A Decade of Antimicrobial Resistance in Human and Animal Campylobacter spp. Isolates
Abstract
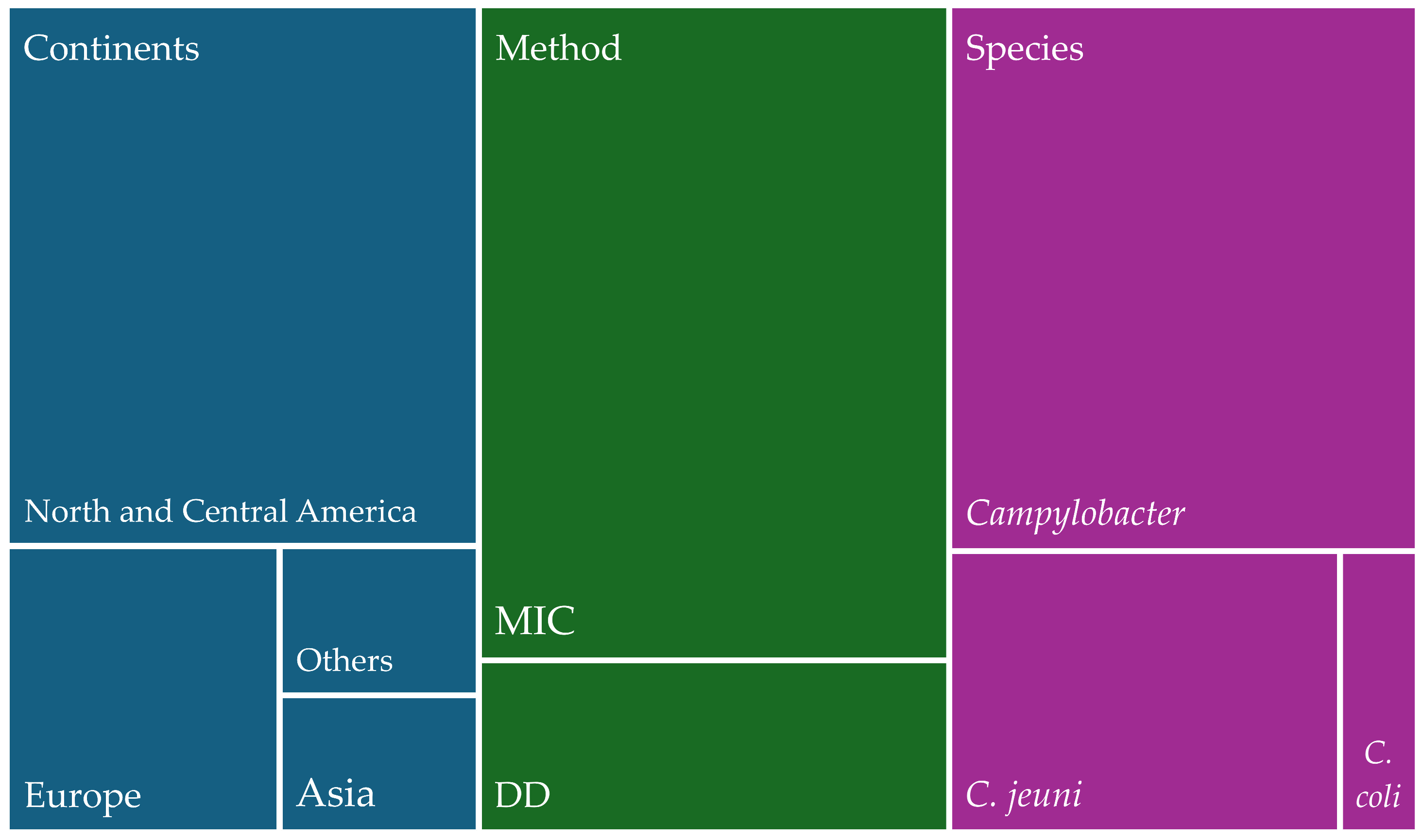
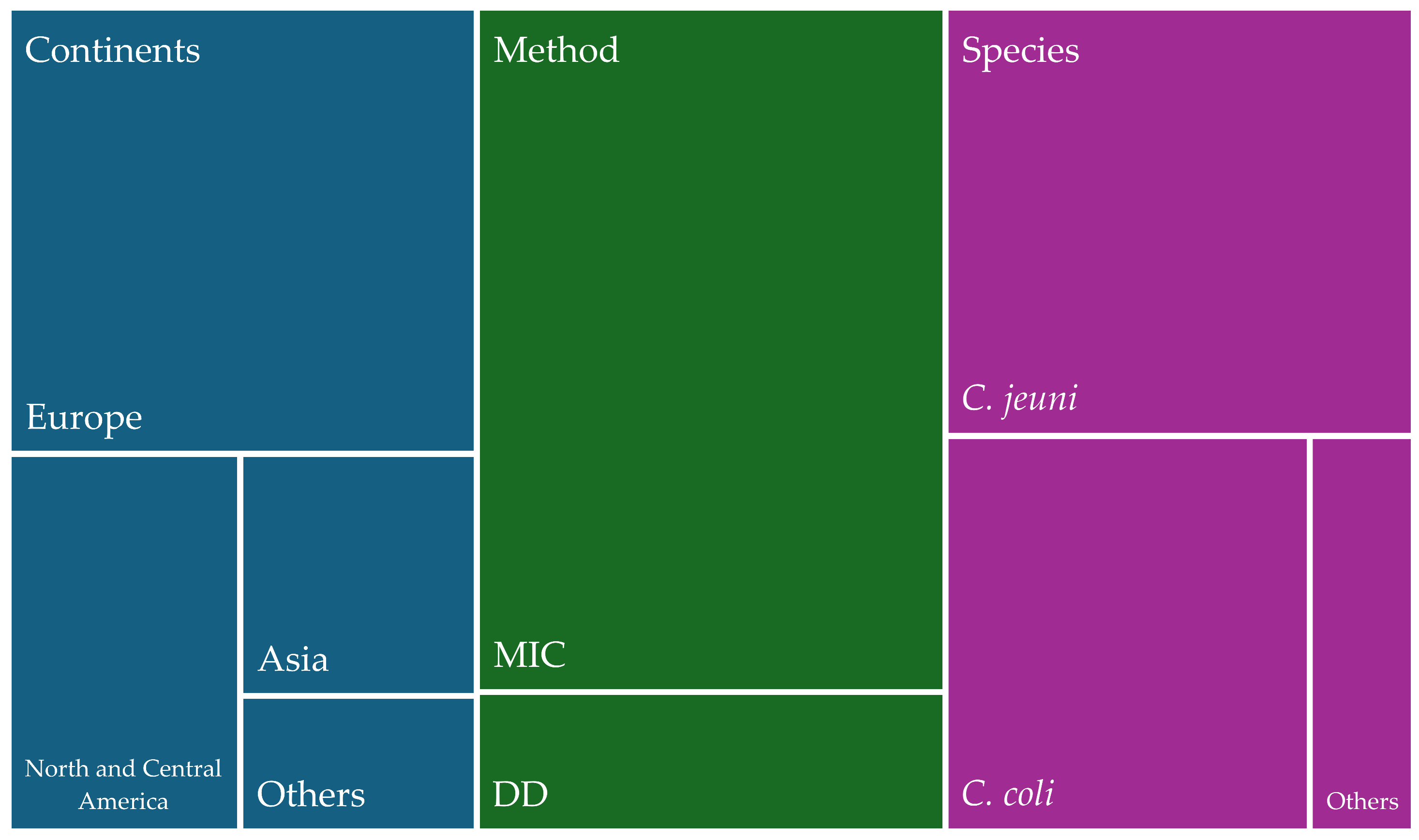
1. Introduction
2. Results
2.1. Studies of Antimicrobial Resistance of Campylobacter spp.
2.1.1. Studies on Antibiotic Resistance in Human Isolates of Campylobacter spp.
2.1.2. Studies on Antibiotic Resistance in Animal Isolates of Campylobacter spp.
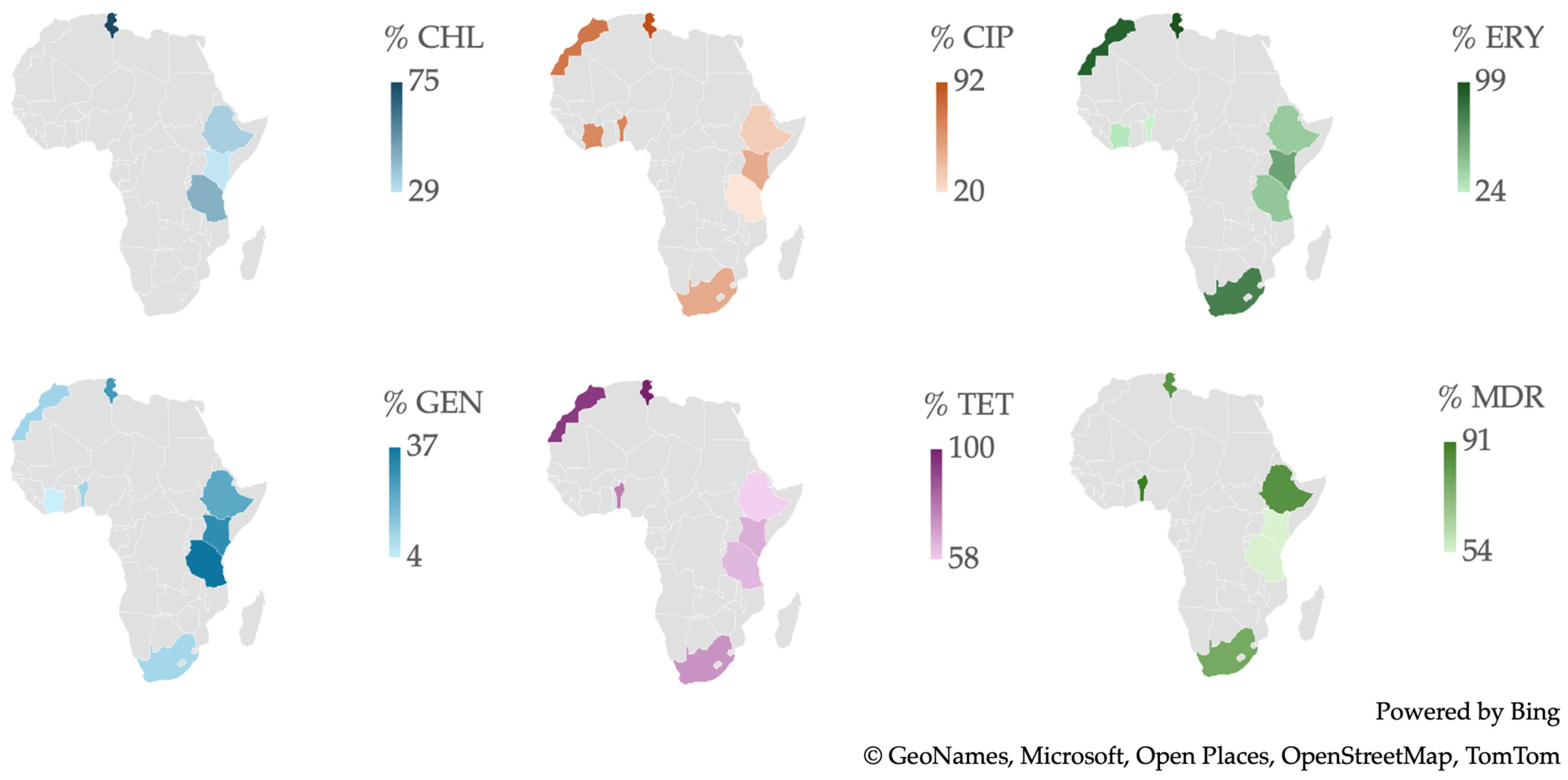
2.2. Resistance of Campylobacter spp. Isolates from African Studies
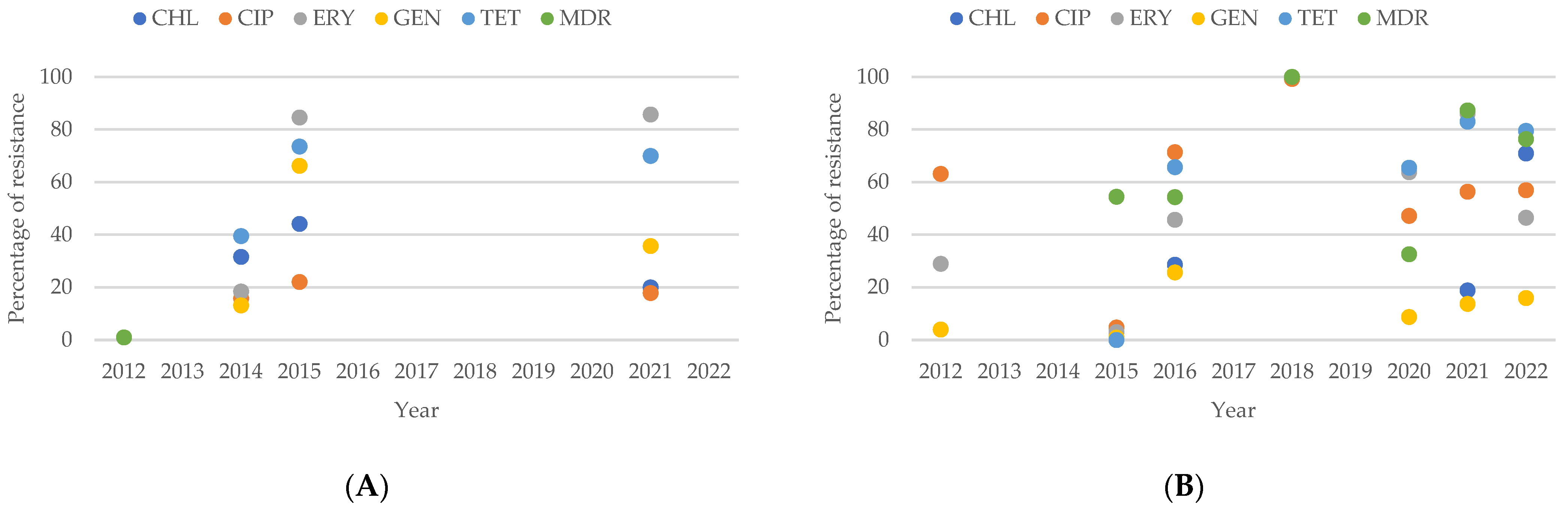
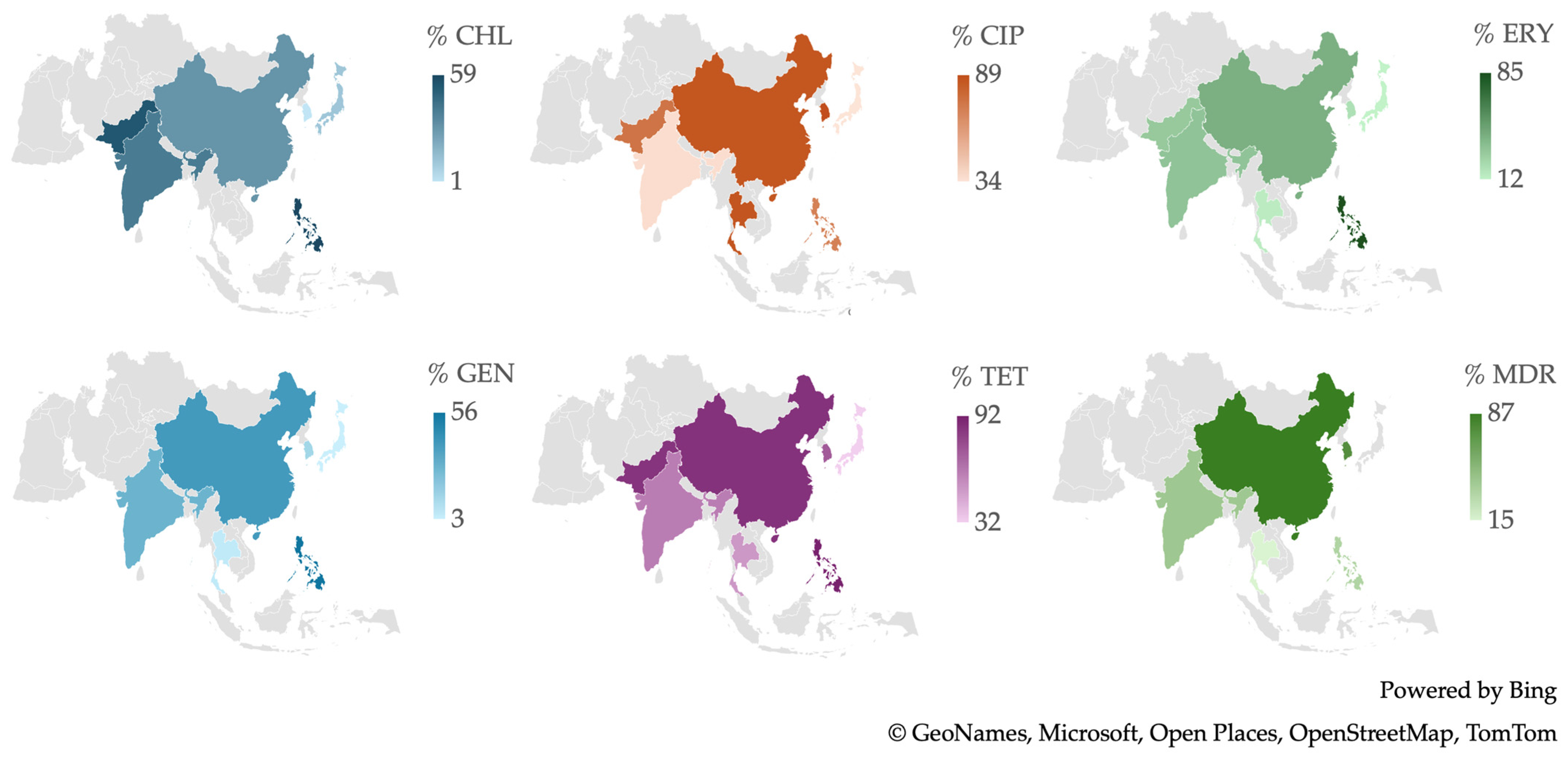
2.3. Resistance of Campylobacter spp. Isolates from Asian Studies
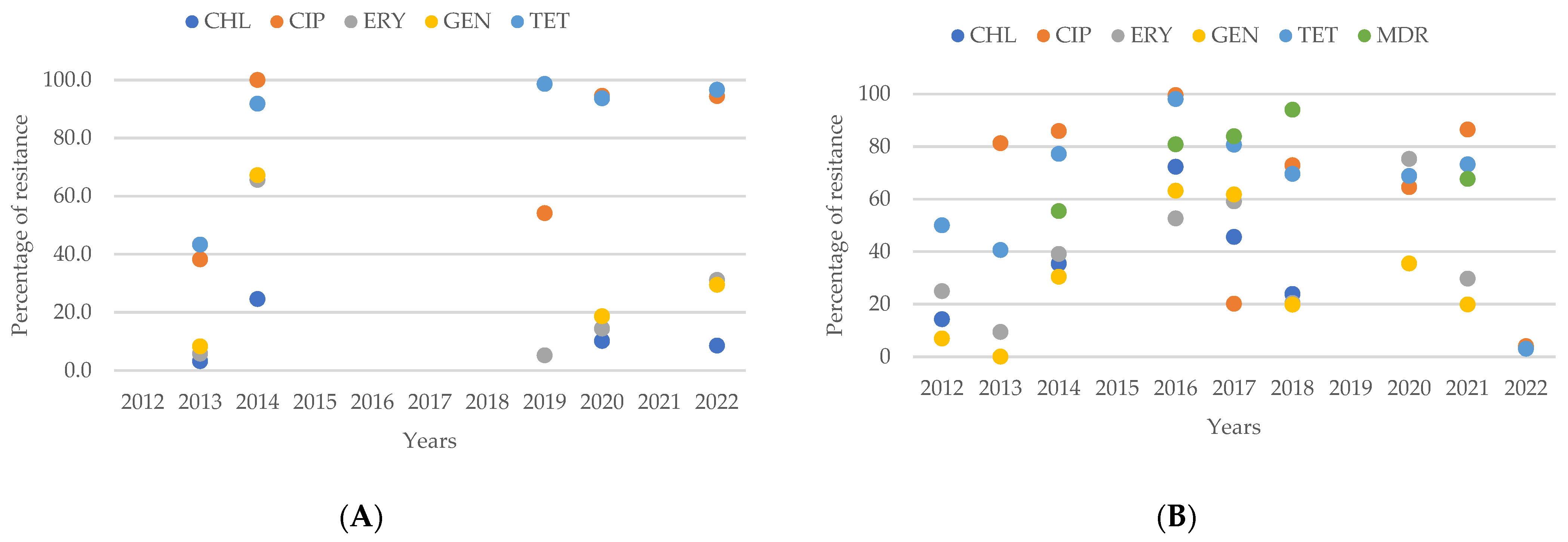
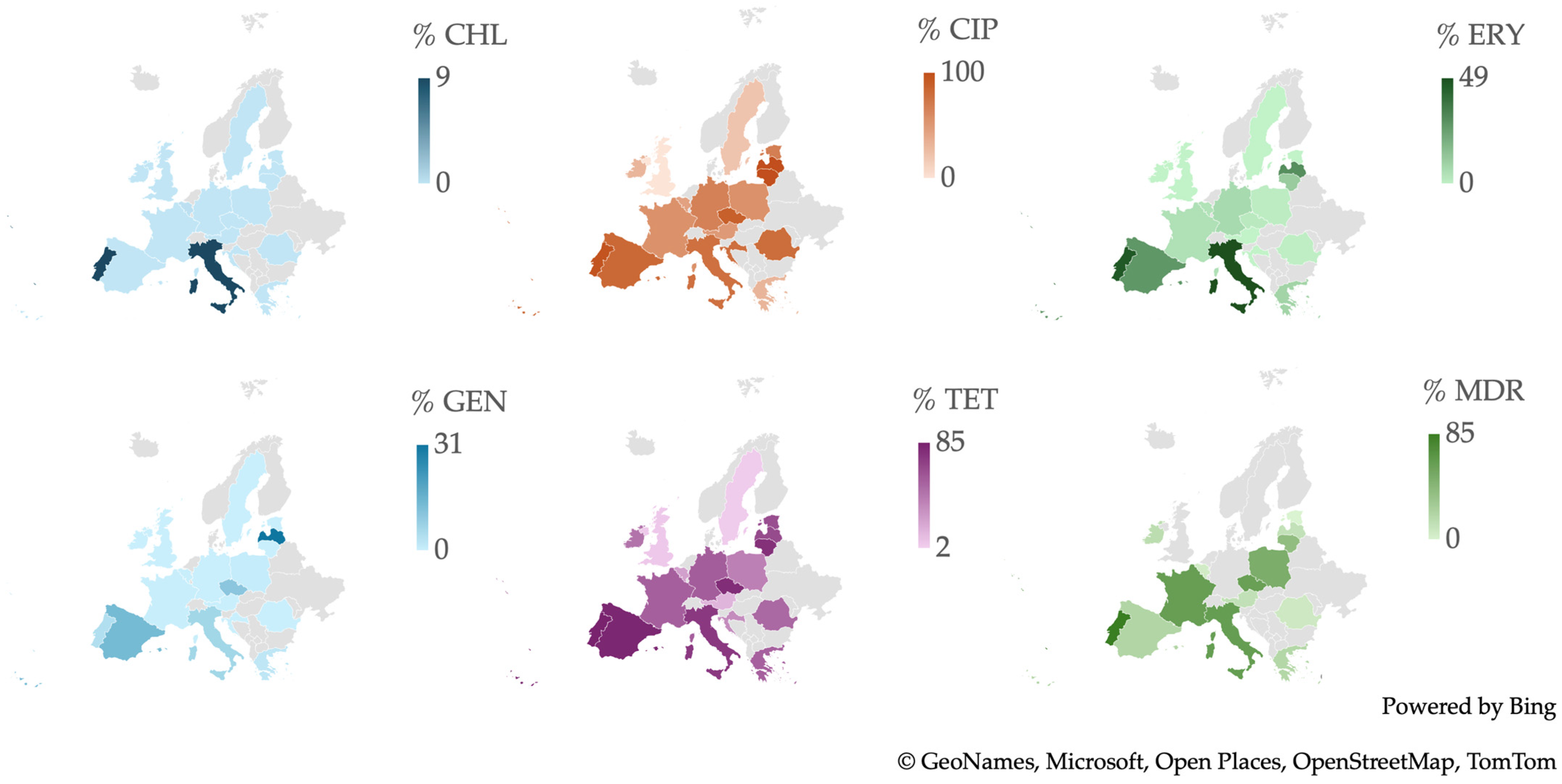
2.4. Resistance of Campylobacter spp. Isolates from European Studies
2.5. Resistance of Campylobacter spp. Isolates from Northern and Central American Studies
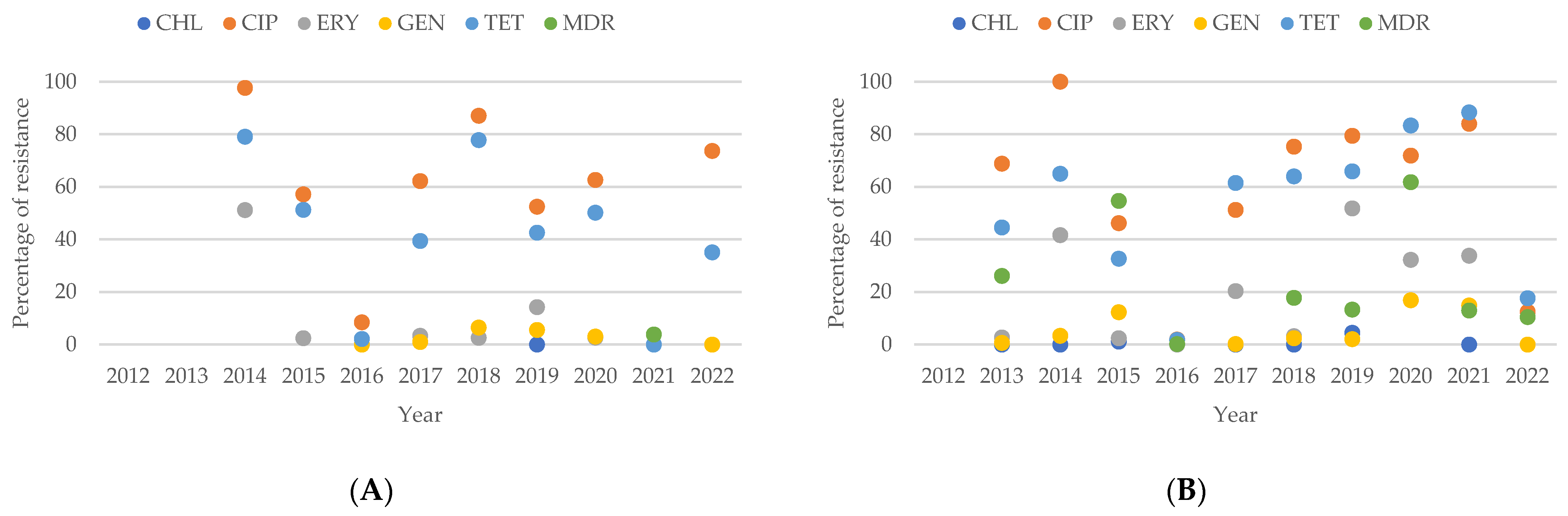
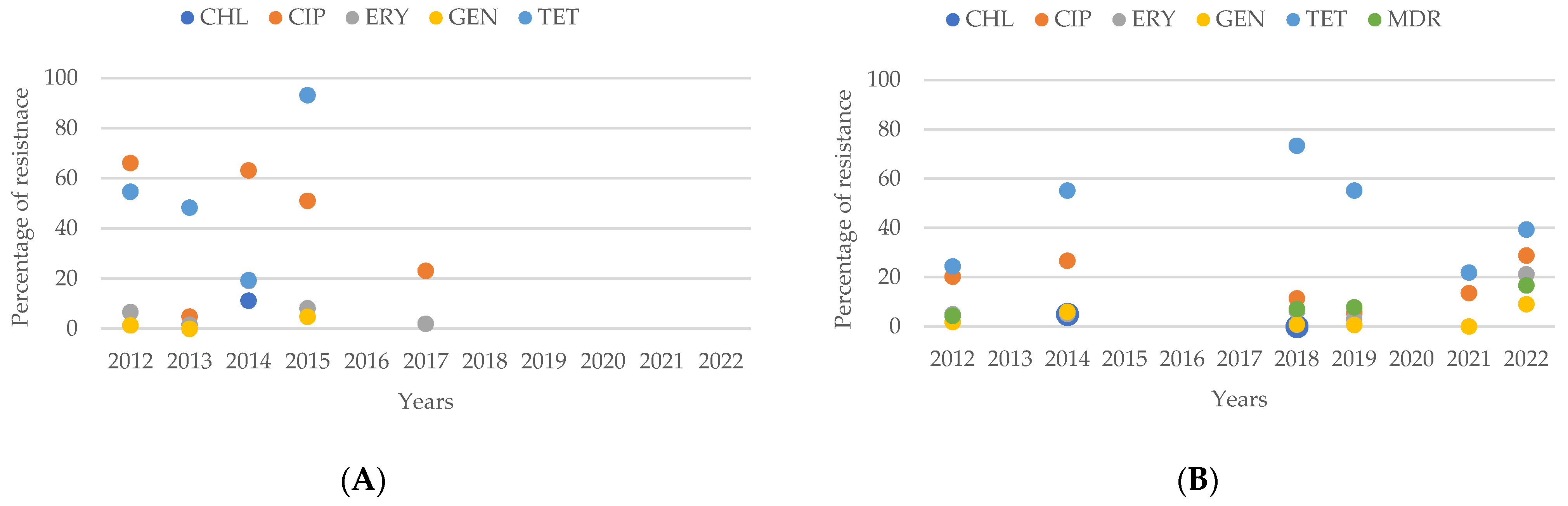
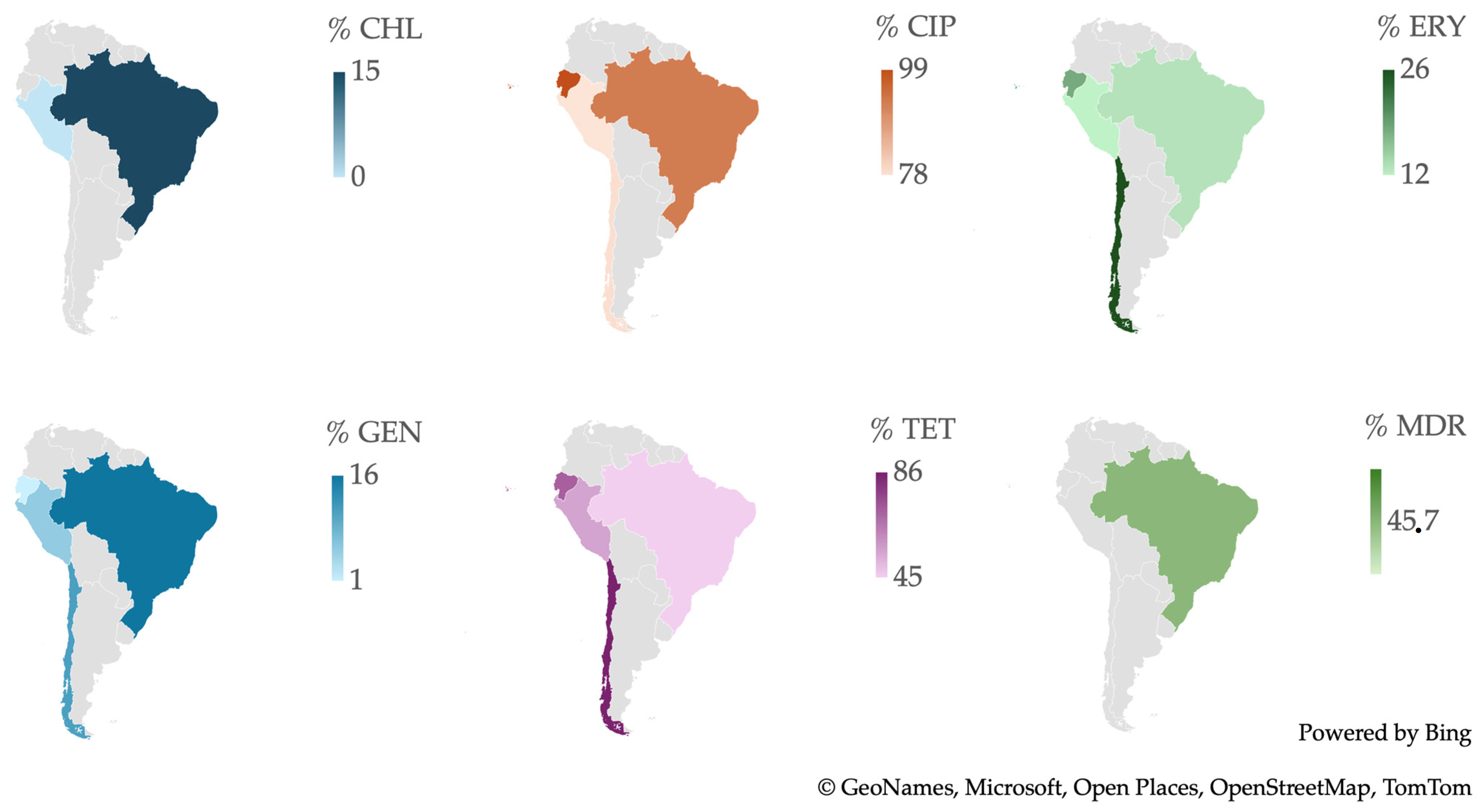
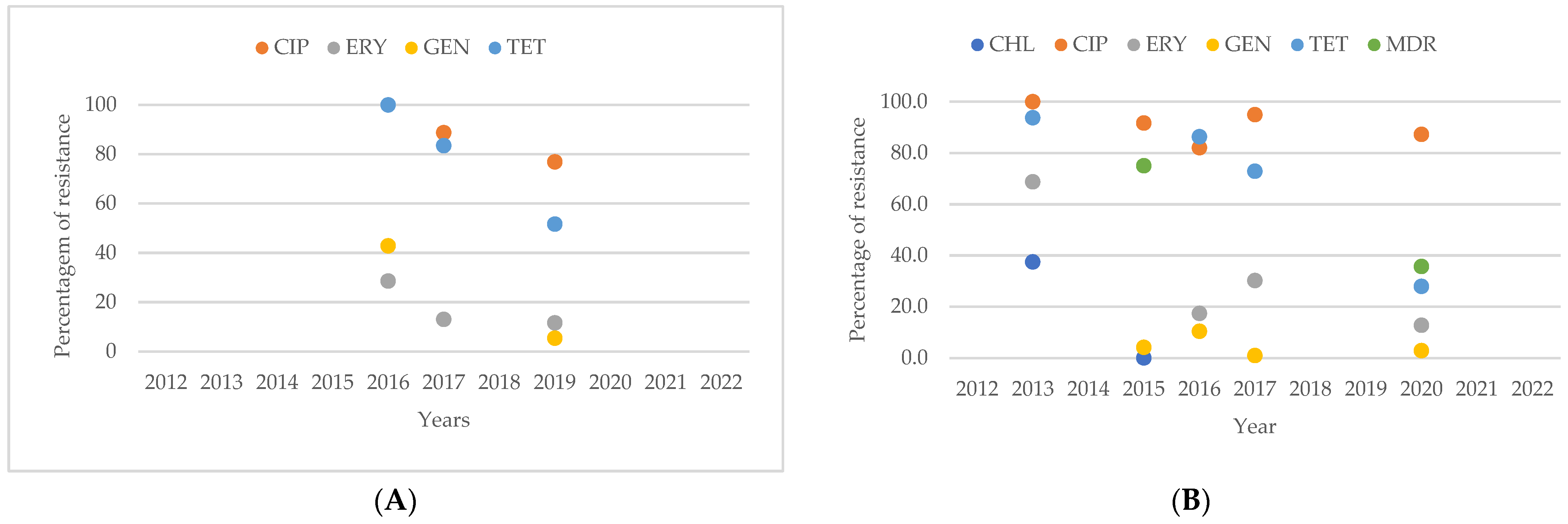
2.6. Resistance of Campylobacter spp. Isolates from Southern American Studies
2.7. Resistance of Campylobacter spp. Isolates from Oceania Studies
3. Discussion
3.1. Global Patterns and Regional Discrepancies in Campylobacter spp. Infections
3.2. MIC Testing Dominates Antimicrobial Resistance Research
3.3. Predominance of Campylobacter jejuni
3.4. Antimicrobial Resistance in Campylobacter spp. Isolates in Africa
3.5. Antimicrobial Resistance in Campylobacter spp. Isolates in Asia
3.6. Antimicrobial Resistance in Campylobacter spp. Isolates in Europe
3.7. Antimicrobial Resistance in Campylobacter spp. Isolates in North and Central America
3.8. Antimicrobial Resistance in Campylobacter spp. Isolates in South America
3.9. Regional MDR Variations
3.10. Global Patterns in Antimicrobial Resistance
4. Materials and Methods
4.1. Literature Study Search and Selection Strategy
4.2. Data Extraction
4.3. Antimicrobial Susceptibility Testing (AST)
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portes, A.B.; Panzenhagen, P.; Pereira dos Santos, A.M.; Junior, C.A.C. Antibiotic Resistance in Campylobacter: A Systematic Review of South American Isolates. Antibiotics 2023, 12, 548. [Google Scholar] [CrossRef]
- EFSA; ECDC. The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Sheppard, S.K.; Maiden, M.C.J. The Evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harb. Perspect. Biol. 2015, 7, a018119. [Google Scholar] [CrossRef]
- Imbrea, A.-M.; Balta, I.; Dumitrescu, G.; McCleery, D.; Pet, I.; Iancu, T.; Stef, L.; Corcionivoschi, N.; Liliana, P.-C. Exploring the Contribution of Campylobacter jejuni to Post-Infectious Irritable Bowel Syndrome: A Literature Review. Appl. Sci. 2024, 14, 3373. [Google Scholar] [CrossRef]
- Kreling, V.; Falcone, F.H.; Kehrenberg, C.; Hensel, A. Campylobacter sp.: Pathogenicity Factors and Prevention Methods—New Molecular Targets for Innovative Antivirulence Drugs? Appl. Microbiol. Biotechnol. 2020, 104, 10409–10436. [Google Scholar] [CrossRef]
- Igwaran, A.; Okoh, A.I. Human Campylobacteriosis: A Public Health Concern of Global Importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef]
- Amore, G.; Beloeil, P.; Fierro, R.G.; Guerra, B.; Papanikolaou, A.; Rizzi, V.; Stoicescu, A. Manual for Reporting 2022 Antimicrobial Resistance Data within the Framework of Directive 2003/99/EC and Decision 2020/1729/EU. EFSA Support. Publ. 2023, 20, 7826E. [Google Scholar] [CrossRef]
- Nielsen, H.L.; Dalager-Pedersen, M.; Nielsen, H. Risk of Inflammatory Bowel Disease after Campylobacter jejuni and Campylobacter Concisus Infection: A Population-Based Cohort Study. Scand. J. Gastroenterol. 2019, 54, 265–272. [Google Scholar] [CrossRef]
- Lazou, T.P.; Chaintoutis, S.C. Comparison of Disk Diffusion and Broth Microdilution Methods for Antimicrobial Susceptibility Testing of Campylobacter Isolates of Meat Origin. J. Microbiol. Methods 2023, 204, 106649. [Google Scholar] [CrossRef]
- Yang, Y.; Feye, K.M.; Shi, Z.; Pavlidis, H.O.; Kogut, M.; Ashworth, A.J.; Ricke, S.C. A Historical Review on Antibiotic Resistance of Foodborne Campylobacter. Front. Microbiol. 2019, 10, 1509. [Google Scholar] [CrossRef]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and Alternative Strategies for the Prevention, Control, and Treatment of Antibiotic-Resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- WHO. Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Almansour, A.M.; Alhadlaq, M.A.; Alzahrani, K.O.; Mukhtar, L.E.; Alharbi, A.L.; Alajel, S.M. The Silent Threat: Antimicrobial-Resistant Pathogens in Food-Producing Animals and Their Impact on Public Health. Microorganisms 2023, 11, 2127. [Google Scholar] [CrossRef]
- EFSA; ECDC. The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar] [CrossRef]
- Agbankpe, A.J.; Kougblenou, S.D.; Dougnon, T.V.; Oussou, A.; Gbotche, E.; Koudokpon, C.H.; Legba, B.B.; Baba-Moussa, L.; Bankole, H.S. Prevalence and Antimicrobial Resistance of Campylobacter coli and Campylobacter jejuni Isolated from Pig Guts, Pig Feces, and Surface Swabs from the Cutting Tables at Slaughterhouse and Taverns in Southern Benin. Int. J. Microbiol. 2022, 2022, 5120678. [Google Scholar] [CrossRef]
- Tafa, B.; Sewunet, T.; Tassew, H.; Asrat, D. Isolation and Antimicrobial Susceptibility Patterns of Campylobacter Species among Diarrheic Children at Jimma, Ethiopia. Int. J. Bacteriol. 2014, 2014, 560617. [Google Scholar] [CrossRef]
- Chala, G.; Eguale, T.; Abunna, F.; Asrat, D.; Stringer, A. Identification and Characterization of Campylobacter Species in Livestock, Humans, and Water in Livestock Owning Households of Peri-Urban Addis Ababa, Ethiopia: A One Health Approach. Front. Public Health 2021, 9, 750551. [Google Scholar] [CrossRef]
- Berhanu, L.; Bedru, H.; Gume, B.; Tolosa, T.; Kassa, T.; Getaneh, A.; Mereta, S.T. Occurrence, Risk Factors, and Antimicrobial Susceptibility Test of Thermophilic Campylobacter Species of Bovine Carcass at Municipal Abattoir and Butcher Shops of Jimma Town, Southwest Ethiopia. Infect. Drug Resist. 2021, 14, 3753–3762. [Google Scholar] [CrossRef]
- Debelo, M.; Mohammed, N.; Tiruneh, A.; Tolosa, T. Isolation, Identification and Antibiotic Resistance Profile of Thermophilic Campylobacter Species from Bovine, Knives and Personnel at Jimma Town Abattoir, Ethiopia. PLoS ONE 2022, 17, e0276625. [Google Scholar] [CrossRef]
- Gblossi Bernadette, G.; Eric Essoh, A.; Elise Solange, K.-N.; Natalie, G.; Souleymane, B.; Lamine Sébastien, N.; Mireille, D. Prevalence and Antimicrobial Resistance of Thermophilic Campylobacter Isolated from Chicken in Côte d’Ivoire. Int. J. Microbiol. 2012, 2012, 150612. [Google Scholar] [CrossRef]
- Zachariah, O.H.; Lizzy, M.A.; Rose, K.; Angela, M.M. Multiple Drug Resistance of Campylobacter jejuni and Shigella Isolated from Diarrhoeic Children at Kapsabet County Referral Hospital, Kenya. BMC Infect. Dis. 2021, 21, 109. [Google Scholar] [CrossRef]
- Nguyen, T.N.M.; Hotzel, H.; Njeru, J.; Mwituria, J.; El-Adawy, H.; Tomaso, H.; Neubauer, H.; Hafez, H.M. Antimicrobial Resistance of Campylobacter Isolates from Small Scale and Backyard Chicken in Kenya. Gut Pathog. 2016, 8, 39. [Google Scholar] [CrossRef]
- Es-soucratti, K.; Hammoumi, A.; Bouchrif, B.; Asmai, R.; En-nassiri, H.; Karraouan, B. Occurrence and Antimicrobial Resistance of Campylobacter jejuni Isolates from Poultry in Casablanca-Settat, Morocco. Ital. J. Food Saf. 2020, 9, 8692. [Google Scholar] [CrossRef]
- Karama, M.; Kambuyi, K.; Cenci-Goga, B.T.; Malahlela, M.; Jonker, A.; He, C.; Ombui, J.; Tshuma, T.; Etter, E.; Kalake, A. Occurrence and Antimicrobial Resistance Profiles of Campylobacter jejuni, Campylobacter coli, and Campylobacter upsaliensis in Beef Cattle on Cow–Calf Operations in South Africa. Foodborne Pathog. Dis. 2020, 17, 440–446. [Google Scholar] [CrossRef]
- Sithole, V.; Amoako, D.G.; Abia, A.L.K.; Perrett, K.; Bester, L.A.; Essack, S.Y. Occurrence, Antimicrobial Resistance, and Molecular Characterization of Campylobacter spp. in Intensive Pig Production in South Africa. Pathogens 2021, 10, 439. [Google Scholar] [CrossRef]
- Komba, E.V.G.; Mdegela, R.H.; Msoffe, P.L.M.; Nielsen, L.N.; Ingmer, H. Prevalence, Antimicrobial Resistance and Risk Factors for Thermophilic Campylobacter Infections in Symptomatic and Asymptomatic Humans in Tanzania. Zoonoses Public Health 2015, 62, 557–568. [Google Scholar] [CrossRef]
- Kashoma, I.P.; Kassem, I.I.; Kumar, A.; Kessy, B.M.; Gebreyes, W.; Kazwala, R.R.; Rajashekara, G. Antimicrobial Resistance and Genotypic Diversity of Campylobacter Isolated from Pigs, Dairy, and Beef Cattle in Tanzania. Front. Microbiol. 2015, 6, 1240. [Google Scholar] [CrossRef]
- Kashoma, I.P.; Kassem, I.I.; John, J.; Kessy, B.M.; Gebreyes, W.; Kazwala, R.R.; Rajashekara, G. Prevalence and Antimicrobial Resistance of Campylobacter Isolated from Dressed Beef Carcasses and Raw Milk in Tanzania. Microb. Drug Resist. 2016, 22, 40–52. [Google Scholar] [CrossRef]
- Gharbi, M.; Béjaoui, A.; Ben Hamda, C.; Jouini, A.; Ghedira, K.; Zrelli, C.; Hamrouni, S.; Aouadhi, C.; Bessoussa, G.; Ghram, A.; et al. Prevalence and Antibiotic Resistance Patterns of Campylobacter spp. Isolated from Broiler Chickens in the North of Tunisia. Biomed. Res. Int. 2018, 2018, 7943786. [Google Scholar] [CrossRef]
- Béjaoui, A.; Gharbi, M.; Bitri, S.; Nasraoui, D.; Ben Aziza, W.; Ghedira, K.; Rfaik, M.; Marzougui, L.; Ghram, A.; Maaroufi, A. Virulence Profiling, Multidrug Resistance and Molecular Mechanisms of Campylobacter Strains from Chicken Carcasses in Tunisia. Antibiotics 2022, 11, 830. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Xu, X.; Gu, Y.; Tao, X.; Yang, X.; Yan, G.; Zhang, J. Molecular Subtyping and Antimicrobial Susceptibilities of Campylobacter coli Isolates from Diarrheal Patients and Food-Producing Animals in China. Foodborne Pathog. Dis. 2014, 11, 610–619. [Google Scholar] [CrossRef]
- Ge, M.-C.; Kuo, S.-F.; Chang, S.-C.; Chien, C.-C.; You, H.-L.; Lu, J.-J. Antimicrobial Susceptibility and Virulence Surveillance of Campylobacter spp. Isolated from Patients in Two Tertiary Medical Centers in Taiwan. Front. Microbiol. 2019, 9, 3186. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Liu, Y.; Jiang, J.; Shen, Z.; Chen, Q.; Ma, X. Multilocus Sequence Types and Antimicrobial Resistance of Campylobacter jejuni and C. coli Isolates of Human Patients from Beijing, China, 2017–2018. Front. Microbiol. 2020, 11, 554784. [Google Scholar] [CrossRef]
- Liao, Y.-S.; Chen, B.-H.; Teng, R.-H.; Wang, Y.-W.; Chang, J.-H.; Liang, S.-Y.; Tsao, C.-S.; Hong, Y.-P.; Sung, H.-Y.; Chiou, C.-S. Antimicrobial Resistance in Campylobacter coli and Campylobacter jejuni from Human Campylobacteriosis in Taiwan, 2016 to 2019. Antimicrob. Agents Chemother. 2022, 66, e0173621. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Liu, Y.; Cui, Q.; Qin, X.; Niu, Y.; Wang, C.; Wang, T.; Chen, Q.; Ding, S.; et al. Genomic Insights into the Increased Occurrence of Campylobacteriosis Caused by Antimicrobial-Resistant Campylobacter coli. mBio 2022, 13, e0283522. [Google Scholar] [CrossRef]
- Bai, J.; Chen, Z.; Luo, K.; Zeng, F.; Qu, X.; Zhang, H.; Chen, K.; Lin, Q.; He, H.; Liao, M.; et al. Highly Prevalent Multidrug-Resistant Campylobacter spp. Isolated from a Yellow-Feathered Broiler Slaughterhouse in South China. Front. Microbiol. 2021, 12, 682741. [Google Scholar] [CrossRef]
- Li, B.; Ma, L.; Li, Y.; Jia, H.; Wei, J.; Shao, D.; Liu, K.; Shi, Y.; Qiu, Y.; Ma, Z. Antimicrobial Resistance of Campylobacter Species Isolated from Broilers in Live Bird Markets in Shanghai, China. Foodborne Pathog. Dis. 2017, 14, 96–102. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, Q.; Zhang, X.; Zhou, S.; Zhang, J.; Tang, X.; Lu, J.; Gao, Y. Antibiotic Resistance Profiles and Molecular Mechanisms of Campylobacter from Chicken and Pig in China. Front. Microbiol. 2020, 11, 592496. [Google Scholar] [CrossRef]
- Ma, H.; Su, Y.; Ma, L.; Ma, L.; Li, P.; Du, X.; Gölz, G.; Wang, S.; Lu, X. Prevalence and Characterization of Campylobacter jejuni Isolated from Retail Chicken in Tianjin, China. J. Food Prot. 2017, 80, 1032–1040. [Google Scholar] [CrossRef]
- Ghosh, R. Increasing Antimicrobial Resistance of Campylobacter jejuni Isolated from Paediatric Diarrhea Cases in A Tertiary Care Hospital of New Delhi, India. J. Clin. Diagn. Res. 2013, 7, 247–249. [Google Scholar] [CrossRef]
- Khan, J.A.; Rathore, R.S.; Abulreesh, H.H.; Qais, F.A.; Ahmad, I. Prevalence and Antibiotic Resistance Profiles of Campylobacter jejuni Isolated from Poultry Meat and Related Samples at Retail Shops in Northern India. Foodborne Pathog. Dis. 2018, 15, 218–225. [Google Scholar] [CrossRef]
- Suman Kumar, M.; Ramees, T.P.; Dhanze, H.; Gupta, S.; Dubal, Z.B.; Kumar, A. Occurrence and Antimicrobial Resistance of Campylobacter Isolates from Broiler Chicken and Slaughter House Environment in India. Anim. Biotechnol. 2023, 34, 199–207. [Google Scholar] [CrossRef]
- Yamada, K.; Saito, R.; Muto, S.; Sasaki, M.; Murakami, H.; Aoki, K.; Ishii, Y.; Tateda, K. Long-Term Observation of Antimicrobial Susceptibility and Molecular Characterisation of Campylobacter jejuni Isolated in a Japanese General Hospital 2000–2017. J. Glob. Antimicrob. Resist. 2019, 18, 59–63. [Google Scholar] [CrossRef]
- Ozawa, M.; Makita, K.; Tamura, Y.; Asai, T. Associations of Antimicrobial Use with Antimicrobial Resistance in Campylobacter coli from Grow-Finish Pigs in Japan. Prev. Veter-Med. 2012, 106, 295–300. [Google Scholar] [CrossRef]
- Haruna, M.; Sasaki, Y.; Murakami, M.; Mori, T.; Asai, T.; Ito, K.; Yamada, Y. Prevalence and Antimicrobial Resistance of Campylobacter Isolates from Beef Cattle and Pigs in Japan. J. Veter-Med. Sci. 2013, 75, 625–628. [Google Scholar] [CrossRef]
- Haruna, M.; Sasaki, Y.; Murakami, M.; Ikeda, A.; Kusukawa, M.; Tsujiyama, Y.; Ito, K.; Asai, T.; Yamada, Y. Prevalence and Antimicrobial Susceptibility of Campylobacter in Broiler Flocks in Japan. Zoonoses Public Health 2012, 59, 241–245. [Google Scholar] [CrossRef]
- Furukawa, I.; Ishihara, T.; Teranishi, H.; Saito, S.; Yatsuyanagi, J.; Wada, E.; Kumagai, Y.; Takahashi, S.; Konno, T.; Kashio, H.; et al. Prevalence and Characteristics of Salmonella and Campylobacter in Retail Poultry Meat in Japan. Jpn. J. Infect. Dis. 2017, 70, 239–247. [Google Scholar] [CrossRef]
- Osaili, T.M.; Alaboudi, A.R.; Al-Akhras, R.R. Prevalence and Antimicrobial Susceptibility of Campylobacter spp. in Live and Dressed Chicken in Jordan. Foodborne Pathog. Dis. 2012, 9, 54–58. [Google Scholar] [CrossRef]
- Shin, E.; Oh, Y.; Kim, M.; Jung, J.; Lee, Y. Antimicrobial Resistance Patterns and Corresponding Multilocus Sequence Types of the Campylobacter jejuni Isolates from Human Diarrheal Samples. Microb. Drug Resist. 2013, 19, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, J.; Lee, H.; Ha, J.; Kim, S.; Choi, Y.; Oh, H.; Seo, K.; Yoon, Y.; Lee, S. Antibiotic Susceptibility, Genetic Diversity, and the Presence of Toxin Producing Genes in Campylobacter Isolates from Poultry. Int. J. Environ. Res. Public Health 2017, 14, 1400. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.; Kim, J.; Kim, J.H.; Jung, J.I.; Cho, S.; Ryu, S.; Jeon, B. Comparative Analysis of Aerotolerance, Antibiotic Resistance, and Virulence Gene Prevalence in Campylobacter jejuni Isolates from Retail Raw Chicken and Duck Meat in South Korea. Microorganisms 2019, 7, 433. [Google Scholar] [CrossRef]
- Chon, J.-W.; Lee, S.-K.; Yoon, Y.; Yoon, K.-S.; Kwak, H.-S.; Joo, I.-S.; Seo, K.-H. Quantitative Prevalence and Characterization of Campylobacter from Chicken and Duck Carcasses from Poultry Slaughterhouses in South Korea. Poult. Sci. 2018, 97, 2909–2916. [Google Scholar] [CrossRef]
- Choi, J.-H.; Moon, D.C.; Mechesso, A.F.; Kang, H.Y.; Kim, S.-J.; Song, H.-J.; Yoon, S.-S.; Lim, S.-K. Antimicrobial Resistance Profiles and Macrolide Resistance Mechanisms of Campylobacter coli Isolated from Pigs and Chickens. Microorganisms 2021, 9, 1077. [Google Scholar] [CrossRef]
- Gahamanyi, N.; Song, D.-G.; Yoon, K.-Y.; Mboera, L.E.G.; Matee, M.I.; Mutangana, D.; Amachawadi, R.G.; Komba, E.V.G.; Pan, C.-H. Antimicrobial Resistance Profiles, Virulence Genes, and Genetic Diversity of Thermophilic Campylobacter Species Isolated From a Layer Poultry Farm in Korea. Front. Microbiol. 2021, 12, 622275. [Google Scholar] [CrossRef]
- Kwon, B.-R.; Wei, B.; Cha, S.-Y.; Shang, K.; Zhang, J.-F.; Kang, M.; Jang, H.-K. Longitudinal Study of the Distribution of Antimicrobial-Resistant Campylobacter Isolates from an Integrated Broiler Chicken Operation. Animals 2021, 11, 246. [Google Scholar] [CrossRef]
- Samad, A.; Abbas, F.; Ahmed, Z.; Akbar, A.; Naeem, M.; Sadiq, M.B.; Ali, I.; Saima; Roomeela; Bugti, F.S.; et al. Prevalence, Antimicrobial Susceptibility, and Virulence of Campylobacter jejuni Isolated from Chicken Meat. J. Food Saf. 2019, 39, e12600. [Google Scholar] [CrossRef]
- Sison, F.B.; Chaisowwong, W.; Alter, T.; Tiwananthagorn, S.; Pichpol, D.; Lampang, K.N.; Baumann, M.P.O.; Gölz, G. Loads and Antimicrobial Resistance of Campylobacter spp. on Fresh Chicken Meat in Nueva Ecija, Philippines. Poult. Sci. 2014, 93, 1270–1273. [Google Scholar] [CrossRef]
- Lim, P.W.N.; Tiam-Lee, D.C.; Paclibare, P.A.P.; Subejano, M.S.E.P.; Cabero-Palma, J.A.S.; Penuliar, G.M. High Rates of Contamination of Poultry Meat Products with Drug-Resistant Campylobacter in Metro Manila, Philippines. Jpn. J. Infect. Dis. 2017, 70, 311–313. [Google Scholar] [CrossRef]
- Chokboonmongkol, C.; Patchanee, P.; Gölz, G.; Zessin, K.-H.; Alter, T. Prevalence, Quantitative Load, and Antimicrobial Resistance of Campylobacter spp. from Broiler Ceca and Broiler Skin Samples in Thailand. Poult. Sci. 2013, 92, 462–467. [Google Scholar] [CrossRef]
- Wangroongsarb, P.; Cheunban, N.; Jittaprasatsin, C.; Kamthalang, T.; Saipradit, N.; Chaichana, P.; Pulsrikarn, C.; Parnmen, S.; Sripichai, O. Prevalence and Antimicrobial Susceptibility of Campylobacter Isolated from Retail Chickens in Thailand. Int. J. Food Microbiol. 2021, 339, 109017. [Google Scholar] [CrossRef]
- Klein-Jöbstl, D.; Sofka, D.; Iwersen, M.; Drillich, M.; Hilbert, F. Multilocus Sequence Typing and Antimicrobial Resistance of Campylobacter jejuni Isolated from Dairy Calves in Austria. Front. Microbiol. 2016, 7, 72. [Google Scholar] [CrossRef]
- Post, A.; Martiny, D.; van Waterschoot, N.; Hallin, M.; Maniewski, U.; Bottieau, E.; Van Esbroeck, M.; Vlieghe, E.; Ombelet, S.; Vandenberg, O.; et al. Antibiotic Susceptibility Profiles among Campylobacter Isolates Obtained from International Travelers between 2007 and 2014. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2101–2107. [Google Scholar] [CrossRef]
- Elhadidy, M.; Miller, W.G.; Arguello, H.; Álvarez-Ordóñez, A.; Dierick, K.; Botteldoorn, N. Molecular Epidemiology and Antimicrobial Resistance Mechanisms of Campylobacter coli from Diarrhoeal Patients and Broiler Carcasses in Belgium. Transbound. Emerg. Dis. 2019, 66, 463–475. [Google Scholar] [CrossRef]
- Elhadidy, M.; Ali, M.M.; El-Shibiny, A.; Miller, W.G.; Elkhatib, W.F.; Botteldoorn, N.; Dierick, K. Antimicrobial Resistance Patterns and Molecular Resistance Markers of Campylobacter jejuni Isolates from Human Diarrheal Cases. PLoS ONE 2020, 15, e0227833. [Google Scholar] [CrossRef]
- Elhadidy, M.; Miller, W.G.; Arguello, H.; Álvarez-Ordóñez, A.; Duarte, A.; Dierick, K.; Botteldoorn, N. Genetic Basis and Clonal Population Structure of Antibiotic Resistance in Campylobacter jejuni Isolated from Broiler Carcasses in Belgium. Front. Microbiol. 2018, 9, 1014. [Google Scholar] [CrossRef]
- Jurinović, L.; Duvnjak, S.; Kompes, G.; Šoprek, S.; Šimpraga, B.; Krstulović, F.; Mikulić, M.; Humski, A. Occurrence of Campylobacter jejuni in Gulls Feeding on Zagreb Rubbish Tip, Croatia; Their Diversity and Antimicrobial Susceptibility in Perspective with Human and Broiler Isolates. Pathogens 2020, 9, 695. [Google Scholar] [CrossRef]
- Šoprek, S.; Duvnjak, S.; Kompes, G.; Jurinović, L.; Tambić Andrašević, A. Resistome Analysis of Campylobacter jejuni Strains Isolated from Human Stool and Primary Sterile Samples in Croatia. Microorganisms 2022, 10, 1410. [Google Scholar] [CrossRef]
- Yan, C.Y.J.C.; Jun, M.J.Z.M.; Ping, Y.P.M.Y.; Rang, J.R.L.J.; Hua, M.H.Y.M.; Hui, H.C.; Yun, C.Y.L.C.; Xin, Y.X.G.Y.; Yan, Y.Y.F.Y.; Xiang, Y.X.D.Y. Genetic and Antibiotic Resistance Characteristics of Campylobacter jejuni Isolated from Diarrheal Patients, Poultry and Cattle in Shenzhen. Biomed. Environ. Sci. 2018, 31, 579–585. [Google Scholar] [CrossRef]
- Tedersoo, T.; Roasto, M.; Mäesaar, M.; Häkkinen, L.; Kisand, V.; Ivanova, M.; Valli, M.H.; Meremäe, K. Antibiotic Resistance in Campylobacter spp. Isolated from Broiler Chicken Meat and Human Patients in Estonia. Microorganisms 2022, 10, 1067. [Google Scholar] [CrossRef]
- Olkkola, S.; Nykäsenoja, S.; Raulo, S.; Llarena, A.-K.; Kovanen, S.; Kivistö, R.; Myllyniemi, A.-L.; Hänninen, M.-L. Antimicrobial Resistance and Multilocus Sequence Types of Finnish Campylobacter jejuni Isolates from Multiple Sources. Zoonoses Public Health 2016, 63, 10–19. [Google Scholar] [CrossRef]
- Sifré, E.; Salha, B.A.; Ducournau, A.; Floch, P.; Chardon, H.; Mégraud, F.; Lehours, P. EUCAST Recommendations for Antimicrobial Susceptibility Testing Applied to the Three Main Campylobacter Species Isolated in Humans. J. Microbiol. Methods 2015, 119, 206–213. [Google Scholar] [CrossRef]
- Guyard-Nicodème, M.; Rivoal, K.; Houard, E.; Rose, V.; Quesne, S.; Mourand, G.; Rouxel, S.; Kempf, I.; Guillier, L.; Gauchard, F.; et al. Prevalence and Characterization of Campylobacter jejuni from Chicken Meat Sold in French Retail Outlets. Int. J. Food Microbiol. 2015, 203, 8–14. [Google Scholar] [CrossRef]
- Kempf, I.; Kerouanton, A.; Bougeard, S.; Nagard, B.; Rose, V.; Mourand, G.; Osterberg, J.; Denis, M.; Bengtsson, B.O. Campylobacter coli in Organic and Conventional Pig Production in France and Sweden: Prevalence and Antimicrobial Resistance. Front. Microbiol. 2017, 8, 955. [Google Scholar] [CrossRef]
- Tenhagen, B.-A.; Flor, M.; Alt, K.; Knüver, M.-T.; Buhler, C.; Käsbohrer, A.; Stingl, K. Association of Antimicrobial Resistance in Campylobacter spp. in Broilers and Turkeys with Antimicrobial Use. Antibiotics 2021, 10, 673. [Google Scholar] [CrossRef]
- von Altrock, A.; Hamedy, A.; Merle, R.; Waldmann, K.-H. Campylobacter spp.—Prevalence on Pig Livers and Antimicrobial Susceptibility. Prev. Veter-Med. 2013, 109, 152–157. [Google Scholar] [CrossRef]
- Döhne, S.; Merle, R.; Altrock, A.V.; Waldmann, K.-H.; Verspohl, J.; Grüning, P.; Hamedy, A.; Kreienbrock, L. Antibiotic Susceptibility of Salmonella, Campylobacter coli, and Campylobacter jejuni Isolated from Northern German Fattening Pigs. J. Food Prot. 2012, 75, 1839–1845. [Google Scholar] [CrossRef]
- Marinou, I.; Bersimis, S.; Ioannidis, A.; Nicolaou, C.; Mitroussia-Ziouva, A.; Legakis, N.J.; Chatzipanagiotou, S. Identification and Antimicrobial Resistance of Campylobacter Species Isolated from Animal Sources. Front. Microbiol. 2012, 3, 58. [Google Scholar] [CrossRef]
- Lazou, T.; Houf, K.; Soultos, N.; Dovas, C.; Iossifidou, E. Campylobacter in Small Ruminants at Slaughter: Prevalence, Pulsotypes and Antibiotic Resistance. Int. J. Food Microbiol. 2014, 173, 54–61. [Google Scholar] [CrossRef]
- Lazou, T.; Dovas, C.; Houf, K.; Soultos, N.; Iossifidou, E. Diversity of Campylobacter in Retail Meat and Liver of Lambs and Goat Kids. Foodborne Pathog. Dis. 2014, 11, 320–328. [Google Scholar] [CrossRef]
- Economou, V.; Zisides, N.; Gousia, P.; Petsios, S.; Sakkas, H.; Soultos, N.; Papadopoulou, C. Prevalence and Antimicrobial Profile of Campylobacter Isolates from Free-Range and Conventional Farming Chicken Meat during a 6-Year Survey. Food Control 2015, 56, 161–168. [Google Scholar] [CrossRef]
- Ladely, S.R.; Berrang, M.E.; Meinersmann, R.J.; Cox, N.A. Campylobacter Multi-locus Sequence Types and Antimicrobial Susceptibility of Broiler Cecal Isolates: A Two Year Study of 143 Commercial Flocks. J. Food Saf. 2017, 37, e12366. [Google Scholar] [CrossRef]
- Natsos, G.; Mouttotou, N.K.; Magiorkinis, E.; Ioannidis, A.; Magana, M.; Chatzipanagiotou, S.; Koutoulis, K.C. Antimicrobial Resistance, FlaA Sequencing, and Phylogenetic Analysis of Campylobacter Isolates from Broiler Chicken Flocks in Greece. Veter-Sci. 2021, 8, 68. [Google Scholar] [CrossRef]
- Papadopoulos, D.; Petridou, E.; Papageorgiou, K.; Giantsis, I.A.; Delis, G.; Economou, V.; Frydas, I.; Papadopoulos, G.; Hatzistylianou, M.; Kritas, S.K. Phenotypic and Molecular Patterns of Resistance among Campylobacter coli and Campylobacter jejuni Isolates, from Pig Farms. Animals 2021, 11, 2394. [Google Scholar] [CrossRef]
- Emanowicz, M.; Meade, J.; Burgess, C.; Bolton, D.; Egan, J.; Lynch, H.; O’Connor, L.; Coffey, A.; Lucey, B.; Gutierrez, M.; et al. Antimicrobial Resistance and Genomic Diversity of Campylobacter jejuni Isolates from Broiler Caeca and Neck Skin Samples Collected at Key Stages during Processing. Food Control 2022, 135, 108664. [Google Scholar] [CrossRef]
- Marotta, F.; Garofolo, G.; di Marcantonio, L.; Di Serafino, G.; Neri, D.; Romantini, R.; Sacchini, L.; Alessiani, A.; Di Donato, G.; Nuvoloni, R.; et al. Antimicrobial Resistance Genotypes and Phenotypes of Campylobacter jejuni Isolated in Italy from Humans, Birds from Wild and Urban Habitats, and Poultry. PLoS ONE 2019, 14, e0223804. [Google Scholar] [CrossRef]
- Giacomelli, M.; Salata, C.; Martini, M.; Montesissa, C.; Piccirillo, A. Antimicrobial Resistance of Campylobacter jejuni and Campylobacter coli from Poultry in Italy. Microb. Drug Resist. 2014, 20, 181–188. [Google Scholar] [CrossRef]
- Pergola, S.; Franciosini, M.P.; Comitini, F.; Ciani, M.; De Luca, S.; Bellucci, S.; Menchetti, L.; Casagrande Proietti, P. Genetic Diversity and Antimicrobial Resistance Profiles of Campylobacter coli and Campylobacter jejuni Isolated from Broiler Chicken in Farms and at Time of Slaughter in Central Italy. J. Appl. Microbiol. 2017, 122, 1348–1356. [Google Scholar] [CrossRef]
- Casagrande Proietti, P.; Pergola, S.; Bellucci, S.; Menchetti, L.; Miraglia, D.; Franciosini, M.P. Occurrence and Antimicrobial Susceptibility of Campylobacter spp. on Fresh and Refrigerated Chicken Meat Products in Central Italy. Poult. Sci. 2018, 97, 2895–2901. [Google Scholar] [CrossRef]
- Di Donato, G.; Marotta, F.; Nuvoloni, R.; Zilli, K.; Neri, D.; Di Sabatino, D.; Calistri, P.; Di Giannatale, E. Prevalence, Population Diversity and Antimicrobial Resistance of Campylobacter coli Isolated in Italian Swine at Slaughterhouse. Microorganisms 2020, 8, 222. [Google Scholar] [CrossRef]
- Marotta, F.; Di Marcantonio, L.; Janowicz, A.; Pedonese, F.; Di Donato, G.; Ardelean, A.; Nuvoloni, R.; Di Giannatale, E.; Garofolo, G. Genotyping and Antibiotic Resistance Traits in Campylobacter jejuni and Coli from Pigs and Wild Boars in Italy. Front. Cell. Infect. Microbiol. 2020, 10, 592512. [Google Scholar] [CrossRef]
- Russo, T.P.; Pace, A.; Varriale, L.; Borrelli, L.; Gargiulo, A.; Pompameo, M.; Fioretti, A.; Dipineto, L. Prevalence and Antimicrobial Resistance of Enteropathogenic Bacteria in Yellow-Legged Gulls (Larus michahellis) in Southern Italy. Animals 2021, 11, 275. [Google Scholar] [CrossRef]
- Kovaļenko, K.; Roasto, M.; Šantare, S.; Bērziņš, A.; Hörman, A. Campylobacter Species and Their Antimicrobial Resistance in Latvian Broiler Chicken Production. Food Control 2014, 46, 86–90. [Google Scholar] [CrossRef]
- Szczepanska, B.; Andrzejewska, M.; Spica, D.; Klawe, J.J. Prevalence and Antimicrobial Resistance of Campylobacter jejuni and Campylobacter coli Isolated from Children and Environmental Sources in Urban and Suburban Areas. BMC Microbiol 2017, 17, 80. [Google Scholar] [CrossRef]
- Wieczorek, K.; Wołkowicz, T.; Osek, J. Antimicrobial Resistance and Virulence-Associated Traits of Campylobacter jejuni Isolated from Poultry Food Chain and Humans With Diarrhea. Front. Microbiol. 2018, 9, 1508. [Google Scholar] [CrossRef]
- Wysok, B.; Wojtacka, J.; Hänninen, M.-L.; Kivistö, R. Antimicrobial Resistance and Virulence-Associated Markers in Campylobacter Strains from Diarrheic and Non-Diarrheic Humans in Poland. Front. Microbiol. 2020, 11, 1799. [Google Scholar] [CrossRef]
- Wieczorek, K.; Denis, E.; Lynch, O.; Osek, J. Molecular Characterization and Antibiotic Resistance Profiling of Campylobacter Isolated from Cattle in Polish Slaughterhouses. Food Microbiol. 2013, 34, 130–136. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Characteristics and Antimicrobial Resistance of Campylobacter Isolated from Pig and Cattle Carcasses in Poland. Pol. J. Veter-Sci. 2013, 16, 501–508. [Google Scholar] [CrossRef]
- Wieczorek, K.; Kania, I.; Osek, J. Prevalence and Antimicrobial Resistance of Campylobacter spp. Isolated from Poultry Carcasses in Poland. J. Food Prot. 2013, 76, 1451–1455. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. A Five-Year Study on Prevalence and Antimicrobial Resistance of Campylobacter from Poultry Carcasses in Poland. Food Microbiol. 2015, 49, 161–165. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Antimicrobial Resistance and Genotypes of Campylobacter jejuni from Pig and Cattle Carcasses Isolated in Poland During 2009–2016. Microb. Drug Resist. 2018, 24, 680–684. [Google Scholar] [CrossRef]
- Woźniak-Biel, A.; Bugla-Płoskońska, G.; Kielsznia, A.; Korzekwa, K.; Tobiasz, A.; Korzeniowska-Kowal, A.; Wieliczko, A. High Prevalence of Resistance to Fluoroquinolones and Tetracycline Campylobacter spp. Isolated from Poultry in Poland. Microb. Drug Resist. 2018, 24, 314–322. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Szczepańska, B.; Śpica, D.; Klawe, J.J. Prevalence, Virulence, and Antimicrobial Resistance of Campylobacter spp. in Raw Milk, Beef, and Pork Meat in Northern Poland. Foods 2019, 8, 420. [Google Scholar] [CrossRef]
- Wysok, B.; Wojtacka, J.; Wiszniewska-Łaszczych, A.; Sołtysiuk, M.; Kobuszewska, A. The Enterotoxin Production and Antimicrobial Resistance of Campylobacter Strains Originating from Slaughter Animals. Pathogens 2022, 11, 1131. [Google Scholar] [CrossRef]
- Santos-Ferreira, N.; Ferreira, V.; Teixeira, P. Occurrence and Multidrug Resistance of Campylobacter in Chicken Meat from Different Production Systems. Foods 2022, 11, 1827. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Ribeiro, S.A.; Pereira, S.C.; Fernandes, M.H.; Fernandes, M.J.; Barreto, A.S. Genetic and Antibiotic Resistance Profiles of Thermophilic Campylobacter spp. Isolated from Quails (Coturnix Coturnix Japonica) in a Portuguese Slaughterhouse. Food Control 2016, 59, 337–344. [Google Scholar] [CrossRef]
- Lemos, A.; Morais, L.; da Conceição Fontes, M.; Pires, I.; Vieira-Pinto, M. Campylobacter spp. Isolation from Infected Poultry Livers with and without Necrotic Lesions. Food Control 2015, 50, 236–242. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Martins, A.; Borges, A.C.; Fernandes, M.H.; Fernandes, M.J.; Vaz, Y.; Bessa, R.J.B.; Barreto, A.S. Antimicrobial Resistance among Campylobacter spp. Strains Isolated from Different Poultry Production Systems at Slaughterhouse Level. Poult. Sci. 2014, 93, 1578–1586. [Google Scholar] [CrossRef]
- Carreira, A.C.; Clemente, L.; Rocha, T.; Tavares, A.; Geraldes, M.; Barahona, M.J.; Botelho, A.; Cunha, M.V. Comparative Genotypic and Antimicrobial Susceptibility Analysis of Zoonotic Campylobacter Species Isolated from Broilers in a Nationwide Survey, Portugal. J. Food Prot. 2012, 75, 2100–2109. [Google Scholar] [CrossRef]
- Tîrziu, E.; Bărbălan, G.; Morar, A.; Herman, V.; Cristina, R.T.; Imre, K. Occurrence and Antimicrobial Susceptibility Profile of Salmonella spp. in Raw and Ready-To-Eat Foods and Campylobacter spp. in Retail Raw Chicken Meat in Transylvania, Romania. Foodborne Pathog. Dis. 2020, 17, 479–484. [Google Scholar] [CrossRef]
- Popa, S.A.; Morar, A.; Ban-Cucerzan, A.; Tîrziu, E.; Herman, V.; Sallam, K.I.; Morar, D.; Acaroz, U.; Imre, M.; Florea, T.; et al. Occurrence of Campylobacter spp. and Phenotypic Antimicrobial Resistance Profiles of Campylobacter jejuni in Slaughtered Broiler Chickens in North-Western Romania. Antibiotics 2022, 11, 1713. [Google Scholar] [CrossRef]
- Ruiz-Castillo, A.; Torres-Sánchez, M.J.; Aznar-Martín, J. Molecular Epidemiology and Antimicrobial Susceptibility of Campylobacter coli Clinical Isolates. Enferm. Infecc. Microbiol. Clin. 2014, 32, 443–445. [Google Scholar] [CrossRef]
- Silvan, J.M.; Zorraquin-Peña, I.; Gonzalez de Llano, D.; Moreno-Arribas, M.V.; Martinez-Rodriguez, A.J. Antibacterial Activity of Glutathione-Stabilized Silver Nanoparticles Against Campylobacter Multidrug-Resistant Strains. Front. Microbiol. 2018, 9, 458. [Google Scholar] [CrossRef]
- Iglesias-Torrens, Y.; Miró, E.; Guirado, P.; Llovet, T.; Muñoz, C.; Cerdà-Cuéllar, M.; Madrid, C.; Balsalobre, C.; Navarro, F. Population Structure, Antimicrobial Resistance, and Virulence-Associated Genes in Campylobacter jejuni Isolated From Three Ecological Niches: Gastroenteritis Patients, Broilers, and Wild Birds. Front. Microbiol. 2018, 9, 1676. [Google Scholar] [CrossRef]
- Nafarrate, I.; Lasagabaster, A.; Sevillano, E.; Mateo, E. Prevalence, Molecular Typing and Antimicrobial Susceptibility of Campylobacter spp. Isolates in Northern Spain. J. Appl. Microbiol. 2021, 130, 1368–1379. [Google Scholar] [CrossRef]
- Rivera-Gomis, J.; Marín, P.; Otal, J.; Galecio, J.S.; Martínez-Conesa, C.; Cubero, M.J. Resistance Patterns to C and D Antibiotic Categories for Veterinary Use of Campylobacter spp., Escherichia coli and Enterococcus spp. Commensal Isolates from Laying Hen Farms in Spain during 2018. Prev. Veter-Med. 2021, 186, 105222. [Google Scholar] [CrossRef]
- Lopez-Chavarrias, V.; Ugarte-Ruiz, M.; Barcena, C.; Olarra, A.; Garcia, M.; Saez, J.L.; de Frutos, C.; Serrano, T.; Perez, I.; Moreno, M.A.; et al. Monitoring of Antimicrobial Resistance to Aminoglycosides and Macrolides in Campylobacter coli and Campylobacter jejuni From Healthy Livestock in Spain (2002–2018). Front. Microbiol. 2021, 12, 689262. [Google Scholar] [CrossRef]
- Rivera-Gomis, J.; Marín, P.; Martínez-Conesa, C.; Otal, J.; Jordán, M.J.; Escudero, E.; Cubero, M.J. Antimicrobial Resistance of Campylobacter jejuni, Escherichia coli and Enterococcus faecalis Commensal Isolates from Laying Hen Farms in Spain. Animals 2021, 11, 1284. [Google Scholar] [CrossRef]
- Ocejo, M.; Oporto, B.; Hurtado, A. Occurrence of Campylobacter jejuni and Campylobacter coli in Cattle and Sheep in Northern Spain and Changes in Antimicrobial Resistance in Two Studies 10-Years Apart. Pathogens 2019, 8, 98. [Google Scholar] [CrossRef]
- Ugarte-Ruiz, M.; Domínguez, L.; Corcionivoschi, N.; Wren, B.W.; Dorrell, N.; Gundogdu, O. Exploring the Oxidative, Antimicrobial and Genomic Properties of Campylobacter jejuni Strains Isolated from Poultry. Res. Vet. Sci. 2018, 119, 170–175. [Google Scholar] [CrossRef]
- García-Sánchez, L.; Melero, B.; Diez, A.M.; Jaime, I.; Rovira, J. Characterization of Campylobacter Species in Spanish Retail from Different Fresh Chicken Products and Their Antimicrobial Resistance. Food Microbiol. 2018, 76, 457–465. [Google Scholar] [CrossRef]
- Torralbo, A.; Borge, C.; García-Bocanegra, I.; Méric, G.; Perea, A.; Carbonero, A. Higher Resistance of Campylobacter coli Compared to Campylobacter jejuni at Chicken Slaughterhouse. Comp. Immunol. Microbiol. Infect. Dis. 2015, 39, 47–52. [Google Scholar] [CrossRef]
- Pérez-Boto, D.; García-Peña, F.J.; Abad-Moreno, J.C.; Echeita, M.A. Antimicrobial Susceptibilities of Campylobacter jejuni and Campylobacter coli Strains Isolated from Two Early Stages of Poultry Production. Microb. Drug Resist. 2013, 19, 323–330. [Google Scholar] [CrossRef]
- Hansson, I.; Ellström, P.; Nilsson, O.; Chaba, M.; Skarin, M.; Fernström, L.-L.; Frosth, S. Differences in Genotype and Antimicrobial Resistance between Campylobacter spp. Isolated from Organic and Conventionally Produced Chickens in Sweden. Pathogens 2021, 10, 1630. [Google Scholar] [CrossRef]
- Wu, Z.; Sippy, R.; Sahin, O.; Plummer, P.; Vidal, A.; Newell, D.; Zhang, Q. Genetic Diversity and Antimicrobial Susceptibility of Campylobacter jejuni Isolates Associated with Sheep Abortion in the United States and Great Britain. J. Clin. Microbiol. 2014, 52, 1853–1861. [Google Scholar] [CrossRef]
- Deckert, A.E.; Reid-Smith, R.J.; Tamblyn, S.E.; Morrell, L.; Seliske, P.; Jamieson, F.B.; Irwin, R.; Dewey, C.E.; Boerlin, P.; McEwen, S.A. Antimicrobial Resistance and Antimicrobial Use Associated with Laboratory-Confirmed Cases of Campylobacter Infection in Two Health Units in Ontario. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, e16. [Google Scholar] [CrossRef]
- Gaudreau, C.; Boucher, F.; Gilbert, H.; Bekal, S. Antimicrobial Susceptibility of Campylobacter jejuni and Campylobacter coli Isolates Obtained in Montreal, Quebec, Canada, from 2002 to 2013. J. Clin. Microbiol. 2014, 52, 2644–2646. [Google Scholar] [CrossRef]
- Riley, A.; Eshaghi, A.; Olsha, R.; Allen, V.G.; Patel, S.N. Antibiotic Susceptibility of Clinical Isolates of Campylobacter jejuni and Campylobacter coli in Ontario, Canada during 2011–2013. Diagn. Microbiol. Infect. Dis. 2015, 83, 292–294. [Google Scholar] [CrossRef]
- Scott, L.; Menzies, P.; Reid-Smith, R.J.; Avery, B.P.; McEwen, S.A.; Moon, C.S.; Berke, O. Antimicrobial Resistance in Campylobacter spp. Isolated from Ontario Sheep Flocks and Associations between Antimicrobial Use and Antimicrobial Resistance. Zoonoses Public Health 2012, 59, 294–301. [Google Scholar] [CrossRef]
- Webb, A.L.; Selinger, L.B.; Taboada, E.N.; Inglis, G.D. Subtype-Specific Selection for Resistance to Fluoroquinolones but Not to Tetracyclines Is Evident in Campylobacter jejuni Isolates from Beef Cattle in Confined Feeding Operations in Southern Alberta, Canada. Appl. Environ. Microbiol. 2018, 84, e02713-17. [Google Scholar] [CrossRef]
- Varga, C.; Guerin, M.T.; Brash, M.L.; Slavic, D.; Boerlin, P.; Susta, L. Antimicrobial Resistance in Campylobacter jejuni and Campylobacter coli Isolated from Small Poultry Flocks in Ontario, Canada: A Two-Year Surveillance Study. PLoS ONE 2019, 14, e0221429. [Google Scholar] [CrossRef]
- Stone, D.M.; Chander, Y.; Bekele, A.Z.; Goyal, S.M.; Hariharan, H.; Tiwari, K.; Chikweto, A.; Sharma, R. Genotypes, Antibiotic Resistance, and ST-8 Genetic Clone in Campylobacter Isolates from Sheep and Goats in Grenada. Veter-Med. Int. 2014, 2014, 212864. [Google Scholar] [CrossRef]
- Benoit, S.R.; Lopez, B.; Arvelo, W.; Henao, O.; Parsons, M.B.; Reyes, L.; Moir, J.C.; Lindblade, K. Burden of Laboratory-Confirmed Campylobacter Infections in Guatemala 2008–2012: Results from a Facility-Based Surveillance System. J. Epidemiol. Glob. Health 2013, 4, 51. [Google Scholar] [CrossRef][Green Version]
- Zaidi, M.B.; McDermott, P.F.; Campos, F.D.; Chim, R.; Leon, M.; Vazquez, G.; Figueroa, G.; Lopez, E.; Contreras, J.; Estrada-Garcia, T. Antimicrobial-Resistant Campylobacter in the Food Chain in Mexico. Foodborne Pathog. Dis. 2012, 9, 841–847. [Google Scholar] [CrossRef]
- Geissler, A.L.; Bustos Carrillo, F.; Swanson, K.; Patrick, M.E.; Fullerton, K.E.; Bennett, C.; Barrett, K.; Mahon, B.E. Increasing Campylobacter Infections, Outbreaks, and Antimicrobial Resistance in the United States, 2004–2012. Clin. Infect. Dis. 2017, 65, 1624–1631. [Google Scholar] [CrossRef]
- Noormohamed, A.; Fakhr, M.K. Prevalence and Antimicrobial Susceptibility of Campylobacter spp. in Oklahoma Conventional and Organic Retail Poultry. Open Microbiol. J. 2014, 8, 130–137. [Google Scholar] [CrossRef][Green Version]
- Whitehouse, C.A.; Young, S.; Li, C.; Hsu, C.-H.; Martin, G.; Zhao, S. Use of Whole-Genome Sequencing for Campylobacter Surveillance from NARMS Retail Poultry in the United States in 2015. Food Microbiol. 2018, 73, 122–128. [Google Scholar] [CrossRef]
- Bailey, M.A.; Taylor, R.M.; Brar, J.S.; Corkran, S.C.; Velásquez, C.; Novoa Rama, E.; Oliver, H.F.; Singh, M. Prevalence and Antimicrobial Resistance of Campylobacter from Antibiotic-Free Broilers during Organic and Conventional Processing. Poult. Sci. 2019, 98, 1447–1454. [Google Scholar] [CrossRef]
- Beier, R.C.; Byrd, J.A.; Andrews, K.; Caldwell, D.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Disinfectant and Antimicrobial Susceptibility Studies of the Foodborne Pathogen Campylobacter jejuni Isolated from the Litter of Broiler Chicken Houses. Poult. Sci. 2021, 100, 1024–1033. [Google Scholar] [CrossRef]
- Poudel, S.; Li, T.; Chen, S.; Zhang, X.; Cheng, W.-H.; Sukumaran, A.T.; Kiess, A.S.; Zhang, L. Prevalence, Antimicrobial Resistance, and Molecular Characterization of Campylobacter Isolated from Broilers and Broiler Meat Raised without Antibiotics. Microbiol. Spectr. 2022, 10, e0025122. [Google Scholar] [CrossRef]
- de Moura, H.M.; Silva, P.R.; da Silva, P.H.C.; Souza, N.R.; Racanicci, A.M.C.; Santana, Â.P. Antimicrobial Resistance of Campylobacter jejuni Isolated from Chicken Carcasses in the Federal District, Brazil. J. Food Prot. 2013, 76, 691–693. [Google Scholar] [CrossRef]
- Ferro, I.D.; Benetti, T.M.; Oliveira, T.C.R.M.; Abrahão, W.M.; Farah, S.M.S.S.; Luciano, F.B.; Macedo, R.E.F. Evaluation of Antimicrobial Resistance of Campylobacter spp. Isolated from Broiler Carcasses. Br. Poult. Sci. 2015, 56, 66–71. [Google Scholar] [CrossRef]
- Sierra-Arguello, Y.M.; Perdoncini, G.; Morgan, R.B.; Salle, C.T.P.; Moraes, H.L.S.; Gomes, M.J.P.; do Nascimento, V.P. Fluoroquinolone and Macrolide Resistance in Campylobacter jejuni Isolated from Broiler Slaughterhouses in Southern Brazil. Avian Pathol. 2016, 45, 66–72. [Google Scholar] [CrossRef]
- Ramires, T.; de Oliveira, M.G.; Kleinubing, N.R.; de Fátima Rauber Würfel, S.; Mata, M.M.; Iglesias, M.A.; Lopes, G.V.; Dellagostin, O.A.; da Silva, W.P. Genetic Diversity, Antimicrobial Resistance, and Virulence Genes of Thermophilic Campylobacter Isolated from Broiler Production Chain. Braz. J. Microbiol. 2020, 51, 2021–2032. [Google Scholar] [CrossRef]
- Dias, T.S.; Nascimento, R.J.; Machado, L.S.; Abreu, D.L.C.; do Nascimento, E.R.; Pereira, V.L.A.; de Aquino, M.H.C. Comparison of Antimicrobial Resistance in Thermophilic Campylobacter Strains Isolated from Conventional Production and Backyard Poultry Flocks. Br. Poult. Sci. 2021, 62, 188–192. [Google Scholar] [CrossRef]
- Lapierre, L.; Gatica, M.A.; Riquelme, V.; Vergara, C.; Yañez, J.M.; San Martín, B.; Sáenz, L.; Vidal, M.; Martínez, M.C.; Araya, P.; et al. Characterization of Antimicrobial Susceptibility and Its Association with Virulence Genes Related to Adherence, Invasion, and Cytotoxicity in Campylobacter jejuni and Campylobacter coli Isolates from Animals, Meat, and Humans. Microb. Drug Resist. 2016, 22, 432–444. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Orellana, D.; Martínez, C.; García Mena, V. Caracterización de Cepas de Campylobacter jejuni Obtenidas Desde Carne de Pollo y Heces de Aves de Corral de La Zona Central de Chile. Rev. Med. Chil. 2017, 145, 1551–1558. [Google Scholar] [CrossRef]
- Vinueza-Burgos, C.; Wautier, M.; Martiny, D.; Cisneros, M.; Van Damme, I.; De Zutter, L. Prevalence, Antimicrobial Resistance and Genetic Diversity of Campylobacter coli and Campylobacter jejuni in Ecuadorian Broilers at Slaughter Age. Poult. Sci. 2017, 96, 2366–2374. [Google Scholar] [CrossRef]
- Lluque, A.; Riveros, M.; Prada, A.; Ochoa, T.J.; Ruiz, J. Virulence and Antimicrobial Resistance in Campylobacter spp. from a Peruvian Pediatric Cohort. Scientifica 2017, 2017, 7848926. [Google Scholar] [CrossRef]
- Schiaffino, F.; Colston, J.M.; Paredes-Olortegui, M.; François, R.; Pisanic, N.; Burga, R.; Peñataro-Yori, P.; Kosek, M.N. Antibiotic Resistance of Campylobacter Species in a Pediatric Cohort Study. Antimicrob. Agents Chemother. 2019, 63, e01911-18. [Google Scholar] [CrossRef]
- Devi, A.; Mahony, T.J.; Wilkinson, J.M.; Vanniasinkam, T. Antimicrobial Susceptibility of Clinical Isolates of Campylobacter jejuni from New South Wales, Australia. J. Glob. Antimicrob. Resist. 2019, 16, 76–80. [Google Scholar] [CrossRef]
- Wallace, R.L.; Bulach, D.; McLure, A.; Varrone, L.; Jennison, A.V.; Valcanis, M.; Smith, J.J.; Polkinghorne, B.G.; Glass, K.; Kirk, M.D. Antimicrobial Resistance of Campylobacter spp. Causing Human Infection in Australia: An International Comparison. Microb. Drug Resist. 2021, 27, 518–528. [Google Scholar] [CrossRef]
- Obeng, A.S.; Rickard, H.; Sexton, M.; Pang, Y.; Peng, H.; Barton, M. Antimicrobial Susceptibilities and Resistance Genes in Campylobacter Strains Isolated from Poultry and Pigs in Australia. J. Appl. Microbiol. 2012, 113, 294–307. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Kubota, K.; Kasuga, F.; Iwasaki, E.; Shunichiinagaki; Sakurai, Y.; Komatsu, M.; Toyofuku, H.; Angulo, F.J.; Scallan, E.; Morikawa, K. Estimating the Burden of Acute Gastroenteritis and Foodborne Illness Caused by Campylobacter, Salmonella, and Vibrio Parahaemolyticus by Using Population-Based Telephone Survey Data, Miyagi Prefecture, Japan, 2005 to 2006. J. Food Prot. 2011, 74, 1592–1598. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Salam, A.; Al-Amin, Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional Methods and Future Trends in Antimicrobial Susceptibility Testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Hoque, N.; Islam, S.S.; Uddin, M.N.; Arif, M.; Haque, A.K.M.Z.; Neogi, S.B.; Hossain, M.M.; Yamasaki, S.; Kabir, S.M.L. Prevalence, Risk Factors, and Molecular Detection of Campylobacter in Farmed Cattle of Selected Districts in Bangladesh. Pathogens 2021, 10, 313. [Google Scholar] [CrossRef]
- Barata, A.R.; Nunes, B.; Oliveira, R.; Guedes, H.; Almeida, C.; Saavedra, M.J.; da Silva, G.J.; Almeida, G. Occurrence and Seasonality of Campylobacter spp. in Portuguese Dairy Farms. Int. J. Food Microbiol. 2022, 383, 109961. [Google Scholar] [CrossRef]
- Zbrun, M.V.; Rossler, E.; Romero-Scharpen, A.; Soto, L.P.; Berisvil, A.; Zimmermann, J.A.; Fusari, M.L.; Signorini, M.L.; Frizzo, L.S. Worldwide Meta-Analysis of the Prevalence of Campylobacter in Animal Food Products. Res. Veter-Sci. 2020, 132, 69–77. [Google Scholar] [CrossRef]
- Wagenaar, J.A.; French, N.P.; Havelaar, A.H. Preventing Campylobacter at the Source: Why Is It So Difficult? Clin. Infect. Dis. 2013, 57, 1600–1606. [Google Scholar] [CrossRef]
- Skarp, C.P.A.; Hänninen, M.-L.; Rautelin, H.I.K. Campylobacteriosis: The Role of Poultry Meat. Clin. Microbiol. Infect. 2016, 22, 103–109. [Google Scholar] [CrossRef]
- Mate, I.; Come, C.E.; Gonçalves, M.P.; Cliff, J.; Gudo, E.S. Knowledge, Attitudes and Practices Regarding Antibiotic Use in Maputo City, Mozambique. PLoS ONE 2019, 14, e0221452. [Google Scholar] [CrossRef]
- Moreno, P.; Cerón, A.; Sosa, K.; Morales, M.; Grajeda, L.M.; Lopez, M.R.; McCraken, J.P.; Cordón-Rosales, C.; Palmer, G.H.; Call, D.R.; et al. Availability of Over-the-Counter Antibiotics in Guatemalan Corner Stores. PLoS ONE 2020, 15, e0239873. [Google Scholar] [CrossRef]
- Rojop, N.; Moreno, P.; Grajeda, L.; Romero, J.; Reynoso, L.; Muñoz, E.; Palmer, G.H.; Cordón-Rosales, C.; Call, D.R.; Ramay, B.M. Informal Sale of Antibiotics in Guatemalan Convenience Stores before and after Implementation of Federal Antibiotic Dispensing Legislation. BMC Pharmacol. Toxicol. 2024, 25, 11. [Google Scholar] [CrossRef]
- Chang, D.; Mao, Y.; Qiu, W.; Wu, Y.; Cai, B. The Source and Distribution of Tetracycline Antibiotics in China: A Review. Toxics 2023, 11, 214. [Google Scholar] [CrossRef]
- Duarte, A.; Pereira, L.; Lemos, M.-L.; Pinto, M.; Rodrigues, J.C.; Matias, R.; Santos, A.; Oleastro, M. Epidemiological Data and Antimicrobial Resistance of Campylobacter spp. in Portugal from 13 Years of Surveillance. Pathogens 2024, 13, 147. [Google Scholar] [CrossRef]
- Belhadj, B.; Kaabi, F. Mathematiques; Editions Universitaires Europeennes: Paris, France, 2020; ISBN 6139572991. [Google Scholar]
- Meyerhoff, A.; Albrecht, R.; Meyer, J.M.; Dionne, P.; Higgins, K.; Murphy, D. US Food and Drug Administration Approval of Ciprofloxacin Hydrochloride for Management of Postexposure Inhalational Anthrax. Clin. Infect. Dis. 2004, 39, 303–308. [Google Scholar] [CrossRef]
- Apangu, T.; Griffith, K.; Abaru, J.; Candini, G.; Apio, H.; Okoth, F.; Okello, R.; Kaggwa, J.; Acayo, S.; Ezama, G.; et al. Successful Treatment of Human Plague with Oral Ciprofloxacin. Emerg. Infect. Dis. 2017, 23, 553–555. [Google Scholar] [CrossRef]
- Unemo, M.; Lahra, M.M.; Cole, M.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.-A.R.; Ramon-Pardo, P.; Bolan, G.; Wi, T. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): Review of New Data and Evidence to Inform International Collaborative Actions and Research Efforts. Sex. Health 2019, 16, 412. [Google Scholar] [CrossRef]
- Inglis, G.D.; Taboada, E.N.; Boras, V.F. Rates of Fluoroquinolone Resistance in Domestically Acquired Campylobacter jejuni Are Increasing in People Living within a Model Study Location in Canada. Can. J. Microbiol. 2021, 67, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Bruyndonckx, R.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S.; Strauss, R.; et al. Consumption of Quinolones in the Community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76, ii37–ii44. [Google Scholar] [CrossRef] [PubMed]
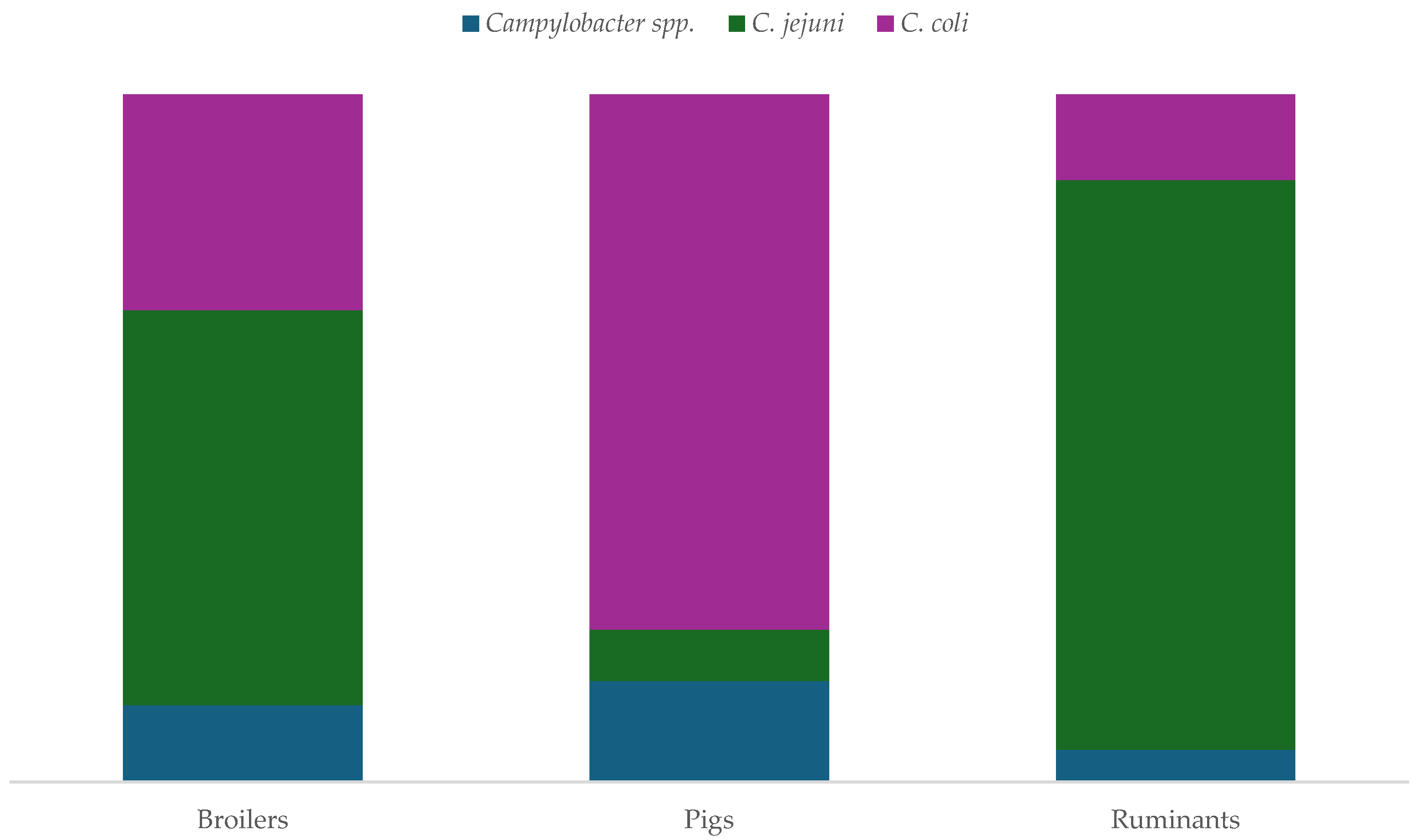


| Country | No. of Isolates | Aim of Sampling | First Study | Last Study | AMR Increase | AMR Decrease | Higher in Last Study | % of MDR | Source |
|---|---|---|---|---|---|---|---|---|---|
| Benin | 109 from animals | Focused sampling | 2022 | 2022 | Na | Na | TET | 90.8 | [16] |
| Ethiopia | 48 from humans | Passive surveillance of diagnostic samples Structured survey | 2014 | 2021 | CIP ERY GEN TET | CHL | TET | Nd | [17,18] |
| 91 from animals | Structured survey Focused sampling Monitoring sample | 2021 | 2022 | CHL | CIP TET | CHL | 84.2 | [18,19,20] | |
| Ivory Coast | 76 from animals | Focused sampling | 2012 | 2012 | Na | Na | CIP | Nd | [21] |
| Kenya | 18 from humans | Passive surveillance of diagnostic samples | 2021 | 2021 | Na | Na | ERY | Nd | [22] |
| 35 from animals | Focused sampling | 2016 | 2016 | Na | Na | CIP | 54.3 | [23] | |
| Morocco | 143 from animals | Focused sampling | 2020 | 2020 | Na | Na | TET | Nd | [24] |
| South Africa | 464 from animals | Monitoring samples | 2020 | 2021 | CHL CIP ERY GEN TET | None | ERY | 87.3 | [25,26] |
| Tanzania | 136 from humans | Passive surveillance of diagnostic samples | 2015 | 2015 | Na | Na | ERY | Nd | [27] |
| 134 from animals | Focused sampling Survey sampling | 2015 | 2016 | CIP ERY | GEN | ERY | 47.6 (in 2016) | [28,29] | |
| Tunisia | 180 from animals | Focused sampling Monitoring samples | 2018 | 2022 | Na | CIP ERY | TET | 37.5 (in 2022) | [30,31] |
| Country | No. of Isolates | Aim of Sampling | First Study | Last Study | AMR Increase | AMR Decrease | Higher in Last Study | % of MDR | Source |
|---|---|---|---|---|---|---|---|---|---|
| China | 805 from humans | Passive surveillance of diagnostic samples Monitoring samples Focused sampling | 2014 | 2022 | TET | CHL CIP ERY GEN | TET | Nd | [32,33,34,35,36] |
| 755 from animals | Focused sampling Structured surveys Monitoring samples | 2014 | 2022 | ERY GEN | CHL CIP TET | CIP | 90.4 (in 2021) | [32,37,38,39,40] | |
| India | 36 from humans | Passive surveillance of diagnostic samples | 2013 | 2013 | Na | Na | CIP | Nd | [41] |
| 508 from animals | Focused sampling | 2018 | 2021 | CIP ERY GEN TET | Na | TET | 41.5 (in 2021) | [42,43] | |
| Japan | 430 from humans | Passive surveillance of diagnostic samples | 2019 | 2019 | Na | Na | CIP | Nd | [44] |
| 602 from animals | Structured surveys Focused sampling | 2012 | 2017 | Na | CHL ERY | TET | Nd | [45,46,47,48] | |
| Jordan | 38 from humans | Monitoring samples | 2012 | 2012 | Na | Na | CIP ERY TET | Nd | [49] |
| Korea | 121 from humans | Passive surveillance of diagnostic samples | 2013 | 2013 | Na | None | ERY | 87.3 | [50] |
| 1721 from animals | Focused sampling Monitoring samples Structured surveys | 2017 | 2021 | CHL CIP ERY GEN TET | None | CIP | 75.5 (in 2021) | [51,52,53,54,55,56] | |
| Pakistan | 80 from humans | Focused sampling | 2018 | 2018 | Na | Na | TET | Nd | [57] |
| Philippines | 251 from animals | Focused sampling Monitoring samples | 2014 | 2017 | ERY GEN TET | Na | TET | 71.4 (in 2014) | [58,59] |
| Thailand | 215 from animals | Focused sampling Structured surveys | 2013 | 2021 | CIP ERY GEN TET | Na | CIP | 15.3 (in 2021) | [60,61] |
| Country | No. of Isolates | Aim of Sampling | First Study | Last Study | AMR Increase | AMR Decrease | Higher in Last Study | % of MDR | Source |
|---|---|---|---|---|---|---|---|---|---|
| Austria | 55 from animals | Focused sampling | 2016 | 2016 | Na | Na | CIP | 12.7 | [62] |
| Belgium | 472 from humans | Passive surveillance of diagnostic samples | 2017 | 2020 | Na | CIP ERY TET | CIP | Nd | [63,64,65] |
| 249 from animals | Focused sampling | 2018 | 2019 | CIP ERY TET | GEN | TET | 5.4 (in 2018) | [64,66] | |
| Croatia | 65 from humans | Passive surveillance of diagnostic samples | 2020 | 2022 | Na | CIP TET | CIP | Nd | [67,68] |
| 51 from animals | Focused sampling | 2020 | 2020 | Na | Na | CIP | Nd | [67] | |
| Czechia | 23 from humans | Passive surveillance of diagnostic samples | 2018 | 2018 | Na | Na | CIP | Nd | [69] |
| 103 from animals | Focused sampling | 2018 | 2018 | Na | Na | CIP | 60.0 | ||
| Estonia | 15 from humans | Passive surveillance of diagnostic samples | 2022 | 2022 | Na | Na | CIP | Nd | [70] |
| 4 from animals | Focused sampling | 2022 | 2022 | Na | Na | Na | Nd | ||
| Finland | 95 from humans | Focused sampling | 2016 | 2016 | Na | Na | CIP | Nd | [71] |
| 579 from animals | Monitoring samples | 2016 | 2016 | Na | Na | CIP | 0.2 | ||
| France | 2416 from humans | Monitoring samples | 2015 | 2015 | Na | Na | CIP | Nd | [72] |
| 276 from animals | Focused sampling Structured surveys | 2015 | 2017 | ERY GEN TET | CIP | TET | 61.5 (2015) | [73,74] | |
| Germany | 737 from animals | Focused sampling Monitoring samples | 2012 | 2021 | CIP ERY GEN TET | Na | CIP | Nd | [75,76,77] |
| Greece | 276 from animals | Focused sampling Monitoring samples Structured surveys | 2015 | 2017 | ERY GEN TET | CIP | TET | 61.5 (in 2015) | [78,79,80,81,82,83,84] |
| Ireland | 96 from animals | Monitoring samples | 2022 | 2022 | Na | Na | TET | 15.6 | [85] |
| Italy | 51 from humans | Structured surveys | 2019 | 2019 | Na | Na | CIP | Nd | [86] |
| 1197 from animals | Structured surveys Focused sampling Monitoring samples | 2014 | 2021 | None | CHL CIP ERY GEN TET | TET | 61.8 | [87,88,89,90,91,92] | |
| Latvia | 51 from animals | Structured surveys Focused sampling | 2014 | 2022 | ERY GEN TET | Na | CIP | 12.5 | [70,93] |
| Lithuania | 26 from animals | Focused sampling | 2022 | 2022 | Na | Na | CIP | 38.5 | [70] |
| Poland | 297 from humans | Passive surveillance of diagnostic samples Structured surveys Focused sampling | 2017 | 2020 | CIP ERY GEN TET | Na | CIP | Nd | [94,95,96] |
| 3689 from animals | Structured surveys Focused sampling Monitoring samples | 2013 | 2022 | GEN | CIP ERY TET | TET | 8.1 (in 2022) | [94,95,97,98,99,100,101,102,103,104] | |
| Portugal | 763 from animals | Structured surveys Focused sampling | 2012 | 2022 | CIP TET | CHL ERY GEN | CIP | 98.5 (in 2022) | [105,106,107,108,109] |
| Romania | 111 from animals | Monitoring samples Passive surveillance of diagnostic samples | 2020 | 2022 | TET | CIP ERY | CIP | 6.9 (in 2022) | [110,111] |
| Spain | 139 from humans | Focused sampling | 2014 | 2021 | Na | CIP ERY GEN TEY | ERY | 3.8 (in 2021) | [112,113,114,115] |
| 5402 from animals | Monitoring samples Focused sampling Structured surveys | 2013 | 2021 | CIP ERY GEN TET | Na | TET | 12.5 (in 2021) | [113,114,115,116,117,118,119,120,121,122,123] | |
| Sweden | 215 from animals | Focused sampling | 2017 | 2021 | TET | CIP | CIP | Nd | [74,124] |
| UK | 41 from animals | Focused sampling | 2014 | 2014 | Na | Na | TET | Nd | [125] |
| Country | No. of Isolates | Aim of Sampling | First Study | Last Study | AMR Increase | AMR Decrease | Higher in Last Study | % of MDR | Source |
|---|---|---|---|---|---|---|---|---|---|
| Canada | 749 from humans | Passive surveillance of diagnostic samples | 2013 | 2015 | CIP ERY GEN TET | Na | TET | Nd | [126,127,128] |
| 1951 from animals | Structured surveys Monitoring samples Passive surveillance of diagnostic samples | 2012 | 2019 | CIP ERY GEN | TET | TET | 4.3 (in 2012) | [129,130,131] | |
| Grenada | 162 from animals | Structured surveys | 2014 | 2014 | Na | Na | Na | Nd | [132] |
| Guatemala | 161 from humans | Passive surveillance of diagnostic samples | 2013 | 2013 | Na | Na | TET | Nd | [133] |
| Mexico | 360 from humans | Structured surveys | 2012 | 2012 | Na | Na | CIP | Nd | [134] |
| 2698 from animals | Focused sampling | 2012 | 2012 | Na | Na | TET | Nd | ||
| USA | 11,726 from humans | Passive surveillance of diagnostic samples | 2017 | 2017 | Na | Na | CIP | Nd | [135] |
| 1396 from animals | Focused sampling Structured survey Passive surveillance of diagnostic samples | 2014 | 2022 | CIP ERY GEN TET | Na | TET | 16.7 (in 2022) | [125,135,136,137,138,139,140] |
| Country | No. of Isolates | Aim of Sampling | First Study | Last Study | AMR Increase | AMR Decrease | Higher in Last Study | % of MDR | Source |
|---|---|---|---|---|---|---|---|---|---|
| Brazil | 176 from animals | Focused sampling Monitoring samples | 2013 | 2020 | None | CHL CIP ERY GEN TET | CIP | 35.7 (in 2020) | [141,142,143,144,145] |
| Chile | 7 from humans | Structured surveys | 2016 | 2016 | Na | Na | CIP TET | Nd | [146] |
| 347 from animals | Focused sampling | 2016 | 2017 | ERY | CIP GEN TET | ERY | Nd | [146,147] | |
| Ecuador | 218 from animals | Structured surveys | 2017 | 2017 | Na | Na | CIP | Nd | [148] |
| Peru | 1032 from animals | Focused sampling Structured surveys | 2017 | 2019 | GEN | CIP ERY TET | CIP | Nd | [149,150] |
| Country | No. of Isolates | Type of Sampling | First Study | Last Study | AMR Increase | AMR Decrease | Higher in Last Study | % of MDR | Source |
|---|---|---|---|---|---|---|---|---|---|
| Australia | 281 from humans | Passive surveillance of diagnostic samples Structured surveys | 2019 | 2020 | ERY | CIP TET | CIP | Nd | [151,152] |
| 237 from animals | Monitoring samples | 2012 | 2012 | Na | Na | TET | Nd | [153] |
| Antimicrobial | C. jejuni EU Surveillance 2021 EUCAST ECOFF | C. coli EU Surveillance 2021 EUCAST ECOFF |
|---|---|---|
| Chloramphenicol (CHL) | >16 | >16 |
| Ciprofloxacin (CIP) | >0.5 | >0.5 |
| Erythromycin (ERY) | >4 | >8 |
| Gentamicin (GEN) | >2 | >2 |
| Tetracycline (TET) | >1 | >2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barata, R.; Saavedra, M.J.; Almeida, G. A Decade of Antimicrobial Resistance in Human and Animal Campylobacter spp. Isolates. Antibiotics 2024, 13, 904. https://doi.org/10.3390/antibiotics13090904
Barata R, Saavedra MJ, Almeida G. A Decade of Antimicrobial Resistance in Human and Animal Campylobacter spp. Isolates. Antibiotics. 2024; 13(9):904. https://doi.org/10.3390/antibiotics13090904
Chicago/Turabian StyleBarata, Rita, Maria José Saavedra, and Gonçalo Almeida. 2024. "A Decade of Antimicrobial Resistance in Human and Animal Campylobacter spp. Isolates" Antibiotics 13, no. 9: 904. https://doi.org/10.3390/antibiotics13090904
APA StyleBarata, R., Saavedra, M. J., & Almeida, G. (2024). A Decade of Antimicrobial Resistance in Human and Animal Campylobacter spp. Isolates. Antibiotics, 13(9), 904. https://doi.org/10.3390/antibiotics13090904








