatment for septic failure of knee arthroplasty. J. Orthop. Traumatol. 2010, 11, 81–88. [Google Scholar] [CrossRef]
- Mabry, T.M.; Jacofsky, D.J.; Haidukewych, G.J.; Hanssen, A.D. Comparison of intramedullary nailing and external fixation knee arthrodesis for the infected knee replacement. Clin. Orthop. Relat. Res. 2007, 464, 11–15. [Google Scholar] [CrossRef]
- Le Vavasseur, B.; Zeller, V. Antibiotic Therapy for Prosthetic Joint Infections: An Overview. Antibiotics 2022, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Rottier, W.; Seidelman, J.; Wouthuyzen-Bakker, M. Antimicrobial treatment of patients with a periprosthetic joint infection: Basic principles. J. Arthroplast. 2023, 5, 10. [Google Scholar] [CrossRef]
- Li, H.-K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef]
- Dudareva, M.; Kümin, M.; Vach, W.; Kaier, K.; Ferguson, J.; McNally, M.; Scarborough, M. Short or Long Antibiotic Regimes in Orthopaedics (SOLARIO): A randomised controlled open-label non-inferiority trial of duration of systemic antibiotics in adults with orthopaedic infection treated operatively with local antibiotic therapy. Trials 2019, 20, 693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrabi, H.; Mamona-Kilu, C.; Meyer, E.; d’Anglejan Chatillon, E.; Dournon, N.; Bouchand, F.; Duran, C.; Perronne, V.; Jaffal, K.; Dinh, A. Treatment Duration in Bacterial Prosthetic Joint Infections: A Narrative Review of Current Evidence. Antibiotics 2025, 14, 1066. https://doi.org/10.3390/antibiotics14111066
Harrabi H, Mamona-Kilu C, Meyer E, d’Anglejan Chatillon E, Dournon N, Bouchand F, Duran C, Perronne V, Jaffal K, Dinh A. Treatment Duration in Bacterial Prosthetic Joint Infections: A Narrative Review of Current Evidence. Antibiotics. 2025; 14(11):1066. https://doi.org/10.3390/antibiotics14111066
Chicago/Turabian StyleHarrabi, Hajer, Christel Mamona-Kilu, Eloïse Meyer, Emma d’Anglejan Chatillon, Nathalie Dournon, Frédérique Bouchand, Clara Duran, Véronique Perronne, Karim Jaffal, and Aurélien Dinh. 2025. "Treatment Duration in Bacterial Prosthetic Joint Infections: A Narrative Review of Current Evidence" Antibiotics 14, no. 11: 1066. https://doi.org/10.3390/antibiotics14111066
APA StyleHarrabi, H., Mamona-Kilu, C., Meyer, E., d’Anglejan Chatillon, E., Dournon, N., Bouchand, F., Duran, C., Perronne, V., Jaffal, K., & Dinh, A. (2025). Treatment Duration in Bacterial Prosthetic Joint Infections: A Narrative Review of Current Evidence. Antibiotics, 14(11), 1066. https://doi.org/10.3390/antibiotics14111066







