The Appearance of Antiphage Antibodies in Sera of Patients Treated with Phages
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Bacteriophages
4.3. Plate Phage Neutralization Test
4.4. Categories of the Results of PT
- A—Pathogen eradication and/or recovery (eradication confirmed by the results of bacterial cultures. Recovery refers to wound healing or complete subsidence of the infection symptoms);
- B—Good clinical result (almost complete subsidence of some infection symptoms, together with significant improvement in the patient’s general condition after completion of PT);
- C—Clinical improvement (discernible reduction in the intensity of some infection symptoms after completion of PT to a degree not observed before PT, when no treatment was used).
- D—Questionable clinical improvement (reduction in the intensity of some infection symptoms to the degree that could also be observed before PT);
- E—Transient clinical improvement (reduction in the intensity of some infection symptoms observed only during the application of phage preparations and not after the termination of PT);
- F—No response to treatment (lack of reduction in the intensity of some infection symptoms observed before PT);
- G—Clinical deterioration (of symptoms of infection at the end of PT).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, C.; Zhou, X.; Guo, X.; Yang, Y.; Liu, W.; Zhao, R.; Song, H. Bacteriophage therapy for drug-resistant Staphylococcus aureus infections. Front. Cell Infect. Microbiol. 2024, 14, 1336821. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Fapohunda, O.; Egbon, E.; Ebiesuwa, O.A.; Usman, S.O.; Faronbi, A.O.; Fidelis, S.C. Phage therapy: A targeted approach to overcoming antibiotic resistance. Microb. Pathog. 2024, 197, 107088. [Google Scholar] [CrossRef] [PubMed]
- Archana, A.; Patel, P.S.; Kumar, R.; Nath, G. Neutralizing antibody response against subcutaneously injected bacteriophages in rabbit model. Virusdisease 2021, 32, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gembara, K.; Dąbrowska, K. Interaction of bacteriophages with the immune system: Induction of bacteriophage-specific antibodies. Methods Mol. Biol. 2024, 2734, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Letkiewicz, S.; Fortuna, W.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; Olchawa, E.; et al. Antiphage activity of sera during phage therapy in relation to its outcome. Future Microbiol. 2017, 12, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Żaczek, M.; Łusiak-Szelachowska, M.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Owczarek, B.; Kopciuch, A.; Fortuna, W.; Rogóż, P.; Górski, A. Antibody production in response to staphylococcal MS-1 phage cocktail in patients undergoing phage therapy. Front. Microbiol. 2016, 7, 1681. [Google Scholar] [CrossRef] [PubMed]
- Nick, J.A.; Dedrick, R.M.; Gray, A.L.; Vladar, E.K.; Smith, B.E.; Freeman, K.G.; Malcolm, K.C.; Epperson, L.E.; Hasan, N.A.; Hendrix, J.; et al. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 2022, 185, 1860–1874.e12. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Nang, S.C.; Zhao, J.; Yu, H.H.; Li, J.; Gill, J.J.; Liu, M.; Aslam, S. Therapeutic potential of intravenous phage as standalone therapy for recurrent drug-resistant urinary tract infections. Antimicrob. Agents Chemother. 2023, 67, e0003723. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Lehman, S.M.; Al-Kolla, R.; Penziner, S.; Afshar, K.; Yung, G.; Golts, E.; Law, N.; Logan, C.; Kovach, Z.; et al. Development of host immune response to bacteriophage in a lung transplant recipient on adjunctive phage therapy for a multidrug-resistant pneumonia. J. Infect. Dis. 2023, 227, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Smith, B.E.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Whitney Brown, A.; Cohen, K.A.; Davidson, R.M.; van Duin, D.; et al. Phage therapy of Mycobacterium infections: Compassionate use of phages in 20 patients with drug-resistant mycobacterial disease. Clin. Infect. Dis. 2023, 76, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bernabéu-Gimeno, M.; Pardo-Freire, M.; Chan, B.K.; Turner, P.E.; Gil-Brusola, A.; Pérez-Tarazona, S.; Carrasco-Hernández, L.; Quintana-Gallego, E.; Domingo-Calap, P. Neutralizing antibodies after nebulized phage therapy in cystic fibrosis patients. Med 2024, 5, 1096–1111.e6. [Google Scholar] [CrossRef] [PubMed]
- Berkson, J.D.; Wate, C.E.; Allen, G.B.; Schubert, A.M.; Dunbar, K.E.; Coryell, M.P.; Sava, R.L.; Gao, Y.; Hastie, J.L.; Smith, E.M.; et al. Phage-specific immunity impairs efficacy of bacteriophage targeting Vancomycin Resistant Enterococcus in a murine model. Nat. Commun. 2024, 15, 2993. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Freeman, K.G.; Nguyen, J.A.; Bahadirli-Talbott, A.; Smith, B.E.; Wu, A.E.; Ong, A.S.; Lin, C.T.; Ruppel, L.C.; Parrish, N.M.; et al. Potent antibody-mediated neutralization limits bacteriophage treatment of a pulmonary Mycobacterium abscessus infection. Nat. Med. 2021, 27, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Freeman, K.G.; Nguyen, J.A.; Bahadirli-Talbott, A.; Cardin, M.E.; Cristinziano, M.; Smith, B.E.; Jeong, S.; Ignatius, E.H.; Lin, C.T.; et al. Nebulized bacteriophage in a patient with refractory Mycobacterium abscessus lung disease. Open Forum Infect. Dis. 2022, 9, ofac194. [Google Scholar] [CrossRef] [PubMed]
- Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóż, P.; Kłak, M.; Wojtasik, E.; et al. Clinical aspects of phage therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar] [CrossRef]
- Łobocka, M.; Hejnowicz, M.S.; Dąbrowski, K.; Gozdek, A.; Kosakowski, J.; Witkowska, M.; Ulatowska, M.I.; Weber-Dąbrowska, B.; Kwiatek, M.; Parasion, S.; et al. Genomics of staphylococcal Twort-like phages--potential therapeutics of the post-antibiotic era. Adv. Virus Res. 2012, 83, 143–216. [Google Scholar] [CrossRef] [PubMed]
- Żaczek, M.; Weber-Dąbrowska, B.; Łusiak-Szelachowska, M.; Międzbrodzki, R.; Górski, A. Polish contribution into the advancement of phage treatment in humans. In Bacterial Viruses: Exploitation for Biocontrol and Therapeutics; Coffey, A., Buttimer, C., Eds.; Caister Academic Press: Poole, UK, 2020; pp. 187–202. [Google Scholar] [CrossRef]
- Żaczek, M.; Górski, A.; Weber-Dąbrowska, B.; Letkiewicz, S.; Fortuna, W.; Rogóż, P.; Pasternak, E.; Międzybrodzki, R. A through synthesis of Phage Therapy Unit activity in Poland—Its history, milestones and international recognition. Viruses 2022, 14, 1170. [Google Scholar] [CrossRef] [PubMed]
- Pescovitz, M.D.; Torgerson, T.R.; Ochs, H.D.; Ocheltree, E.; McGee, P.; Krause-Steinrauf, H.; Lachin, J.M.; Canniff, J.; Greenbaum, C.; Herold, K.C.; et al. Effect of rituximab on human in vivo antibody immune responses. J. Allergy Clin. Immunol. 2011, 128, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.H. Methods of study of bacterial viruses. In Bacteriophages; Adams, M.H., Ed.; Interscience: New York, NY, USA, 1959; pp. 443–522. [Google Scholar]
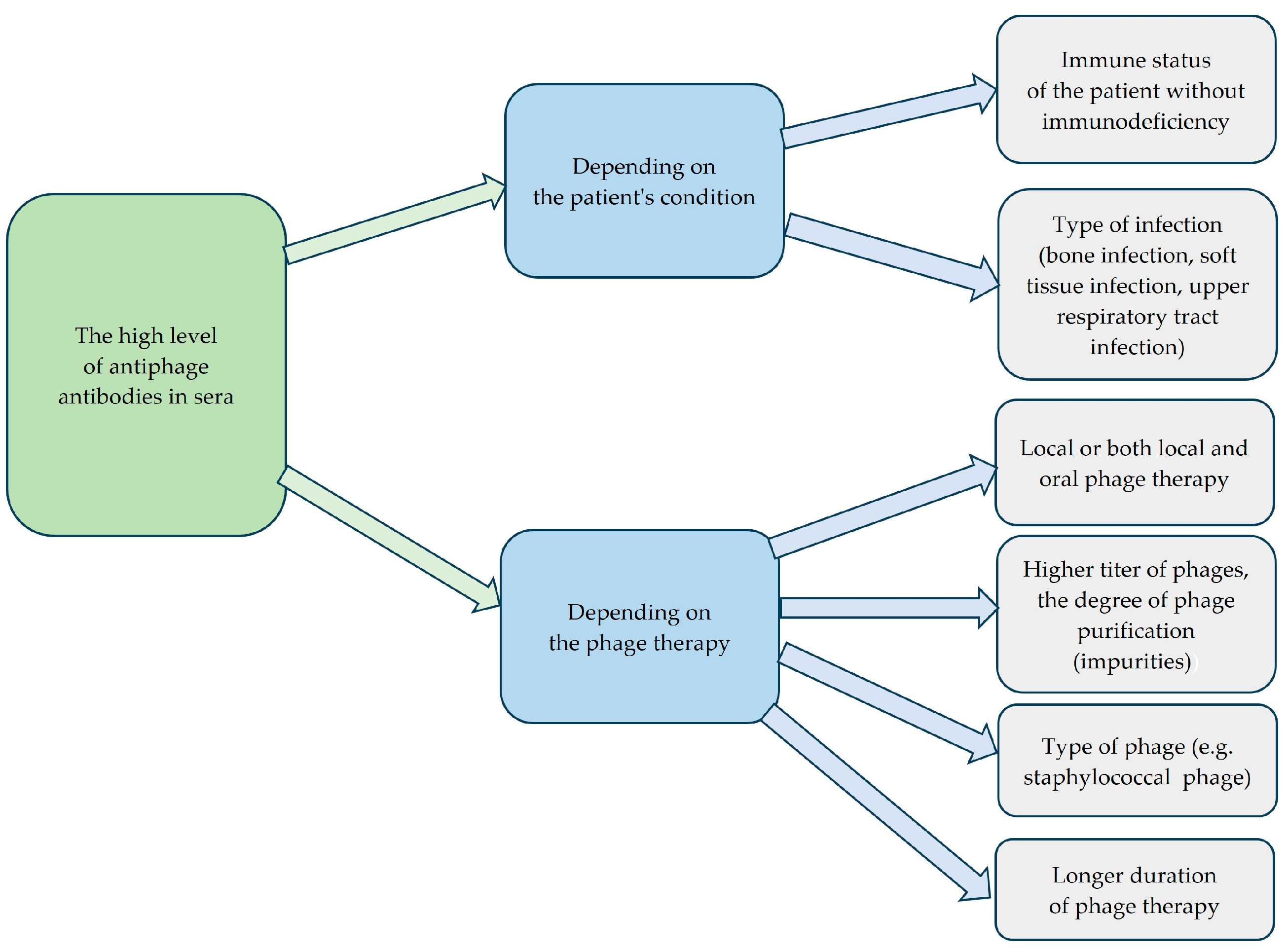
| Patient No. | Type of Infection | Phages Used in PT | K Before PT | K During PT | K After PT | Days of PT | Clinical Outcome of PT a |
|---|---|---|---|---|---|---|---|
| 1 | soft tissue infection | Staph_1N | 0.27 | 0.36 | n.s. | 14 | D |
| 2 | bone infection | Staph_1N | 0.15 | 0.29 | n.s. | 18 | F |
| 3 | upper respiratory tract infection | Staph_1N | 0.19 | 0.67 | n.s. | 18 | F |
| 4 | bone infection | Staph_A5L | 0.04 | 0.38 | n.s. | 21 | F |
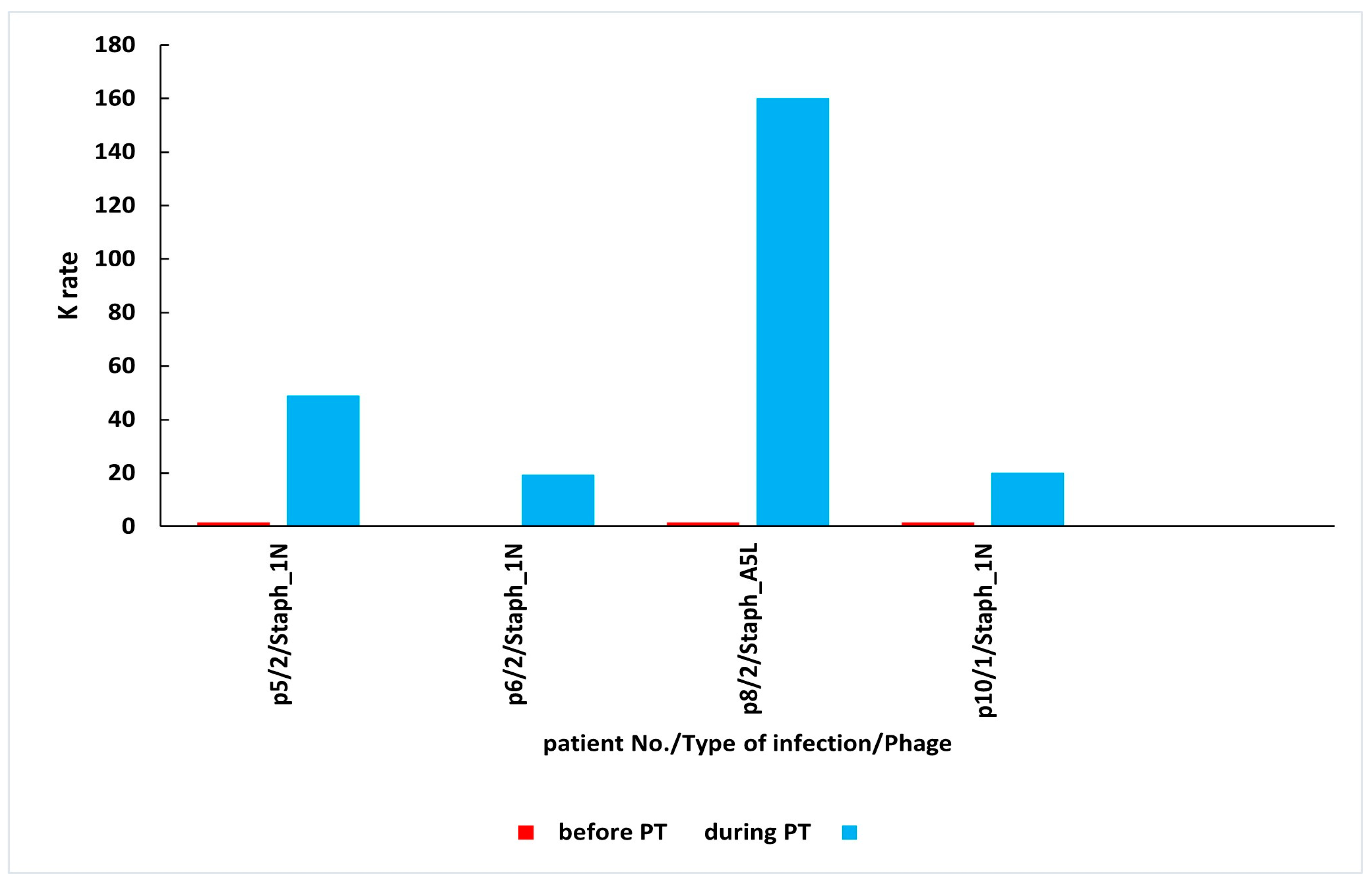
| 5 | bone infection | Staph_1N | 0.05 | 48.86 | n.s. | 21 | E |
| 6 | bone infection | Staph_1N | 0.00 | 19.51 | n.s. | 28 | D |
| 7 | bone infection | Staph_1N | 0.02 | 0.02 | 0.02 | 28 | C |
| 8 | bone infection | Staph_A5L | 0.25 | 160.08 | 303.99 | 35 | D |
| 9 | soft tissue infection | Staph_A5L | 0.05 | 0.25 | n.s. | 56 | D |
| 10 | soft tissue infection | Staph_1N | 0.36 | 20.15 | n.s. | 56 | C |
| 11 | bone infection | Staph_1N | 0.02 | 143.12 (2.5 months after PT) | 22.18 (1 year and 8 months after PT) | 24 | C |
| Mean K ± SD | 0.12 ± 0.11 | 35.79 ± 56.56 |
| Patient No. | Type of Infection | Phages Used in PT | K Before PT | K During PT | K After PT | Days of PT | Clinical Outcome of PT a |
|---|---|---|---|---|---|---|---|
| 1 | soft tissue infection | Staph_1N | 0.06 | 0.32 | n.s | 12 | E |
| 2 | soft tissue infection | Staph_A5L | 0.14 | 0.15 | 0.05 | 14 | E |
| 3 | soft tissue infection | Staph_A5L | 0.47 | 2.59 | n.s. | 14 | B |
| 4 | soft tissue infection | Staph_1N | 0.08 | 0.10 | n.s. | 14 | B |
| 5 | upper respiratory tract infection | Staph_A5L | 0.03 | 0.72 | n.s. | 14 | C |
| 6 | soft tissue infection | Staph_1N | 0.18 | 0.12 | n.s. | 17 | B |
| 7 | upper respiratory tract infection | Staph_1N | 0.02 | 1.42 | n.s. | 17 | E |
| 8 | soft tissue infection | Staph_1N | 0.02 | 0.27 | 0.01 | 18 | A |
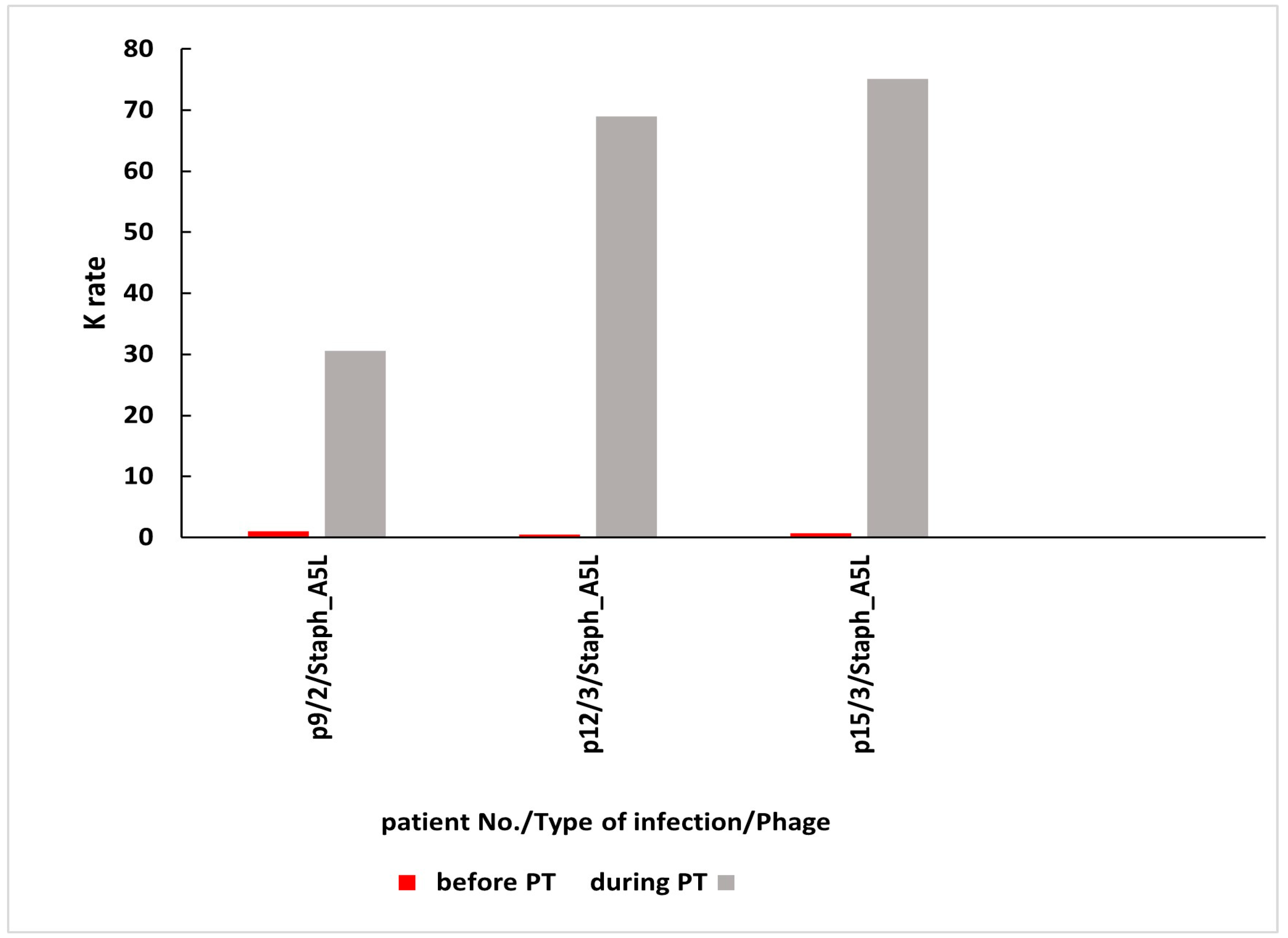
| 9 | bone infection | Staph_A5L | 0.52 | 30.56 | n.s. | 21 | C |
| 10 | bone infection | Staph_A5L | 0.03 | 0.34 | n.s. | 24 | D |
| 11 | soft tissue infection | Staph_A5L | 0.26 | 2.62 | n.s. | 28 | C |
| 12 | upper respiratory tract infection | Staph_A5L | 0.02 | 68.94 | 28.04 | 30 | E |
| 13 | upper respiratory tract infection | Staph_1N | 0.34 | 0.53 | n.s. | 47 | F |
| 14 | soft tissue infection | Staph_1N | 0.46 | 0.58 | n.s. | 55 | F |
| 15 | upper respiratory tract infection | Staph_A5L | 0.11 | 75.12 | n.s. | 56 | E |
| 16 | bone infection | Staph_1N | 0.14 | 2.86 | 71.79 (1 month after PT) | 35 | D |
| Mean K ± SD | 0.18 ± 0.17 | 11.70 ± 23.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Żaczek, M.; Międzybrodzki, R.; Górski, A. The Appearance of Antiphage Antibodies in Sera of Patients Treated with Phages. Antibiotics 2025, 14, 87. https://doi.org/10.3390/antibiotics14010087
Łusiak-Szelachowska M, Weber-Dąbrowska B, Żaczek M, Międzybrodzki R, Górski A. The Appearance of Antiphage Antibodies in Sera of Patients Treated with Phages. Antibiotics. 2025; 14(1):87. https://doi.org/10.3390/antibiotics14010087
Chicago/Turabian StyleŁusiak-Szelachowska, Marzanna, Beata Weber-Dąbrowska, Maciej Żaczek, Ryszard Międzybrodzki, and Andrzej Górski. 2025. "The Appearance of Antiphage Antibodies in Sera of Patients Treated with Phages" Antibiotics 14, no. 1: 87. https://doi.org/10.3390/antibiotics14010087
APA StyleŁusiak-Szelachowska, M., Weber-Dąbrowska, B., Żaczek, M., Międzybrodzki, R., & Górski, A. (2025). The Appearance of Antiphage Antibodies in Sera of Patients Treated with Phages. Antibiotics, 14(1), 87. https://doi.org/10.3390/antibiotics14010087






