Nuclear Magnetic Resonance Fingerprinting and Principal Component Analysis Strategies Lead to Anti-Tuberculosis Natural Product Discovery from Actinomycetes
Abstract
1. Introduction
2. Results
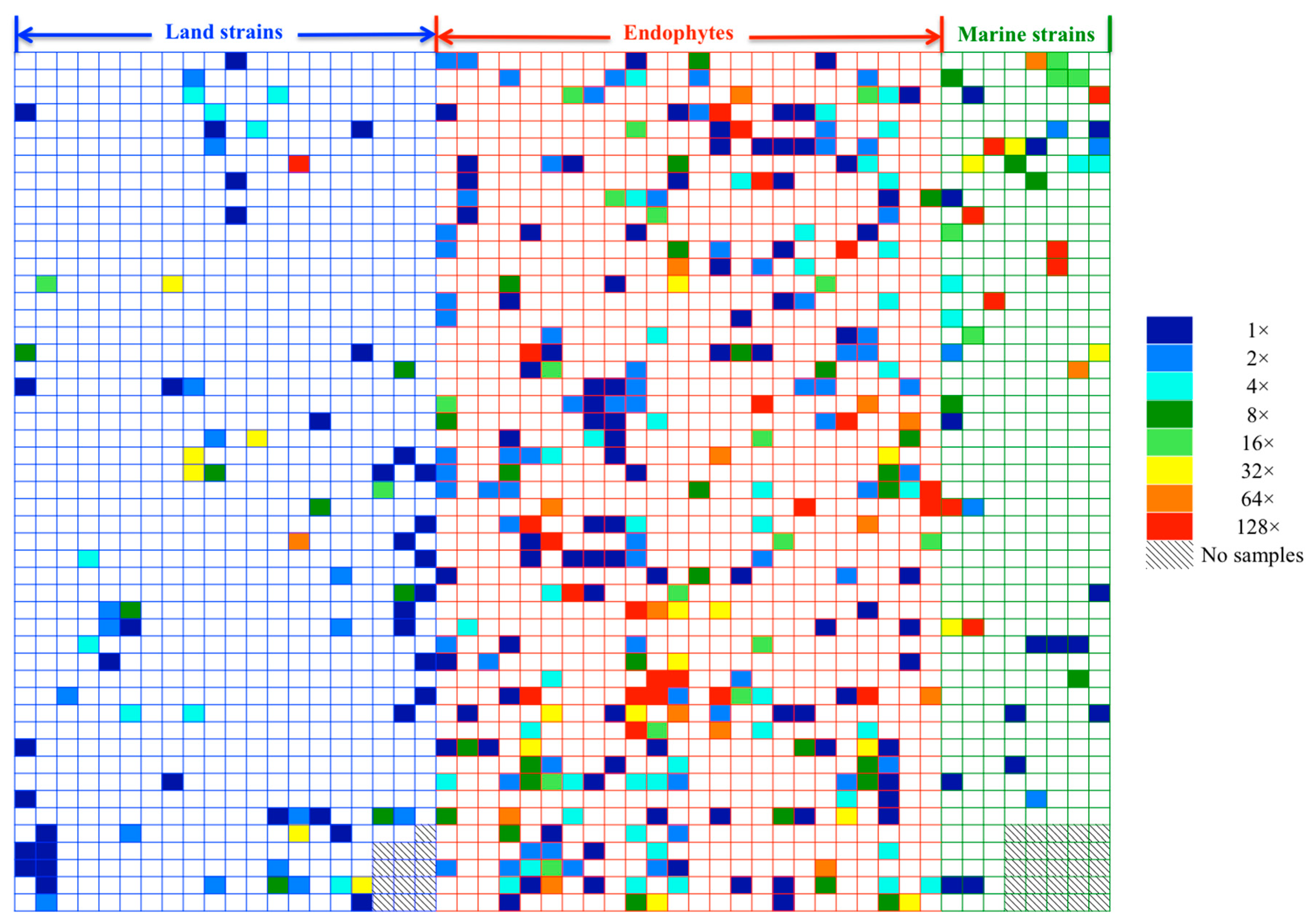
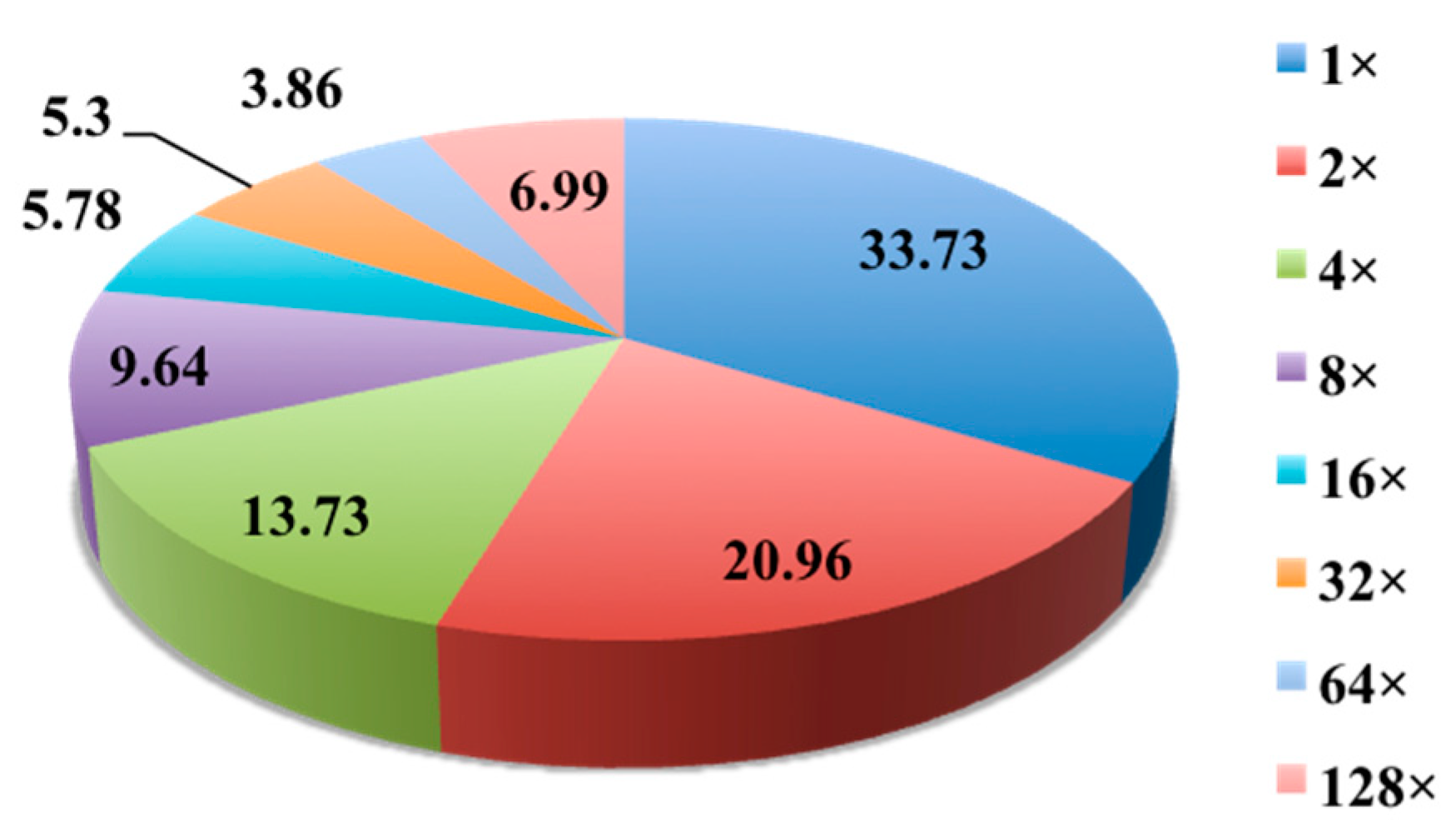
2.1. Evaluation of Anti-Mycobacterial Activity of 654 Actinomycete Cultures in Six Media
2.2. Chemical and Biological Investigation of Five Selected Strains Cultured in 32 Conditions
2.2.1. Platform for Discovery of Unique Bioactive Secondary Metabolites
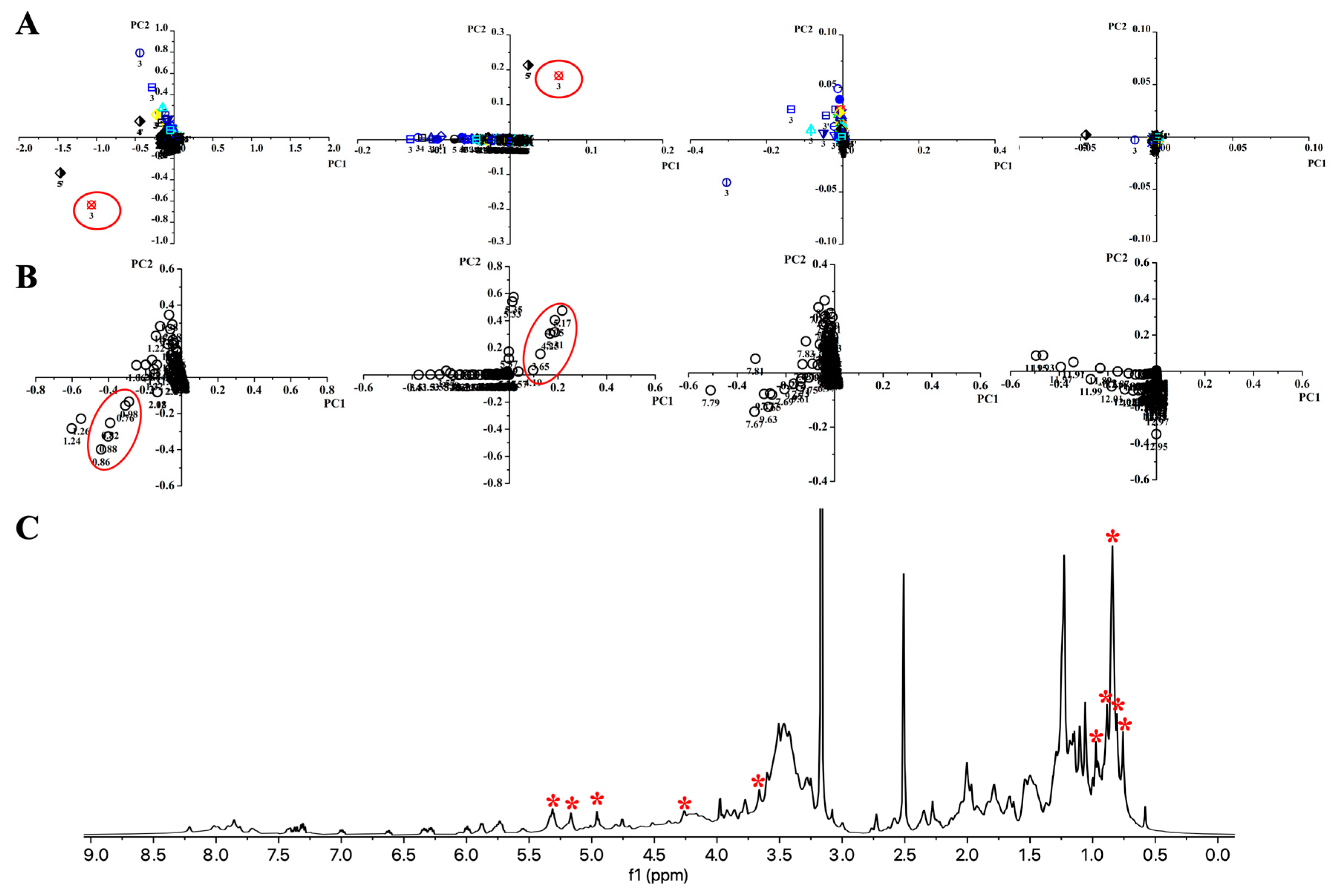
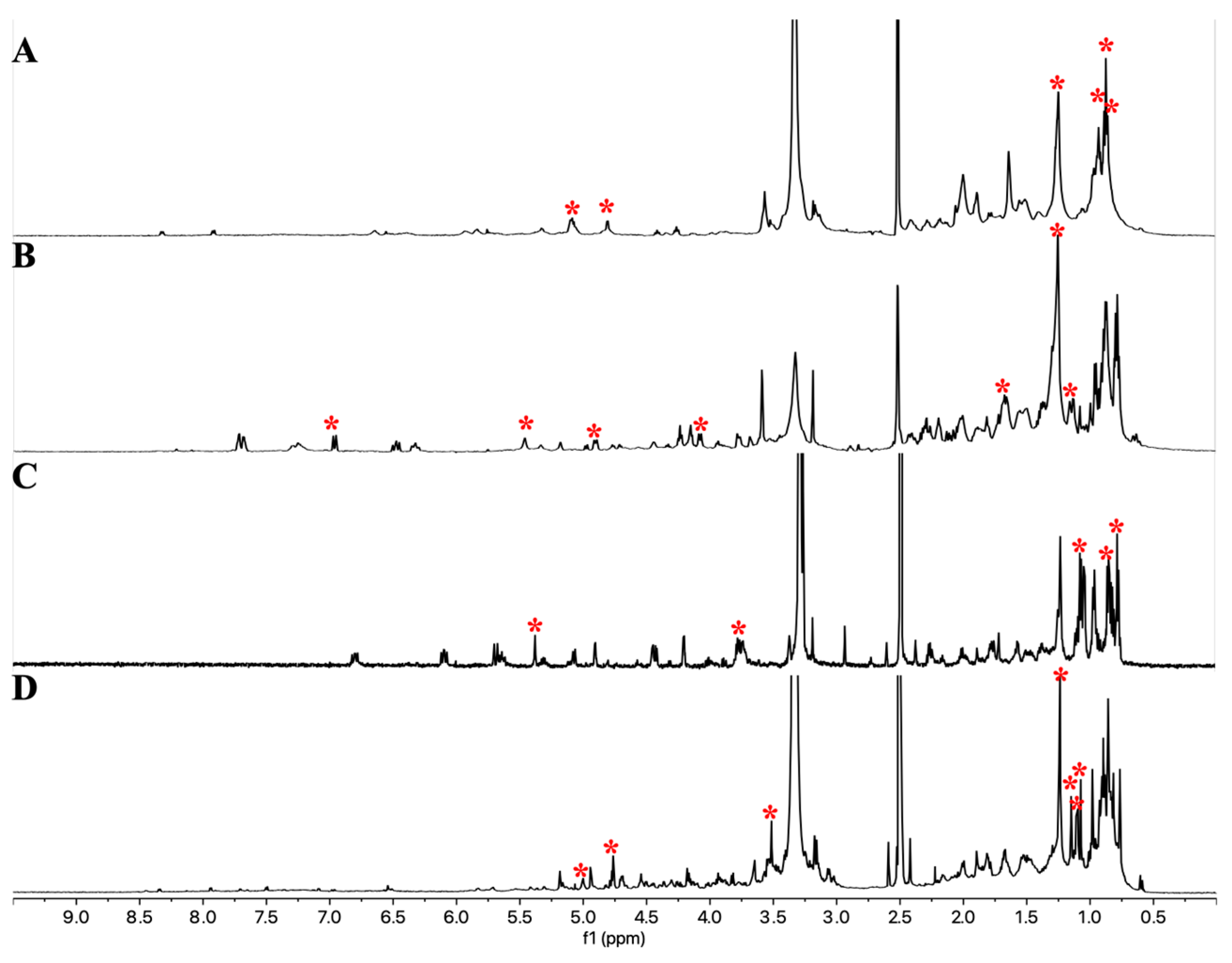
2.2.2. PCA Analysis to Highlight the Metabolites Corresponding to the Bioactivity
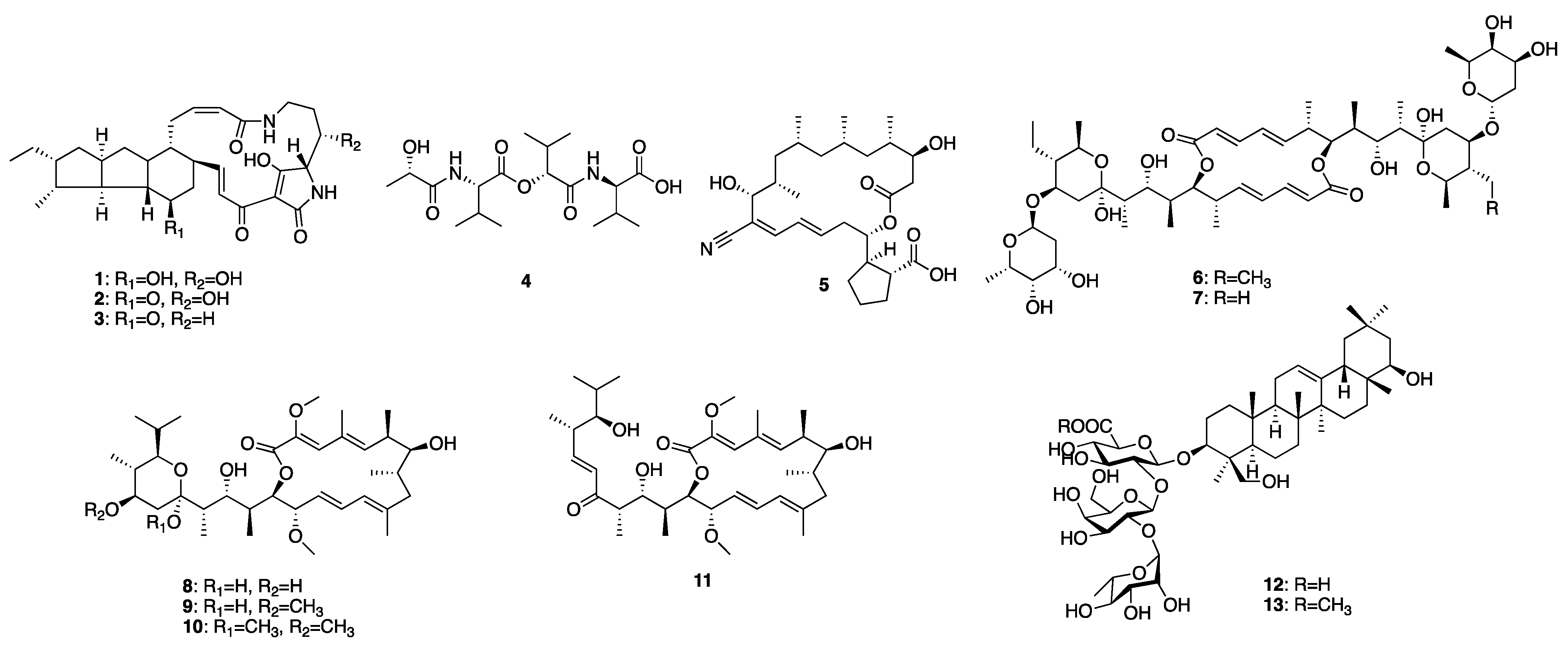
2.2.3. Purification and Identification of Newly-Generated Secondary Metabolites from the Selected Four Microbes
2.2.4. Antimicrobial Activity Evaluation
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Strain Identification
4.3. Fermentation and Extraction
4.4. Media
4.5. HPLC Fractionation Procedure
4.6. NMR Fingerprinting
4.7. NMR Data Processing and Multivariate Analysis
4.8. Anti-BCG Assay
4.9. Anti-Mtb Assay
4.10. Antimicrobial Assay
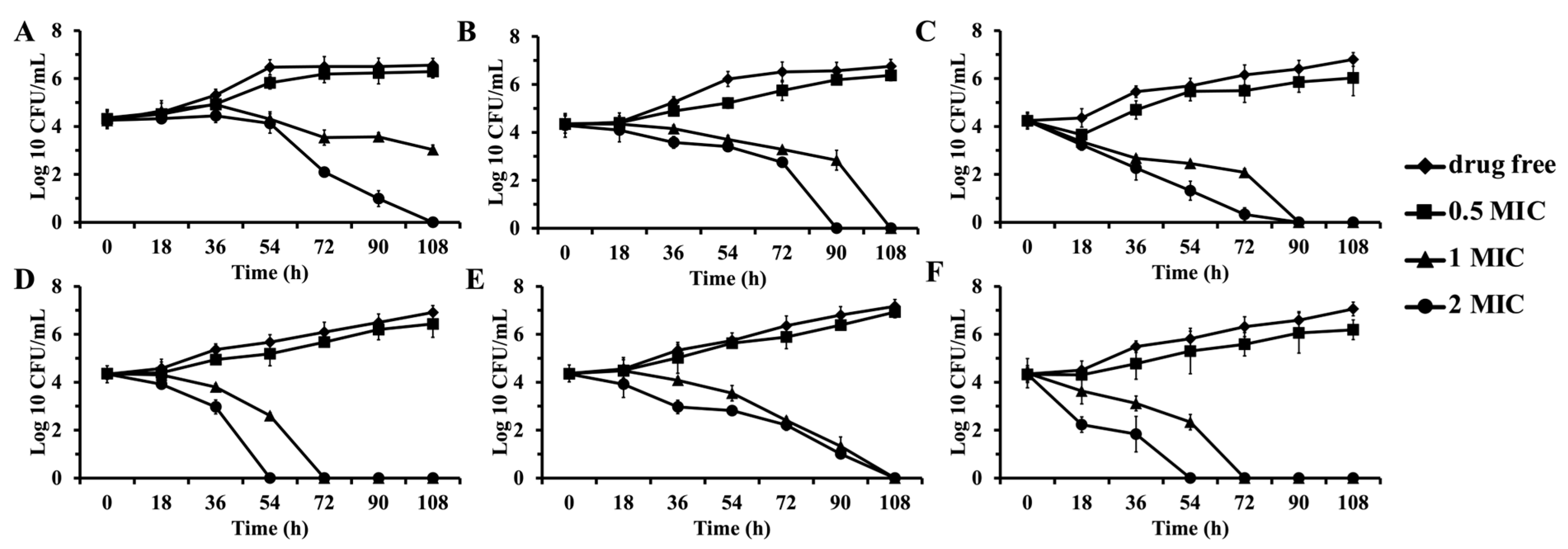
4.11. Time-Kill Assay
4.12. Compounds 1–11
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024; pp. 1–50. [Google Scholar]
- Schraufnagel, D.E. Tuberculosis treatment for the beginning of the next century. Int. J. Tuberc. Lung Dis. 1999, 3, 651–662. [Google Scholar] [PubMed]
- Brigden, G.; Hewison, C.; Varaine, F. New developments in the treatment of drug-resistant tuberculosis: Clinical utility of bedaquiline and delamanid. Infect. Drug Resist. 2015, 8, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Quan, D.; Nagalingam, G.; Payne, R.; Triccas, J.A. New tuberculosis drug leads from naturally occurring compounds. Int. J. Infect. Dis. 2017, 56, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Liu, X.T.; Zhang, L.X.; Quinn, R.J.; Feng, Y.J. Anti-mycobacterial natural products and mechanisms of action. Nat. Prod. Rep. 2022, 39, 77–89. [Google Scholar] [CrossRef]
- Sayed, A.M.; Hassan, M.H.A.; Alhadrami, H.A.; Hassan, H.M.; Goodfellow, M.; Rateb, M.E. Extreme environments: Microbiology leading to specialized metabolites. J. Appl. Microbiol. 2020, 128, 630–657. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J. Amphidinolides and its related macrolides from marine dinoflagellates. J. Antibiot. 2008, 61, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef]
- Liu, M.M.; Grkovic, T.; Zhang, L.X.; Liu, X.T.; Quinn, R.J. A model to predict anti-tuberculosis activity: Value proposition for marine microorganisms. J. Antibiot. 2016, 69, 594–599. [Google Scholar] [CrossRef]
- Alvin, A.; Miller, K.I.; Neilan, B.A. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol. Res. 2014, 169, 483–495. [Google Scholar] [CrossRef]
- Xie, F.Y.; Pathom-aree, W. Actinobacteria from desert: Diversity and biotechnological applications. Front. Microbiol. 2021, 12, 765531. [Google Scholar] [CrossRef]
- Sivakumar, N.; Selvakumar, G. Marine cyanobacteria A prolific source of antimicrobial natural products. In Antimicrobials: Synthetic and Natural Compounds; CRC Press: Boca Raton, FL, USA, 2016; pp. 203–231. [Google Scholar]
- Bode, H.B.; Bethe, B.; Hofs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chembiochem 2002, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Doull, J.L.; Ayer, S.W.; Singh, A.K.; Thibault, P. Production of a novel polyketide antibiotic, jadomycin B, by Streptomyces venezuelae following heat-shock. J. Antibiot. 1993, 46, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Ohnishi-Kameyama, M.; Muramatsu, H.; Murakami, K.; Tsurumi, Y.; Kodani, S.; Yoshida, M.; Fujie, A.; Ochi, K. Antibacterial discovery in actinomycetes strains with mutations in RNA polymerase or ribosomal protein S12. Nat. Biotechnol. 2009, 27, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Recio, E.; Colinas, A.; Rumbero, A.; Aparicio, J.F.; Martín, J.F. PI factor, a novel type quorum-sensing inducer elicits pimaricin production in Streptomyces natalensis. J. Biol. Chem. 2004, 279, 41586–41593. [Google Scholar] [CrossRef]
- Tevyashova, A.N.; Efimova, S.S.; Alexandrov, A.I.; Ghazy, E.S.M.O.; Bychkova, E.N.; Solovieva, S.E.; Zatonsky, G.B.; Grammatikova, N.E.; Dezhenkova, L.G.; Pereverzeva, E.R.; et al. Semisynthetic amides of polyene antibiotic natamycin. ACS Infect. Dis. 2023, 9, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Christian, O.E.; Compton, J.; Christian, K.R.; Mooberry, S.L.; Valeriote, F.A.; Crews, P. Using jasplakinolide to turn on pathways that enable the isolation of new chaetoglobosins from Phomospis asparagi. J. Nat. Prod. 2005, 68, 1592–1597. [Google Scholar] [CrossRef]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef]
- Xue, D.F.; Wang, Q.X.; Chen, Z.H.; Cai, L.; Bao, L.; Qi, Q.Y.; Liu, L.H.; Wang, X.H.; Jin, H.J.; Wang, J.; et al. 3-Anhydro-6-hydroxy-ophiobolin A, a fungal sesterterpene from induced autophagy and promoted the degradation of α-synuclein in PC12 cells. Bioorg. Med. Chem. Lett. 2015, 25, 1464–1470. [Google Scholar] [CrossRef]
- Camp, D.; Davis, R.A.; Campitelli, M.; Ebdon, J.; Quinn, R.J. Drug-like Properties: Guiding principles for the design of natural product libraries. J. Nat. Prod. 2012, 75, 72–81. [Google Scholar] [CrossRef]
- Liu, M.M.; Han, J.Y.; Feng, Y.J.; Guymer, G.; Forster, P.I.; Quinn, R.J. Antimicrobial benzyltetrahydroisoquinoline-derived alkaloids from the leaves of Doryphora aromatica. J. Nat. Prod. 2021, 84, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Grkovic, T.; Liu, X.T.; Han, J.Y.; Zhang, L.X.; Quinn, R.J. A systems approach using OSMAC, Log P and NMR fingerprinting: An approach to novelty. Synth. Syst. Biotechnol. 2017, 2, 276–286. [Google Scholar] [CrossRef]
- Liu, M.M. Actinomycetes Sourced from Unique Environments as a Promising Source of New TB-Active Natural Products. Ph.D. Thesis, Griffith University, Nathan, Australia, 2017. [Google Scholar]
- Kumari, P.; Deepa, N.; Trivedi, P.K.; Singh, B.K.; Srivastava, V.; Singh, A. Plants and endophytes interaction: A “secret wedlock” for sustainable biosynthesis of pharmaceutically important secondary metabolites. Microb. Cell Fact. 2023, 22, 226. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Bolla, K.; Ashforth, E.J.; Zhuo, Y.; Gao, H.; Huang, P.; Stanley, S.A.; Hung, D.T.; Zhang, L.X. Systematics-guided bioprospecting for bioactive microbial natural products. Anton. Leeuw. Int. J. Microbiol. 2012, 101, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Liu, X.T.; Zhang, L.X.; Van Voorhis, W.C.; Quinn, R.J.; Liu, M.M. Rapid discovery of antimicrobial and antimalarial agents from natural product fragments. Separations 2024, 11, 194. [Google Scholar] [CrossRef]
- Quezada, M.; Licona-Cassani, C.; Cruz-Morales, P.; Salim, A.A.; Marcellin, E.; Capon, R.J.; Barona-Gómez, F. Diverse cone-snail species harbor closely related Streptomyces species with conserved chemical and cenetic profiles, including polycyclic tetramic acid macrolactams. Front. Microbiol. 2017, 8, 2305. [Google Scholar] [CrossRef]
- Sun, W.B.; Tang, B.; Dong, L.L.; Xu, J.H.; Zhao, Y.C.; Liu, F.Q. A novel and high-efficient method for the preparation of heat-stable antifungal factor from Lysobacter enzymogenes by high-speed counter-current chromatography. Front. Microbiol. 2023, 14, 1227244. [Google Scholar] [CrossRef]
- Graupner, P.R.; Thornburgh, S.; Mathieson, J.T.; Chapin, E.L.; Kemmitt, G.M.; Brown, J.M.; Snipes, C.E. Dihydromaltophilin; A novel fungicidal tetramic acid containing metabolite from Streptomyces sp. J. Antibiot. 1997, 50, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.X.; Wu, P.; Wright, S.J.; Du, L.C.; Wei, X.Y. Bioactive polycyclic tetramate macrolactams from Lysobacter enzymogenes and their absolute configurations by theoretical ECD calculations. J. Nat. Prod. 2015, 78, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Hashidoko, Y.; Nakayama, T.; Homma, Y.; Tahara, S. Structure elucidation of xanthobaccin A, a new antibiotic produced from Stenotrophomonas sp. strain SB-K88. Tetrahedron Lett. 1999, 40, 2957–2960. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, S.D.; Haidar, A.K.; Bajimaya, M.; Guo, Y.J.; Larsen, T.O.; Gram, L. Polycyclic tetramate macrolactams-a group of natural bioactive metallophores. Front. Chem. 2021, 9, 772858. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, M.; Winkelmann, G.; Kaiser, D.; Kempter, C.; Jung, G.; Berg, G.; Bahl, H. Maltophilin: A new antifungal compound produced by Stenotrophomonas maltophilia R3089. J. Antibiot. 1996, 49, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.G.; Wang, L.P.; Wang, D.Y.; Fan, J.; Zhu, W.M. Polycyclic tetramate macrolactams from the marine-derived Actinoalloteichus cyanogriseus WH1-2216-6. Chin. J. Org. Chem. 2017, 37, 2352–2360. [Google Scholar] [CrossRef]
- Hu, Y.C.; MacMillan, J.B. A new peptide isolated from a marine derived Streptomyces bacillaris. Nat. Prod. Commun. 2012, 7, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.S.; Khodakaramian, G.; Arakawa, K.; Kinashi, H. Isolation of borrelidin as a phytotoxic compound from a potato pathogenic Streptomyces strain. Biosci. Biotechnol. Biochem. 2012, 76, 353–357. [Google Scholar] [CrossRef]
- Gao, X.X.; Jiang, Y.; Han, L.; Chen, X.; Hu, C.J.; Su, H.; Mu, Y.; Guan, P.P.; Huang, X.S. Effect of borrelidin on hepatocellular carcinoma cells in vitro and in vivo. RSC Adv. 2017, 7, 44401–44409. [Google Scholar] [CrossRef]
- Hassan, R.; Shaaban, M.I.; Bar, F.M.A.; El-Mahdy, A.M.; Shokralla, S. Quorum sensing inhibiting activity of Streptomyces coelicoflavus isolated from soil. Front. Microbiol. 2016, 7, 659. [Google Scholar] [CrossRef]
- Kaiser, H.; Keller-Schierlein, W. Stoffwechselprodukte von mikroorganismen. 202. mitteilung. atrukturaufklärung von elaiophylin: Spektroskopische untersuchungen und abbau. Helv. Chim. Acta 1981, 64, 407–424. [Google Scholar] [CrossRef]
- Hammannt, P.; Kretzschmar, G. Secondary metabolites by chemical screening. 12. 13C NMR studies of elaiophylin derivatives. Magn. Reson. Chem. 1991, 29, 667470. [Google Scholar]
- Supong, K.; Thawai, C.; Choowong, W.; Kittiwongwattana, C.; Thanaboripat, D.; Laosinwattana, C.; Koohakan, P.; Parinthawong, N.; Pittayakhajonwut, P. Antimicrobial compounds from endophytic Streptomyces sp. BCC72023 isolated from rice (Oryza sativa L.). Res. Microbiol. 2016, 167, 290–298. [Google Scholar] [CrossRef]
- Scheidt, K.A.; Bannister, T.D.; Tasaka, A.; Wendt, M.D.; Savall, B.M.; Fegley, G.J.; Roush, W.R. Total synthesis of (-)-bafilomycin A. J. Am. Chem. Soc. 2002, 124, 6981–6990. [Google Scholar] [CrossRef]
- Baker, G.H.; Brown, P.J.; Dorgan, R.J.J.; Everett, J.R. The conformational-analysis of bafilomycin-A1. J. Chem. Soc. Perkin Trans. 2 1989, 8, 1073–1079. [Google Scholar] [CrossRef]
- Carr, G.; Williams, D.E.; Díaz-Marrero, A.R.; Patrick, B.O.; Bottriell, H.; Balgi, A.D.; Donohue, E.; Roberge, M.; Andersen, R.J. Bafilomycins produced in culture by Streptomyces spp. isolated from marine habitats are potent inhibitors of autophagy. J. Nat. Prod. 2010, 73, 422–427. [Google Scholar] [CrossRef]
- Axel Kretschmer, M.D.; Deeg, M.; Hagenmaier, H. The structures of novel insecticidal macrolides: Bafilomycins D and E, and oxohygrolidin. Agric. Biol. Chem. 1985, 49, 2509–2511. [Google Scholar]
- Yu, Z.G.; Zhao, L.X.; Jiang, C.L.; Duan, Y.W.; Wong, L.; Carver, K.C.; Schuler, L.A.; Shen, B. Bafilomycins produced by an endophytic actinomycete Streptomyces sp. YIM56209. J. Antibiot. 2011, 64, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Berger, J.; Jampolsky, L.M.; Goldberg, M.W. Borrelidin, a new antibiotic with antiborrelia activity and penicillin enhancement properties. Arch. Biochem. 1949, 22, 476–478. [Google Scholar]
- Fang, P.F.; Yu, X.; Jeong, S.J.; Mirando, A.; Chen, K.; Chen, X.; Kim, S.; Francklyn, C.S.; Guo, M. Structural basis for full-spectrum inhibition of translational functions on a tRNA synthetase. Nat. Commun. 2015, 6, 6402. [Google Scholar] [CrossRef]
- Habibi, D.; Ogloff, N.; Jalili, R.B.; Yost, A.; Weng, A.P.; Ghahary, A.; Ong, C.J. Borrelidin, a small molecule nitrile-containing macrolide inhibitor of threonyl-tRNA synthetase, is a potent inducer of apoptosis in acute lymphoblastic leukemia. Investig. New Drugs 2012, 30, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Otoguro, K.; Ui, H.; Ishiyama, A.; Kobayashi, M.; Togashi, H.; Takahashi, Y.; Masuma, R.; Tanaka, H.; Tomoda, H.; Yamada, H.; et al. In vitro and in vivo antimalarial activities of a non-glycosidic 18-membered macrolide antibiotic, borrelidin, against drug-resistant strains of Plasmodia. J. Antibiot. 2003, 56, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, B.; Gregory, M.A.; Moss, S.J.; Carletti, I.; Sheridan, R.M.; Kaja, A.; Ward, M.; Olano, C.; Mendez, C.; Salas, J.A.; et al. Separation of anti-angiogenic and cytotoxic activities of borrelidin by modification at the C17 side chain. Bioorg. Med. Chem. Lett. 2006, 16, 5814–5817. [Google Scholar] [CrossRef] [PubMed]
- Lumb, M.; Macey, P.E.; Spyvee, J.; Whitmarsh, J.M.; Wright, R.D. Isolation of vivomycin and borrelidin, two antibiotics with anti-viral activity, from a species of Streptomyces (C2989). Nature 1965, 206, 263–265. [Google Scholar] [CrossRef]
- Bhikshapathi, D.V.R.N.; Krishna, D.R.; Kishan, V. Anti-HIV, anti-tubercular and mutagenic activities of borrelidin. Indian J. Biotechnol. 2010, 9, 265–270. [Google Scholar]
- Wu, C.Y.; Tan, Y.; Gan, M.L.; Wang, Y.G.; Guan, Y.; Hu, X.X.; Zhou, H.X.; Shang, X.Y.; You, X.F.; Yang, Z.Y.; et al. Identification of elaiophylin derivatives from the marine-derived Actinomycete Streptomyces sp. 7-145 using PCR-based screening. J. Nat. Prod. 2013, 76, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.R.D.; Muniz, D.F.; Pereira, P.S.; Lima, S.M.D.; Tintino, C.D.D.O.; De Souza, V.C.A.; Da Silva, J.M.A.; Da Costa, R.H.S.; Pinheiro, J.C.A.; De Matos, Y.M.L.S.; et al. Evaluation of elaiophylin extracted from Streptomyces hygroscopicus as a potential inhibitor of the NorA efflux protein in Staphylococcus aureus: An in vitro and in silico approach. Bioorg. Med. Chem. Lett. 2021, 50, 128334. [Google Scholar]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Zhao, X.J.; Fang, Y.; Yang, Y.; Qin, Y.; Wu, P.; Wang, T.; Lai, H.L.; Meng, L.; Wang, D.W.; Zheng, Z.H.; et al. Elaiophylin, a novel autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells. Autophagy 2015, 11, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Buedenbender, L.; Robertson, L.P.; Lucantoni, L.; Avery, V.M.; Kurtböke, D.I.; Carroll, A.R. HSQC-TOCSY fingerprinting-directed discovery of antiplasmodial polyketides from the marine ascidian-derived Streptomyces sp. (USC-16018). Mar. Drugs 2018, 16, 189. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.S.; Kim, Y.H.; Han, S.B.; Kim, H.M.; Hong, S.D.; Lee, J.J. Immunosuppressive activity of elaiophylins. J. Microbiol. Biotechnol. 1997, 7, 272–277. [Google Scholar]
- Jomon, K.; Kuroda, Y.; Ajisaka, M.; Sakai, H. A new antibiotic, ikarugamycin. J. Antibiot. 1972, 25, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Schobert, R.; Schlenk, A. Tetramic and tetronic acids: An update on new derivatives and biological aspects. Bioorg. Med. Chem. 2008, 16, 4203–4221. [Google Scholar] [CrossRef]
- Nakayama, T.; Homma, Y.; Hashidoko, Y.; Mizutani, J.; Tahara, S. Possible role of xanthobaccins produced by Stenotrophomonas sp. strain SB-K88 in suppression of sugar beet damping-off disease. Appl. Environ. Microbiol. 1999, 65, 4334–4339. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Huffman, J.; Li, Y.; Du, L.C.; Shen, Y.M. 3-Hydroxylation of the polycyclic tetramate macrolactam in the biosynthesis of antifungal HSAF from Lysobacter enzymogenes C3. MedChemComm 2012, 3, 982–986. [Google Scholar] [CrossRef]
- Li, S.; Jochum, C.C.; Yu, F.; Zaleta-Rivera, K.; Du, L.; Harris, S.D.; Yuen, G.Y. An antibiotic complex from Lysobacter enzymogenes strain C3: Antimicrobial activity and role in plant disease control. Phytopathology 2008, 98, 695–701. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, W.J.; Jin, H.B.; Zhang, Q.B.; Chen, Y.C.; Jiang, X.D.; Zhang, G.T.; Zhang, L.P.; Zhang, W.M.; She, Z.G.; et al. Genome mining of marine-derived Streptomyces sp. SCSIO 40010 leads to cytotoxic new polycyclic tetramate macrolactams. Mar. Drugs 2019, 17, 663. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.G.; Blodgett, J.A.V.; Clardy, J. Targeted discovery of polycyclic tetramate macrolactams from an environmental Streptomyces strain. Org. Lett. 2010, 12, 4652–4654. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Jiang, Y.; Han, L.; Chen, X.; Ma, J.; Qu, X.D.; Mu, Y.; Liu, J.; Li, L.Y.; Jiang, C.L.; et al. Bafilomycins and odoriferous sesquiterpenoids from Streptomyces albolongus isolated from Elephas maximus Feces. J. Nat. Prod. 2016, 79, 799–805. [Google Scholar] [CrossRef]
- Lu, C.H.; Shen, Y.M. A new macrolide antibiotic with antitumor activity produced by Streptomyces sp. CS, a commensal microbe of Maytenus hookeri. J. Antibiot. 2003, 56, 415–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilton, J.H.; Hokanson, G.C.; French, J.C. Pd-118,576—A new antitumor macrolide antibiotic. J. Antibiot. 1985, 38, 1449–1452. [Google Scholar] [CrossRef]
- Frandberg, E.; Petersson, C.; Lundgren, L.N.; Schnurer, J. Streptomyces halstedii K122 produces the antifungal compounds bafilomycin B1 and C1. Can. J. Microbiol. 2000, 46, 753–758. [Google Scholar] [CrossRef]
- Goetz, M.A.; Mccormick, P.A.; Monaghan, R.L.; Ostlind, D.A.; Hensens, O.D.; Liesch, J.M.; Albersschonberg, G. L-155,175: A new antiparasitic macrolide. Fermentation, isolation and structure. J. Antibiot. 1985, 38, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Vanek, Z.; Mateju, J.; Curdova, E. Immunomodulators isolated from microorganisms. Folia Microbiol. 1991, 36, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Bowman, E.J.; Siebers, A.; Altendorf, K. Bafilomycins—A class of inhibitors of membrane atpases from microorganisms, animal-cells, and plant-cells. Proc. Natl. Acad. Sci. USA 1988, 85, 7972–7976. [Google Scholar] [CrossRef]
- Yan, Y.M.; Jiang, K.; Liu, P.; Zhang, X.B.; Dong, X.; Gao, J.C.; Liu, Q.T.; Barr, M.P.; Zhang, Q.; Hou, X.K.; et al. Bafilomycin A1 induces caspase-independent cell death in hepatocellular carcinoma cells via targeting of autophagy and MAPK pathways. Sci. Rep. 2016, 6, 37052. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.F.; Empadinhas, N.; Mendes, V. Autophagy in the fight against tuberculosis. DNA Cell Biol. 2015, 34, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Saqib, M.; Gupta, A.; Kumar, P.; Bhaskar, S. Autophagy induction by Mycobacterium indicus pranii promotes Mycobacterium tuberculosis clearance from RAW 264.7 macrophages. PLoS ONE 2017, 12, e0189606. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]


| OSMAC Strategy | Microorganisms | Induced Metabolites | Bioactivity |
|---|---|---|---|
| Heat shock [14] | Streptomyces venezuelae | 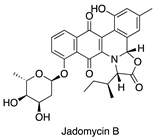 | Antibacterial |
| Addition of antibiotics [15] | Streptomyces sp. | 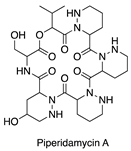 | Antibacterial |
| Addition of inducers [16,17] | S. natalensis | 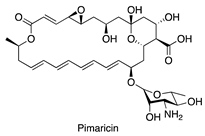 | Antifungal |
| Addition of inhibitors [18] | Phomospis asparagi | 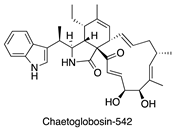 | Cytotoxic |
| Co-culture [19] | Pestalotia sp. |  | Antibacterial |
| Media variation [20] | Bipolaris oryzae | 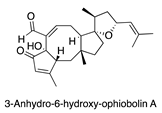 | Antibacterial |
| Strains | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Positive Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. bovis BCG | 3.125 | 25 | 100 | 3.125 | 0.78 | 1.56 | 6.25 | 6.25 | 6.25 | 6.25 | 12.5 | 0.05 a |
| Mtb mc2 6220 | >100 | >100 | >100 | 50% at 50 μM | 50% at 50 μM | 3.125 | 25 | 3.89 | 45% at 50 μM | >100 | >100 | 0.1 a |
| Mtb H37Rv | NT # | NT | NT | NT | NT | >100 | >100 | >100 | NT | NT | NT | 0.1 a |
| Staphylococcus aureus (SA) | >100 | >100 | >100 | 3.125 | 100 | 0.78 | 1.56 | 50 | 50 | 50 | 50 | 1 b |
| Methicillin-resistant S. aureus (MRSA) | >100 | >100 | >100 | 12.5 | >100 | 1.56 | 6.25 | >100 | >100 | >100 | >100 | 1 b |
| Bacillus subtilis (BS) | >100 | >100 | >100 | 3.125 | 25 | 1.56 | 6.25 | >100 | >100 | >100 | >100 | 0.5 b |
| Pseudomonas aeruginosa (PA) | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | 1 c |
| Conditions | Media variation (1–10) | Media variation (11–20) | Heat shock (21–23) | pH shock (24–26) | Ethanol shock (27–29) | Inducer addition (30–31) | Standard (32) |
| Medium | Media 1–10 | Media 1–10 | AM2 | AM2 | AM2 | AM2 | AM2 |
| Culture length | 7 days | 14 days | 7 days | 7 days | 7 days | 7 days | 7 days |
| pH | 7.0–7.4 | 7.0–7.4 | 7.3 | 5.5, 7.5, 9.5 | 7.3 | 7.3 | 7.3 |
| Temperature | 28 °C | 28 °C | 37 °C, 42 °C, or 42 °C for one hour (then 28 °C) | 28 °C | 28 °C | 28 °C | 28 °C |
| Ethanol | 0 | 0 | 0 | 0 | 1 mM, 10 mM, or 100 mM | 0 | 0 |
| Inducers | 0 | 0 | 0 | 0 | 0 | 200 nM of L-homoserine lactone hydrochloride or N-carbobenzoxy-L-homoserine lactone | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Liu, X.; Zhang, L.; Quinn, R.J.; Liu, M. Nuclear Magnetic Resonance Fingerprinting and Principal Component Analysis Strategies Lead to Anti-Tuberculosis Natural Product Discovery from Actinomycetes. Antibiotics 2025, 14, 108. https://doi.org/10.3390/antibiotics14010108
Han J, Liu X, Zhang L, Quinn RJ, Liu M. Nuclear Magnetic Resonance Fingerprinting and Principal Component Analysis Strategies Lead to Anti-Tuberculosis Natural Product Discovery from Actinomycetes. Antibiotics. 2025; 14(1):108. https://doi.org/10.3390/antibiotics14010108
Chicago/Turabian StyleHan, Jianying, Xueting Liu, Lixin Zhang, Ronald J. Quinn, and Miaomiao Liu. 2025. "Nuclear Magnetic Resonance Fingerprinting and Principal Component Analysis Strategies Lead to Anti-Tuberculosis Natural Product Discovery from Actinomycetes" Antibiotics 14, no. 1: 108. https://doi.org/10.3390/antibiotics14010108
APA StyleHan, J., Liu, X., Zhang, L., Quinn, R. J., & Liu, M. (2025). Nuclear Magnetic Resonance Fingerprinting and Principal Component Analysis Strategies Lead to Anti-Tuberculosis Natural Product Discovery from Actinomycetes. Antibiotics, 14(1), 108. https://doi.org/10.3390/antibiotics14010108







