From Guidelines to Action: Tackling Risk Factors for Surgical Site Infections
Abstract
1. Introduction
2. Results
3. Discussion
Limitations of This Study
4. Materials and Methods
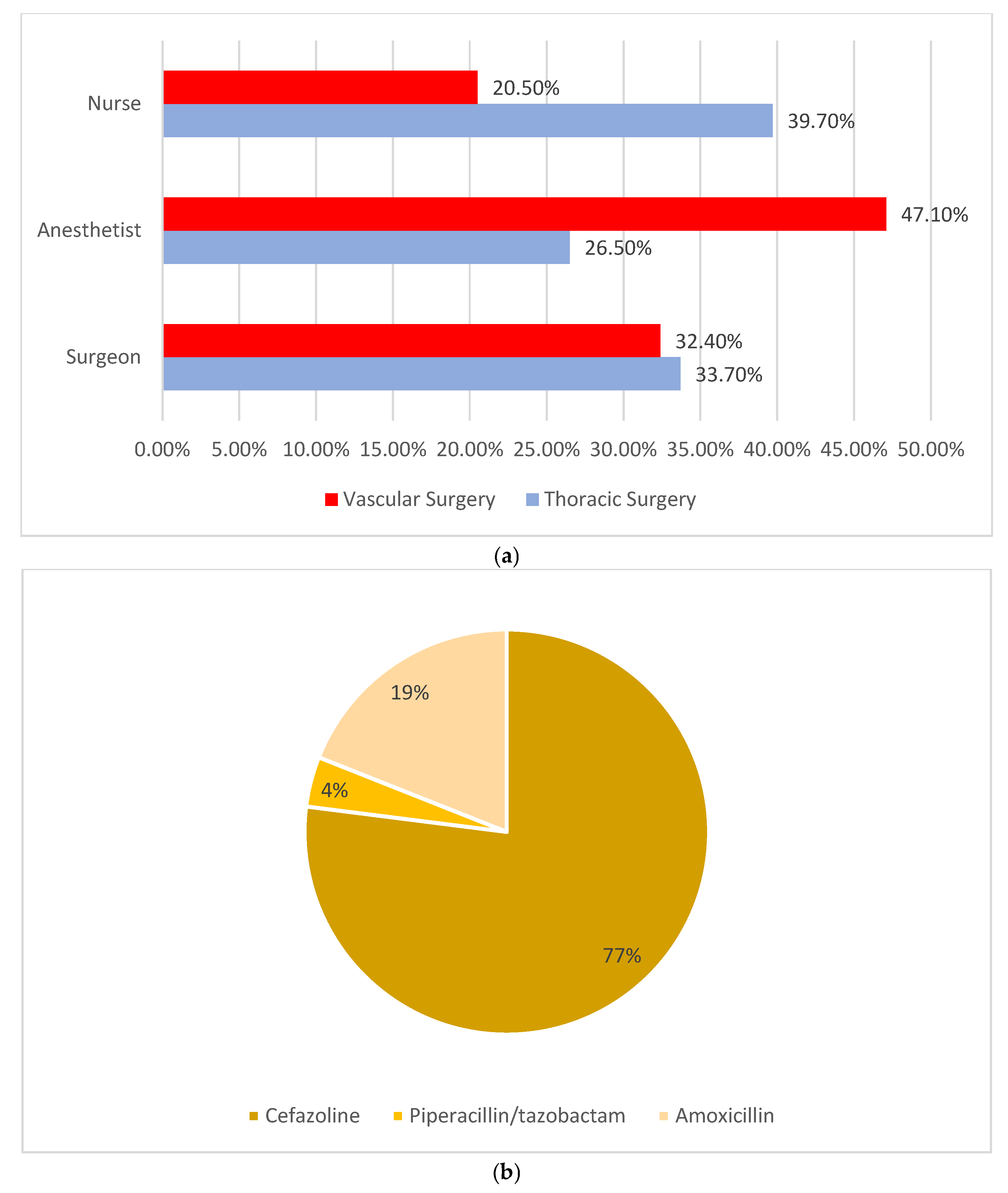
- (a)
- Socio-demographic data (age, gender and BMI);
- (b)
- Risk factors (smoking, diabetes and immunosuppressive therapy);
- (c)
- Clinical and surgical data, including ongoing infections, ICD9-CM codes, whether the surgery was urgent or elective, the use of video-endoscopic techniques, surgery classification, duration, the ASA Physical Status Classification System score and the use of blood transfusion or derivatives;
- (d)
- Details regarding the administration of antibiotics, including the person responsible for their administration, type of antibiotic and time of administration, whether before, during or after the surgical procedure.
- (1)
- patients undergoing thoracic or vascular surgery at the hospital during the study period
- (2)
- who provided informed consent to participate and
- (3)
- were aged 18 or older.
- (1)
- patients under 18
- (2)
- who refused to participate in the study
- (3)
- with severe cognitive impairments or language barriers that would hinder survey completion and
- (4)
- with no available preoperative antibiotic administration data.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO Recommendations on Intraoperative and Postoperative Measures for Surgical Site Infection Prevention: An Evidence-Based Global Perspective. Lancet Infect. Dis. 2016, 16, e288–e303. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Podgorny, K.; Berríos-Torres, S.I.; Bratzler, D.W.; Dellinger, E.P.; Greene, L.; Nyquist, A.-C.; Saiman, L.; Yokoe, D.S.; Maragakis, L.L.; et al. Strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2014 Update. Infect. Control Hosp. Epidemiol. 2014, 35, 605–627. [Google Scholar] [CrossRef] [PubMed]
- Young, P.Y.; Khadaroo, R.G. Surgical Site Infections. Surg. Clin. N. Am. 2014, 94, 1245–1264. [Google Scholar] [CrossRef]
- Marchi, M.; Pan, A.; Gagliotti, C.; Morsillo, F.; Parenti, M.; Resi, D.; Moro, M.L. Sorveglianza Nazionale Infezioni in Chirurgia (SNICh) Study Group The Italian National Surgical Site Infection Surveillance Programme and Its Positive Impact, 2009 to 2011. Eurosurveillance 2014, 19, 20815. [Google Scholar] [CrossRef]
- Guest, J.F.; Fuller, G.W.; Griffiths, B. Cohort Study to Characterise Surgical Site Infections after Open Surgery in the UK’s National Health Service. BMJ Open 2023, 13, e076735. [Google Scholar] [CrossRef]
- Akhter, M.S.J.; Verma, R.; Madhukar, K.P.; Vaishampayan, A.R.; Unadkat, P.C. Incidence of Surgical Site Infection in Postoperative Patients at a Tertiary Care Centre in India. J. Wound Care 2016, 25, 210–217. [Google Scholar] [CrossRef]
- Giufrè, M.; Mazzolini, E.; Cerquetti, M.; Brusaferro, S.; Accogli, M.; Agnoletti, F.; Agodi, A.; Alborali, G.L.; Arghittu, M.; Auxilia, F.; et al. Extended-Spectrum β-Lactamase-Producing Escherichia Coli from Extraintestinal Infections in Humans and from Food-Producing Animals in Italy: A “One Health” Study. Int. J. Antimicrob. Agents 2021, 58, 106433. [Google Scholar] [CrossRef]
- Bartolek Hamp, D.; Cavrić, G.; Prkačin, I.; Houra, K.; Houra, K.; Perović, D.; Ljubičić, T.; Elezović, A. Device-Associated Healthcare Infection and Sepsis in Intensive Care Unit. Acta Med. Croat. 2015, 69, 203–209. [Google Scholar]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784. [Google Scholar] [CrossRef]
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.L.; Apisarnthanarak, A.; Abbas, A.; Morikane, K.; Lee, K.Y.; Warrier, A.; Yamada, K. APSIC Guidelines for the Prevention of Surgical Site Infections. Antimicrob. Resist. Infect. Control 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.-Y.; Gan, H.-Q.; Zhou, J.-F.; Gong, Y.-J.; Li, L.-Y.; Zhang, X.-Q.; Meng, Y.; Chen, J.-R.; Liu, W.-J.; Ye, L.; et al. Incidence of and Risk Factors for Surgical Site Infection after Colorectal Surgery: A Multiple-Center Prospective Study of 3,663 Consecutive Patients in China. Int. J. Infect. Dis. 2020, 96, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Tholany, J.; Kobayashi, T.; Marra, A.R.; Schweizer, M.L.; Samuelson, R.J.; Suzuki, H. Impact of Infectious Diseases Consultation on the Outcome of Patients with Enterococcal Bacteremia: A Systematic Literature Review and Meta-Analysis. Open Forum. Infect. Dis. 2022, 9, ofac200. [Google Scholar] [CrossRef]
- Jung, H.D.; Cho, K.S.; Moon, Y.J.; Chung, D.Y.; Kang, D.H.; Lee, J.Y. Antibiotic Prophylaxis for Percutaneous Nephrolithotomy: An Updated Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0267233. [Google Scholar] [CrossRef]
- O’Hara, L.M.; Thom, K.A.; Preas, M.A. Update to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee Guideline for the Prevention of Surgical Site Infection, A Summary, Review, and Strategies for Implementation. Am. J. Infect. Control 2018, 46, 602–609. [Google Scholar] [CrossRef]
- Albano, G.D.; Rifiorito, A.; Malta, G.; Sorrentino, E.S.; Falco, V.; Firenze, A.; Argo, A.; Zerbo, S. The Impact on Healthcare Workers of Italian Law n. 24/2017 “Gelli-Bianco” on Patient Safety and Medical Liability: A National Survey. Int. J. Environ. Res. Public Health 2022, 19, 8448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Health Institute. Surveillance of Surgical Site Infections. Available online: https://www.epicentro.iss.it/sorveglianza-ica/sorveglianza-infezioni-sito-chirurgico (accessed on 30 August 2024).
- Langelotz, C.; Mueller-Rau, C.; Terziyski, S.; Rau, B.; Krannich, A.; Gastmeier, P.; Geffers, C. Gender-Specific Differences in Surgical Site Infections: An Analysis of 438,050 Surgical Procedures from the German National Nosocomial Infections Surveillance System. Viszeralmedizin 2014, 30, 114–117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aghdassi, S.J.S.; Schröder, C.; Gastmeier, P. Gender-related risk factors for surgical site infections. Results from 10 years of surveillance in Germany. Antimicrob. Resist. Infect. Control 2019, 3, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gachabayov, M.; Senagore, A.J.; Abbas, S.K.; Yelika, S.B.; You, K.; Bergamaschi, R. Perioperative hyperglycemia: An unmet need within a surgical site infection bundle. Tech. Coloproctol. 2018, 22, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Lubelski, D.; Westbroek, E.M.; Ahmed, A.K.; Passias, P.G.; Sciubba, D.M. Persistent Postoperative Hyperglycemia as a Risk Factor for Operative Treatment of Deep Wound Infection After Spine Surgery. Neurosurgery 2020, 87, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.T.; Kaye, K.S.; Knott, C.; Nguyen, H.; Santarossa, M.; Evans, R.; Bertran, E.; Jaber, L. Diabetes and Risk of Surgical Site Infection: A Systematic Review and Meta-analysis. Infect. Control Hosp. Epidemiol. 2016, 7, 88–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ranson, W.A.; White, S.J.W.; Cheung, Z.B.; Mikhail, C.; Ye, I.; Kim, J.S.; Cho, S.K. The Effects of Chronic Preoperative Steroid Therapy on Perioperative Complications Following Elective Posterior Lumbar Fusion. Glob. Spine J. 2018, 8, 834–841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olsen, M.A.; Ball, K.E.; Nickel, K.B.; Wallace, A.E.; Fraser, V.J. Validation of ICD-9-CM Diagnosis Codes for Surgical Site Infection and Noninfectious Wound Complications After Mastectomy. Infect. Control Hosp. Epidemiol. 2017, 38, 334–339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onyekwelu, I.; Yakkanti, R.; Protzer, L.; Pinkston, C.M.; Tucker, C.; Seligson, D. Surgical Wound Classification and Surgical Site Infections in the Orthopaedic Patient. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2017, 3, e022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, A.P. Surveillance of Antibiotic Resistance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140080. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Machairas, N.; Tsourouflis, G.; Chouliaras, C.; Manioti, E.; Broutas, D.; Kykalos, S.; Daikos, G.L.; Samarkos, M.; Vagianos, C. Risk Factors for Surgical Site Infections in Patients Undergoing Emergency Surgery: A Single-centre Experience. In Vivo 2021, 35, 3569–3574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinchera, B.; Buonomo, A.R.; Schiano Moriello, N.; Scotto, R.; Villari, R.; Gentile, I. Update on the Management of Surgical Site Infections. Antibiotics 2022, 11, 1608. [Google Scholar] [CrossRef]
- Sartelli, M.; Boermeester, M.A.; Cainzos, M.; Coccolini, F.; de Jonge, S.W.; Rasa, K.; Dellinger, E.P.; McNamara, D.A.; Fry, D.E.; Cui, Y.; et al. Six Long-Standing Questions about Antibiotic Prophylaxis in Surgery. Antibiotics 2023, 12, 908. [Google Scholar] [CrossRef]
- Taheriazam, A.; Saeidinia, A. Two-Stage Revision of Infected Hip Prosthesis after Post-Operative Antibiotic Therapy: An Observational Study. Medicine 2023, 102, e32878. [Google Scholar] [CrossRef]
- Salminen, P.; Paajanen, H.; Rautio, T.; Nordström, P.; Aarnio, M.; Rantanen, T.; Tuominen, R.; Hurme, S.; Virtanen, J.; Mecklin, J.-P.; et al. Antibiotic Therapy vs Appendectomy for Treatment of Uncomplicated Acute Appendicitis. JAMA 2015, 313, 2340. [Google Scholar] [CrossRef]
- Crader, M.F.; Varacallo, M. Preoperative Antibiotic Prophylaxis. [Updated 4 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK442032/ (accessed on 30 August 2024).
- Misha, G.; Chelkeba, L.; Melaku, T. Incidence, risk factors and outcomes of surgical site infections among patients admitted to Jimma Medical Center, South West Ethiopia: Prospective cohort study. Ann. Med. Surg. 2021, 65, 102247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Z.; Zhou, K.; Tang, K.; Quan, Z.; Liu, S.; Su, B. Perioperative Hypoalbuminemia Is a Risk Factor for Wound Complications Following Posterior Lumbar Interbody Fusion. J. Orthop. Surg. Res. 2020, 15, 538. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Collinsworth, A.; Hasa, F.; Griffin, L. Incidence and Impact of Surgical Site Infections on Length of Stay and Cost of Care for Patients Undergoing Open Procedures. Surg. Open Sci. 2022, 11, 1–18. [Google Scholar] [CrossRef]
- Atesok, K.; Papavassiliou, E.; Heffernan, M.J.; Tunmire, D.; Sitnikov, I.; Tanaka, N.; Rajaram, S.; Pittman, J.; Gokaslan, Z.L.; Vaccaro, A.; et al. Current Strategies in Prevention of Postoperative Infections in Spine Surgery. Glob. Spine J. 2020, 10, 183–194. [Google Scholar] [CrossRef]
- Piednoir, E.; Robert-Yap, J.; Baillet, P.; Lermite, E.; Christou, N. The Socioeconomic Impact of Surgical Site Infections. Front. Public. Health 2021, 9, 712461. [Google Scholar] [CrossRef]
- Iskandar, K.; Sartelli, M.; Tabbal, M.; Ansaloni, L.; Baiocchi, G.L.; Catena, F.; Coccolini, F.; Haque, M.; Labricciosa, F.M.; Moghabghab, A.; et al. Highlighting the Gaps in Quantifying the Economic Burden of Surgical Site Infections Associated with Antimicrobial-Resistant Bacteria. World J. Emerg. Surg. 2019, 14, 50. [Google Scholar] [CrossRef]
- Perencevich, E.N.; Sands, K.E.; Cosgrove, S.E.; Guadagnoli, E.; Meara, E.; Platt, R. Health and Economic Impact of Surgical Site Infections Diagnosed after Hospital Discharge. Emerg. Infect. Dis. 2003, 9, 196–203. [Google Scholar] [CrossRef]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of Surgical Site Infection on Healthcare Costs and Patient Outcomes: A Systematic Review in Six European Countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef]
- Smith, D.; Dushoff, J.; Perencevich, E.N.; Harris, A.; Levin, S.A. Persistent Colonization and the Spread of Antibiotic Resistance in Nosocomial Pathogens: Resistance Is a Regional Problem. Proc. Natl. Acad. Sci. USA 2004, 101, 3709–3714. [Google Scholar] [CrossRef]
- Marrone, M.; Caricato, P.; Mele, F.; Leonardelli, M.; Duma, S.; Gorini, E.; Stellacci, A.; Bavaro, D.F.; Diella, L.; Saracino, A.; et al. Analysis of Italian Requests for Compensation in Cases of Responsibility for Healthcare-Related Infections: A Retrospective Study. Front. Public. Health 2023, 10, 1078719. [Google Scholar] [CrossRef]
- EpiCentro. Piano Nazionale Di Contrasto All’Antibiotico-Resistenza (PNCAR) 2022–2025. Available online: https://www.epicentro.iss.it/antibiotico-resistenza/pncar-2022 (accessed on 12 August 2024).
- Silvestri, M.; Dobrinja, C.; Scomersi, S.; Giudici, F.; Turoldo, A.; Princic, E.; Luzzati, R.; de Manzini, N.; Bortul, M. Modifiable and Non-Modifiable Risk Factors for Surgical Site Infection after Colorectal Surgery: A Single-Center Experience. Surg. Today 2017, 48, 338–345. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 12 August 2024).
- Lopuzzo, M.; Montagna, M.T.; Triggiano, F.; Caggiano, G. Effectiveness of hydrogen peroxide wipes for surface disinfection in healthcare facilities. Ann. Ig. 2024, 36, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qiao, Y.; Jin, R.; Jia, M.; Liu, J.; He, Z.; Liu, Z. Application of chlorine dioxide and its disinfection mechanism. Arch. Microbiol. 2024, 206, 400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, W.; Deng, X.; Bo, L. High-dose ascorbic acid potentiates immune modulation through STAT1 phosphorylation inhibition and negative regulation of PD-L1 in experimental sepsis. Inflammopharmacology 2024, 32, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Klenner, F.R. Observations on the dose and administration of ascorbic acid when employed beyond the range of a vitamin in human pathology. J. Appl. Nutr. 1971, 23, 60–89. [Google Scholar]
- Klenner, F.R. Significance of high daily intake of ascorbic acid in preventive medicine. J. Int. Acad. Prev. Med. 1974, 1, 45–69. [Google Scholar]
- Johnson, A.P.; Woodford, N. Global Spread of Antibiotic Resistance: The Example of New Delhi Metallo-β-Lactamase (NDM)-Mediated Carbapenem Resistance. J. Med. Microbiol. 2013, 62, 499–513. [Google Scholar] [CrossRef]
- Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goossens, H. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) Point Prevalence Survey: Developing Hospital-Quality Indicators of Antibiotic Prescribing for Children. J. Antimicrob. Chemother. 2016, 71, 1106–1117. [Google Scholar] [CrossRef]
| n (117) | % | |
|---|---|---|
| Gender | ||
| Male | 85 | 72.6 |
| Female | 32 | 27.4 |
| Body mass index | ||
| <17 | 4 | 3 |
| 18–24 | 35 | 29.9 |
| 25–29 | 44 | 37.6 |
| >30 | 15 | 12.8 |
| Unknown | 19 | 16.7 |
| Smoking habit | ||
| Smokers | 39 | 33.3 |
| Non-smokers (including ex-smokers) | 66 | 56.4 |
| Unknown | 27 | 10.3 |
| Diabetes | ||
| Yes | 26 | 22.2 |
| No | 91 | 77.8 |
| Immunosuppressive therapy | ||
| Yes | 11 | 9.4 |
| No | 106 | 90.6 |
| n (117) | % | |
|---|---|---|
| Clinical infection at admission | ||
| Yes | 8 | 6.8 |
| No | 109 | 93.2 |
| Type of operation (NAS-NCR) | ||
| Clean | 18 | 15.4 |
| Clean–contaminated | 97 | 82.9 |
| Contaminated | 2 | 1.7 |
| Procedure type | ||
| Elective | 88 | 75.2 |
| Emergency | 28 | 23.9 |
| Unknown | 1 | 0.9 |
| Use of blood transfusion or derivatives | ||
| Yes | 5 | 4.3 |
| No | 101 | 86.3 |
| Unknown | 11 | 9.4 |
| Prosthesis implants | ||
| Yes | 8 | 6.8 |
| No | 102 | 87.2 |
| Unknown | 7 | 6.0 |
| Video endoscopy | ||
| Yes | 53 | 45.3 |
| No | 63 | 53.8 |
| Unknown | 1 | 0.9 |
| ASA score | ||
| 1 | 4 | 3.4 |
| 2 | 31 | 26.5 |
| ≥3 | 82 | 70.1 |
| Variable | Antibiotic Administration Before Surgery | p-Value | Antibiotic Administration During Surgery | p-Value | Antibiotic Administration After Surgery | p-Value |
|---|---|---|---|---|---|---|
| Total % (n) | 40.2% (47) | 2.6% (3) | 43.6% (51) | |||
| Gender | ||||||
| Male | 68.1% (32) | 0.662 | 66.7% (2) | 0.851 | 68.6% (35) | 0.391 |
| Female | 31.9% (15) | 33.3% (1) | 31.4% (16) | |||
| Diabetes | ||||||
| Yes | 21.3% (10) | 0.740 | 33.3% (1) | 0.326 | 17.6% (9) | 0.295 |
| No | 78.7% (37) | 66.7% (2) | 82.4% (42) | |||
| Immunosuppressive therapy | ||||||
| Yes | 12.8% (6) | 0.510 | 0% (0) | 0.356 | 19.6% (10) | 0.001 |
| No | 87.2% (41) | 100% (3) | 80.4% (41) | |||
| ICD9 CM Code | ||||||
| Fiberoptic endoscopy | 21.3% (10) | 0.049 | 33.3% (1) | 0.733 | 21.6% (11) | 0.076 |
| Drainage | 27.7% (13) | 33.3% (1) | 33.3% (17) | |||
| Angioplasty | 36.2% (17) | 33.3% (1) | 27.5% (14) | |||
| Lung resections | 10.6% (5) | 0% (0) | 9.8% (5) | |||
| Biopsies | 4.3% (2) | 0% (0) | 7.8% (4) | |||
| Type of surgical procedure | ||||||
| Emergency | 34% (16) | 0.001 | 33.3% (1) | 0.005 | 19.6% (10) | 0.346 |
| Elective | 63.8% (30) | 66.7% (2) | 78.4% (40) | |||
| Unknown | 2.1% (1) | 0% (0) | 2% (1) | |||
| Classification of surgical procedure | ||||||
| Clean | 12.8% (6) | 0.280 | 0% (0) | 0.928 | 9.8% (5) | 0.337 |
| Clean–contaminated | 87.2% (41) | 100% (3) | 88.2% (45) | |||
| Contaminated | 0% (0) | 0% (0) | 2% (1) | |||
| Prosthesis implant | ||||||
| Yes | 17% (8) | 0.001 | 0% (0) | 0.418 | 3.9% (2) | 0.364 |
| No | 80.9% (38) | 100% (3) | 92.2% (47) | |||
| Unknown | 2.1% (1) | 0% (0) | 3.9% (2) | |||
| Blood transfusion | ||||||
| Yes | 8.5% (4) | 0.001 | 0% (0) | 0.001 | 7.8% (4) | 0.060 |
| No | 87.2% (41) | 100% (3) | 88.2% (45) | |||
| Unknown | 4.3% (2) | 0% (0) | 3.9% (2) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, C.E.; Venuto, R.; Tripodi, P.; Bartucciotto, L.; Ventura Spagnolo, E.; Nirta, A.; Genovese, G.; La Spina, I.; Sortino, S.; Nicita, A.; et al. From Guidelines to Action: Tackling Risk Factors for Surgical Site Infections. Antibiotics 2025, 14, 40. https://doi.org/10.3390/antibiotics14010040
Rizzo CE, Venuto R, Tripodi P, Bartucciotto L, Ventura Spagnolo E, Nirta A, Genovese G, La Spina I, Sortino S, Nicita A, et al. From Guidelines to Action: Tackling Risk Factors for Surgical Site Infections. Antibiotics. 2025; 14(1):40. https://doi.org/10.3390/antibiotics14010040
Chicago/Turabian StyleRizzo, Caterina Elisabetta, Roberto Venuto, Paola Tripodi, Linda Bartucciotto, Elvira Ventura Spagnolo, Antonio Nirta, Giovanni Genovese, Isabella La Spina, Sabrina Sortino, Alessandro Nicita, and et al. 2025. "From Guidelines to Action: Tackling Risk Factors for Surgical Site Infections" Antibiotics 14, no. 1: 40. https://doi.org/10.3390/antibiotics14010040
APA StyleRizzo, C. E., Venuto, R., Tripodi, P., Bartucciotto, L., Ventura Spagnolo, E., Nirta, A., Genovese, G., La Spina, I., Sortino, S., Nicita, A., Loddo, F., Romeo, B., Squeri, R., & Genovese, C. (2025). From Guidelines to Action: Tackling Risk Factors for Surgical Site Infections. Antibiotics, 14(1), 40. https://doi.org/10.3390/antibiotics14010040








