Cefiderocol Comparative Resistance and Clinical Predictors in CRE-BSI: Data from an OXA-48–Endemic Region with Rising OXA-48/NDM Coproducers
Abstract
1. Introduction
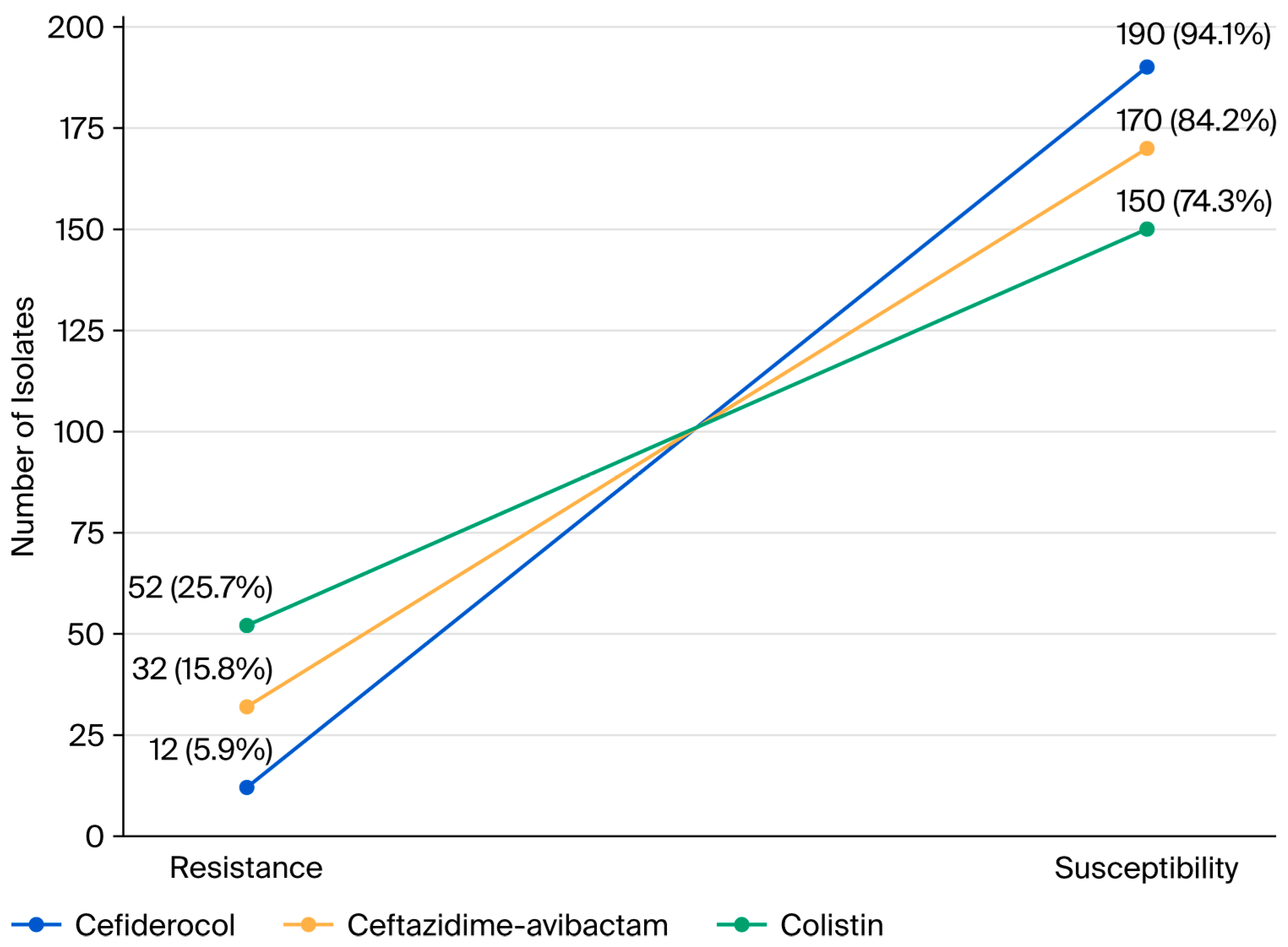
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Inclusion Criteria
- -
- Adults aged ≥ 18 years;
- -
- A diagnosis of a CRE-BSI confirmed by blood culture and antimicrobial susceptibility testing;
- -
- Availability of antimicrobial susceptibility testing results for cefiderocol, CAZ-AVI, and colistin.
4.3. Exclusion Criteria
- Patients were excluded if they had
- Polymicrobial bloodstream infections;
- Incomplete clinical or microbiological data.
4.4. Study Approval
- -
- The study was approved by the Istanbul Medipol University Clinical Research Ethics Committee (date/number: 4 August 2025/E-10840098–202.3.02–4982).
- -
- This retrospective observational study was conducted in accordance with the World Medical Association’s Declaration of Helsinki and the Ethical Principles for Medical Research Involving Human Subjects.
- -
- The Non-invasive Clinical Research Ethics Committee of Medipol University waived the requirement for informed consent as the study was retrospective in design.
4.5. Data Collection
4.6. Microbiological Analysis
4.7. Definitions
- -
- CRE-BSI: Defined as the growth of an Enterobacterales isolate resistant to at least one carbapenem in blood culture [5].
- -
- Sepsis and septic shock: Defined according to the Sepsis-3 criteria based on the Sequential Organ Failure Assessment (SOFA) score [28].
- -
- Classification of BSIs: Determined according to CDC/NHSN definitions [30].
- -
- Cefiderocol resistance: Determined according to the EUCAST 2023 [31] breakpoints. Isolates with inhibition zones < 17 mm were classified as resistant, while those with zones between 17 and 22 mm were considered within ATU and retested by broth microdilution; isolates with a minimum inhibitory concentration (MIC) > 2 µg/mL were defined as resistant [31].
- -
- Colistin resistance: Defined as an MIC > 2 µg/mL according to the EUCAST criteria [31].
- -
- CAZ-AVI resistance: Defined as an inhibition zone < 17 mm according to the EUCAST criteria [31].
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oueslati, S.; Bogaerts, P.; Dortet, L.; Bernabeu, S.; Ben Lakhal, H.; Longshaw, C.; Glupczynski, Y.; Naas, T. In Vitro activity of cefiderocol and comparators against carbapenem-resistant Gram-negative pathogens from France and Belgium. Antibiotics 2022, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Centers for Disease Control and Prevention. About Carbapenem-Resistant Enterobacterales (CRE); CDC: Atlanta, Georgia, 2024.
- World Health Organization Regional Office for Europe. Central Asian and European Surveillance of Antimicrobial Resistance: Annual Report 2023–2021 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2025; Available online: https://www.who.int/europe/publications/i/item/9789289058537 (accessed on 20 August 2025).
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 guidance on the treatment of antimicrobial-resistant Gram-negative infections. Clin. Infect. Dis. 2024, 79, ciae403. [Google Scholar] [CrossRef] [PubMed]
- Isler, B.; Özer, B.; Çınar, G.; Aslan, A.T.; Vatansever, C.; Falconer, C.; Dolapçı, I.; Şimşek, F.; Tülek, N.; Demirkaya, H.; et al. Characteristics and outcomes of carbapenemase-harbouring carbapenem-resistant Klebsiella spp. bloodstream infections: A multicentre prospective cohort study in an OXA-48 endemic setting. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Dumlu, R.; Şahin, M.; Derin, O.; Gül, Ö.; Başgönül, S.; Zengin, R.; Arabacı, Ç.; Şimşek, F.; Gençer, S.; Kocagöz, A.S.; et al. Ceftazidime–avibactam versus polymyxin-based combination therapies: A study on 30-day mortality in carbapenem-resistant Enterobacterales bloodstream infections in an OXA-48-endemic region. Antibiotics 2024, 13, 990. [Google Scholar] [CrossRef]
- Yao, J.; Wang, J.; Chen, M.; Cai, Y. Cefiderocol: An overview of its in vitro and in vivo activity and underlying resistant mechanisms. Front. Med. 2021, 8, 741940. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Golden, A.R.; Zelenitsky, S.; Wiebe, K.; Lawrence, C.K.; Adam, H.J.; Idowu, T.; Domalaon, R.; Schweizer, F.; Zhanel, M.A.; et al. Cefiderocol: A siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant Gram-negative bacilli. Drugs. 2019, 79, 271–289. [Google Scholar] [CrossRef]
- Başaran, S.N.; Öksüz, L. Newly developed antibiotics against multidrug-resistant and carbapenem-resistant Gram-negative bacteria: Action and resistance mechanisms. Arch. Microbiol. 2025, 207, 110. [Google Scholar] [CrossRef]
- Ozyurt, O.; Tufanoglu, P.; Cetinkaya, O.; Ozhak, B.; Yazisiz, H.; Ongut, G.; Turhan, O.; Ogunc, D. In vitro activity of cefiderocol and ceftazidime–avibactam against carbapenemase-producing Enterobacterales. Clin. Lab. 2023, 69, e220827. [Google Scholar] [CrossRef]
- Borde, K.; Kareem, M.A.; Sharma, R.M.; Dass, S.M.; Ravi, V.; Mathai, D. In vitro activity of cefiderocol against comparators against clinical isolates of meropenem-resistant Klebsiella pneumoniae from India. Microbiol. Spectr. 2023, 11, e0084723. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic review of mechanisms of resistance, heteroresistance and in vivo emergence of resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef]
- Isler, B.; Vatansever, C.; Özer, B.; Çınar, G.; Aslan, A.T.; Falconer, C.; Bauer, M.J.; Forde, B.; Şimşek, F.; Tülek, N.; et al. Higher rates of cefiderocol resistance among NDM-producing Klebsiella bloodstream isolates applying EUCAST over CLSI breakpoints. Infect. Dis. 2023, 55, 607–613. [Google Scholar] [CrossRef]
- Shortridge, D.; Streit, J.M.; Mendes, R.; Castanheira, M. In vitro activity of cefiderocol against U.S. and European Gram-Negative clinical isolates collected in 2020 as part of the SENTRY Antimicrobial Surveillance Program. Microbiol. Spectr. 2022, 10, e0271221. [Google Scholar] [CrossRef]
- Kaye, K.S.; Naas, T.; Pogue, J.M.; Rossolii, G.M. Cefiderocol, a siderophore cephalosporin, as a treatment option for infections caused by carbapenem-resistant Enterobacterales. Infect. Dis. Ther. 2023, 12, 777–806. [Google Scholar] [CrossRef] [PubMed]
- Dumlu, R.; Uyar, N.Y.; Ayaş, M.; Aksoy, N.; Öztürk, N.; Kocagöz, A.S. Investigation of ceftazidime–avibactam susceptibility in clinical isolates of Gram-negative bacteria. Turk. J. Med. Sci. 2022, 52, 1839–1844. [Google Scholar] [CrossRef]
- Yakut, S.; Doğan, S.; Yıldız Zeyrek, F. Investigation of colistin and polymyxin B susceptibility in Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii isolates. ANKEM Derg. 2025, 39, 14–21. Available online: https://dergipark.org.tr/tr/download/article-file/4723430 (accessed on 20 August 2025). [CrossRef]
- Kaya, F.; Ölçü, M. Evaluation of ceftazidime–avibactam resistance rates in multi-drug resistant Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa strains in intensive care units. Flora 2024, 29, 45–51. Available online: https://www.floradergisi.org/managete/fu_folder/2024-01/2024-29-01-45-51.pdf (accessed on 22 August 2025). [CrossRef]
- Koçak, C.Ö.; Hazırolan, G. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae clinical isolates. Turk. Mikrobiyol. Cem. Derg. 2019, 49, 17–23. Available online: https://tmc.dergisi.org/pdf/pdf_TMC_639.pdf (accessed on 23 August 2025).
- Lan, P.; Lu, Y.; Chen, Z.; Wu, X.; Hua, X.; Jiang, Y.; Zhou, J.; Yu, Y. Emergence of high-level cefiderocol resistance in carbapenem-resistant Klebsiella pneumoniae from bloodstream infections in patients with hematologic malignancies in China. Microbiol. Spectr. 2022, 10, e0008422. [Google Scholar] [CrossRef]
- Smith, T.; Salgado, C.; Mauldin, P.D.; Zhang, J.; Bosso, J.A. Relationship between days in hospital and infection with a multidrug-resistant Gram-negative pathogen. In Proceedings of the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Washington, DC, USA, 5–9 September 2014. Poster Presentation K-667. [Google Scholar]
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. Healthcare-associated infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK577288/ (accessed on 25 August 2025).
- Centers for Disease Control and Prevention (CDC). Antimicrobial Resistance Threats in the United States, 2021–2022; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2025. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/update-2022.html (accessed on 12 July 2025).
- Kriz, R.; Spettel, K.; Pichler, A.; Kittinger, C.; Zarfel, G. In vitro resistance development gives insights into molecular resistance mechanisms against cefiderocol. J. Antibiot. 2024, 77, 757–767. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, M.N.; Baddour, L.M. Resilience of the Pitt Bacteremia Score: Three decades and counting. Clin. Infect. Dis. 2020, 70, 1834–1836. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC/NHSN Surveillance Definitions for Specific Types of Infections; CDC: Atlanta, GA, USA, 2023. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf (accessed on 10 July 2025).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.0. Växjö: EUCAST. 2023. Available online: http://www.eucast.org (accessed on 20 July 2025).
| Variable | Overall n = 202 (100%) 1 | Cefiderocol-Resistant n = 12 (5.9%) | Cefiderocol-Susceptible n = 190 (94.1%) | p-Value 2 |
|---|---|---|---|---|
| Age, median (IQR) | 52.50 (35.75–70) | 63.50 (42.25–74.5) | 52.00 (35–70) | 0.260 |
| Age ≥ 65 | 68 (33.7) | 6 (50) | 62 (32.6) | 0.217 |
| Male sex | 106 (52.5) | 3 (25) | 103 (54.2) | 0.051 |
| CCI, median (IQR) | 5 (2–7) | 2.5 (1.25–6) | 5 (2–7) | 0.134 |
| CCI ≥ 4 | 124 (61.4) | 5 (41.7) | 119 (62.6) | 0.148 |
| Hematological malignancy | 29 (14.4) | 9 (75) | 20 (10.5) | <0.001 |
| HSCT | 11 (5.4) | 6 (50.0) | 5 (2.6) | <0.001 |
| Neutropenia | 18 (8.9) | 2 (16.7) | 16 (8.4) | 0.331 |
| ICU | 77 (38.1) | 4 (33.3) | 73 (38.4) | 0.725 |
| Invasive device | 96 (47.5) | 5 (41.7) | 91 (47.9) | 0.675 |
| Sepsis | 102 (50.5) | 8 (66.7) | 94 (49.5) | 0.248 |
| Septic shock | 61 (30.2) | 4 (33.3) | 57 (30) | 0.807 |
| PBS, median (IQR) | 4 (2–6) | 4 (1–5.75) | 4 (2–6) | 0.890 |
| Prior CRE infection | 34 (16.8) | 2 (16.7) | 32 (16.8) | 0.987 |
| CRE colonization | 33 (16.3) | 1 (8.3) | 32 (16.8) | 0.439 |
| Prior CAZ-AVI ≤ 90 days | 50 (24.8) | 10 (83.3) | 40 (21.1) | <0.001 |
| Prior polymyxin ≤ 90 days | 53 (26.2) | 9 (75) | 44 (23.2) | <0.001 |
| Hospitalization ≤ 1 year | 71 (35.1) | 9 (75) | 62 (32.6) | 0.003 |
| LOS, median (IQR) | 11 (7–16) | 18 (15–22.25) | 11 (7–15.25) | <0.001 |
| LOS ≥ 14 days | 80 (39.6) | 11 (91.7) | 69 (36.3) | <0.001 |
| Variable | Overall n = 202 (100%) 1 | Cefiderocol-Resistant n = 12 (5.9%) | Cefiderocol-Susceptible n = 190 (94.1%) | p-Value 2 |
|---|---|---|---|---|
| Source of Infection | ||||
| Primary BSI | 40 (19.8) | 2 (16.7) | 38 (20) | 0.779 |
| Secondary BSI | 162 (80.2) | 10 (83.3) | 152 (80) | 0.232 |
| Pneumonia | 50 (24.8) | 4 (33.3) | 46 (24.2) | |
| Catheter-related BSI | 30 (14.9) | 0 (0) | 30 (15.8) | |
| Intra-abdominal infection | 45 (22.3) | 5 (41.7) | 40 (21.1) | |
| Urinary tract infection | 37 (18.3) | 1 (8.3) | 36 (18.9) | |
| Type of CRE | 0.642 | |||
| Klebsiella pneumoniae | 190 (94.1) | 11 (91.7) | 179 (94.2) | |
| Escherichia coli | 10 (5) | 1 (8.3) | 9 (4.7) | |
| Enterobacter spp. | 2 (1) | 0 (0) | 2 (1.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumlu, R.; Mert, A. Cefiderocol Comparative Resistance and Clinical Predictors in CRE-BSI: Data from an OXA-48–Endemic Region with Rising OXA-48/NDM Coproducers. Antibiotics 2025, 14, 1057. https://doi.org/10.3390/antibiotics14111057
Dumlu R, Mert A. Cefiderocol Comparative Resistance and Clinical Predictors in CRE-BSI: Data from an OXA-48–Endemic Region with Rising OXA-48/NDM Coproducers. Antibiotics. 2025; 14(11):1057. https://doi.org/10.3390/antibiotics14111057
Chicago/Turabian StyleDumlu, Rıdvan, and Ali Mert. 2025. "Cefiderocol Comparative Resistance and Clinical Predictors in CRE-BSI: Data from an OXA-48–Endemic Region with Rising OXA-48/NDM Coproducers" Antibiotics 14, no. 11: 1057. https://doi.org/10.3390/antibiotics14111057
APA StyleDumlu, R., & Mert, A. (2025). Cefiderocol Comparative Resistance and Clinical Predictors in CRE-BSI: Data from an OXA-48–Endemic Region with Rising OXA-48/NDM Coproducers. Antibiotics, 14(11), 1057. https://doi.org/10.3390/antibiotics14111057





