Abstract
Background: Vancomycin is a first-line drug for the treatment of MRSA infection. However, overuse of vancomycin can cause bacteria to become resistant, forming resistant strains and making infections more difficult to treat. This study aimed to evaluate the efficacy and safety of different antibiotics in the treatment of MRSA infections and to compare them, mainly with vancomycin, to find better vancomycin alternatives. Methods: All studies were obtained from the PubMed and Embase databases from inception to 13 April 2023. The three comprehensive indicators of clinical cure success rate, clinical microbiological success rate, and adverse reactions were evaluated, and the clinical cure success rates of three disease types, complex skin and skin structure infections (cSSSIs), complex skin and soft tissue infections (cSSTIs), and pneumonia, were analyzed in subgroups. All statistical analyses were performed using R and STATA 14.0 software for network meta-analysis. Results: A total of 38 trials with 6281 patients were included, and 13 drug treatments were evaluated. For MRSA infections, the results of network meta-analysis showed that the clinical success rates of linezolid, the combination of vancomycin and rifampin, and the combination of minocycline and rifampin were better than that of vancomycin (RR 1.71; 95%-CI 1.45–2.02), (RR 2.46; 95%-CI 1.10–5.49) (RR, 2.77; 95%-CI 1.06–7.21). The success rate of clinical microbiological treatment with vancomycin was inferior to that with telavancin (RR 0.74; 95%-CI 0.55–0.99). Linezolid had a higher rate of adverse reactions than teicoplanin (RR 5.35; 95%-CI 1.10–25.98). Subgroup analysis showed that vancomycin had a lower clinical success rate than linezolid in the treatment of MRSA-induced cSSSIs, cSSTIs, and pneumonia (RR 0.59; 95%-CI 0.44–0.80) (RR 0.55; 95%-CI 0.35–0.89) (RR 0.55; 95%-CI 0.32–0.93). Conclusions: This systematic review and NMA provide a new comparison framework for the clinical treatment of MRSA infection. The NMA suggests that linezolid may be the antibiotic of choice for the treatment of MRSA infections, with the ability to improve clinical and microbiological success rates despite its disadvantage in terms of adverse effects. At the same time, the combination of minocycline and rifampicin may be the most effective drug to treat MRSA-induced cSSSIs, tedizolid may be the best drug to treat MRSA-induced cSSTIs, and the combination of vancomycin and rifampicin may be the most effective treatment for MRSA-induced pneumonia. More high-quality studies are still needed in the future to further identify alternatives to vancomycin. Trial registration: PROSPERO registration number CRD42023416788.
1. Background
Infection caused by methicillin-resistant Staphylococcus aureus (MRSA) was first identified in 1961 [1]. For nearly 60 years, because of the overuse of antibiotics, rates of hospital- and community-acquired infections caused by MRSA have risen [2,3]. At present, the main reasons for which MRSA infection has become a global problem include complex skin and skin structure infections (cSSSIs), complex skin and soft tissue infections (cSSTIs), pneumonia, and bacteremia. A study in the United States found that MRSA was isolated from nearly 60% of the patients with cSSSIs, which are among the most common treatment-associated infections in the medical system, with high morbidity and healthcare costs [4,5]. At the same time, MRSA infection accounts for 60% of cSSTIs, which are one of the fastest-growing causes of hospitalization and impose a large economic burden [6,7]. With this series of problems that threaten the lives and health of patients, medical institutions are in urgent need of more effective treatment programs to solve these problems.
Vancomycin is the most commonly used antibiotic in the treatment of MRSA infection, but the overuse of vancomycin has led to the emergence of resistant strains, influencing the treatment’s efficacy [8]. Currently, available antibiotics include rifampin, doxycycline, minocycline, ceftaroline, clindamycin, teicoplanin, TMP-SMX, vancomycin, daptomycin, tigecycline, quapritin/dalfopritin, and linezolid [9,10,11]. The European and American guidelines recommend vancomycin and linezolid as first-line treatments [12,13]. The curative effect of this class of antibiotics is mostly for patients with suspected or confirmed MRSA infection, as well as patients not diagnosed with MRSA infection.
In this study, we conducted a network meta-analysis (NMA) to compare the efficacy and safety of different antibiotics against vancomycin in the treatment of MRSA infection in order to determine which antibiotic is the best alternative to vancomycin.
2. Objective
The main objective of this review was to evaluate the efficacy and safety of clinical antibiotic drugs in patients with MRSA infection. In this paper, we comprehensively evaluate the efficacy and safety of antibiotic drugs in the clinical treatment of MRSA-infected patients. This study aims to guide the current clinical use of antibiotics for the treatment of MRSA infections.
3. Results
3.1. Study Selection
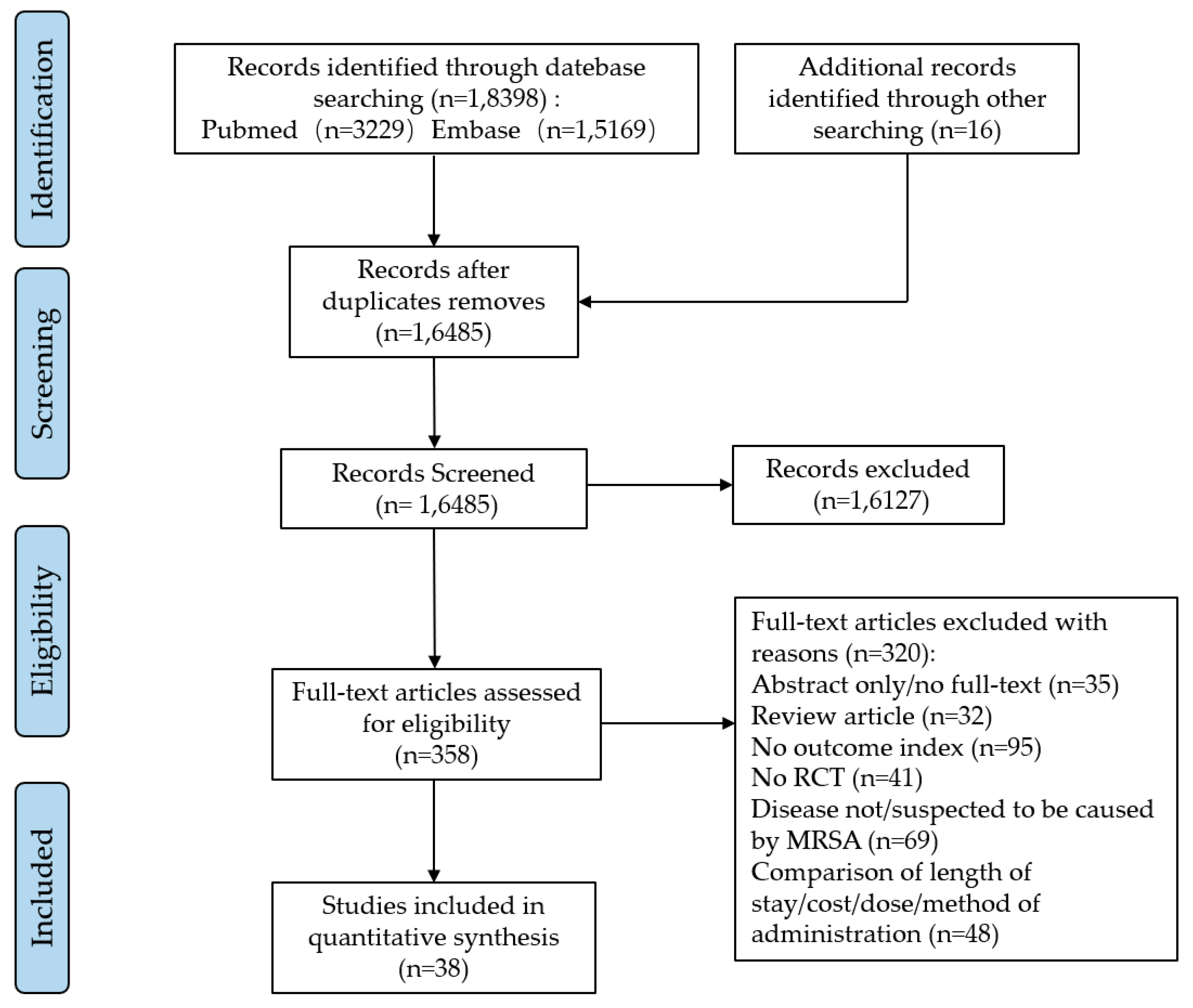
We searched the PubMed and Embase databases for a total of 18,398 potentially relevant articles. After excluding 1913 duplicate articles, the full-text contents of 358 articles were reviewed after reading the titles and abstracts. These articles were evaluated in full, and finally, we included 38 eligible studies that met the criteria (see Figure 1).

Figure 1.
The flowchart of literature filtering.
3.2. Study Characteristics
The main characteristics of the included studies are shown in Table 1, with 38 articles including a total of 6281 patients, and all study participants were patients with confirmed MRSA infection. For treatment of the related diseases caused by MRSA infection, a total of 17 antibiotics were evaluated as monotherapy or combination therapy.

Table 1.
Characteristics of the included studies (RCT: randomized controlled trial. AE: adverse event. CS: clinical success. ME: microbiologically evaluable. NP: nosocomial pneumonia.).
3.3. Bias Risk Assessment
Of the 38 articles included, 37 were clear RCT research studies. One of the studies was not an RCT and was not double-blinded, posing a high risk of bias. Overall, the included articles had a low-to-moderate risk of bias (see Figures S1 and S2).
3.4. The Clinical Cure Rate
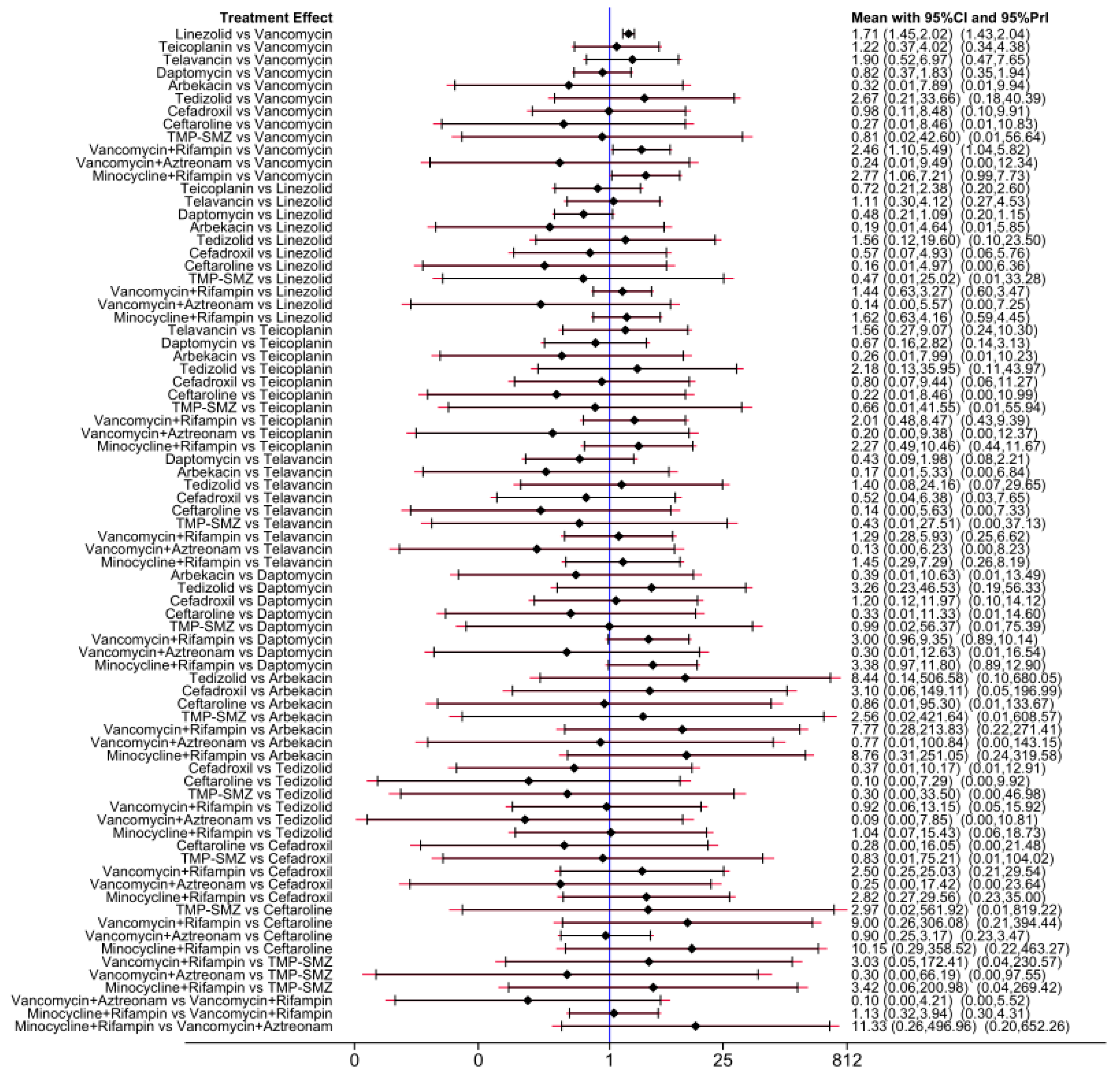
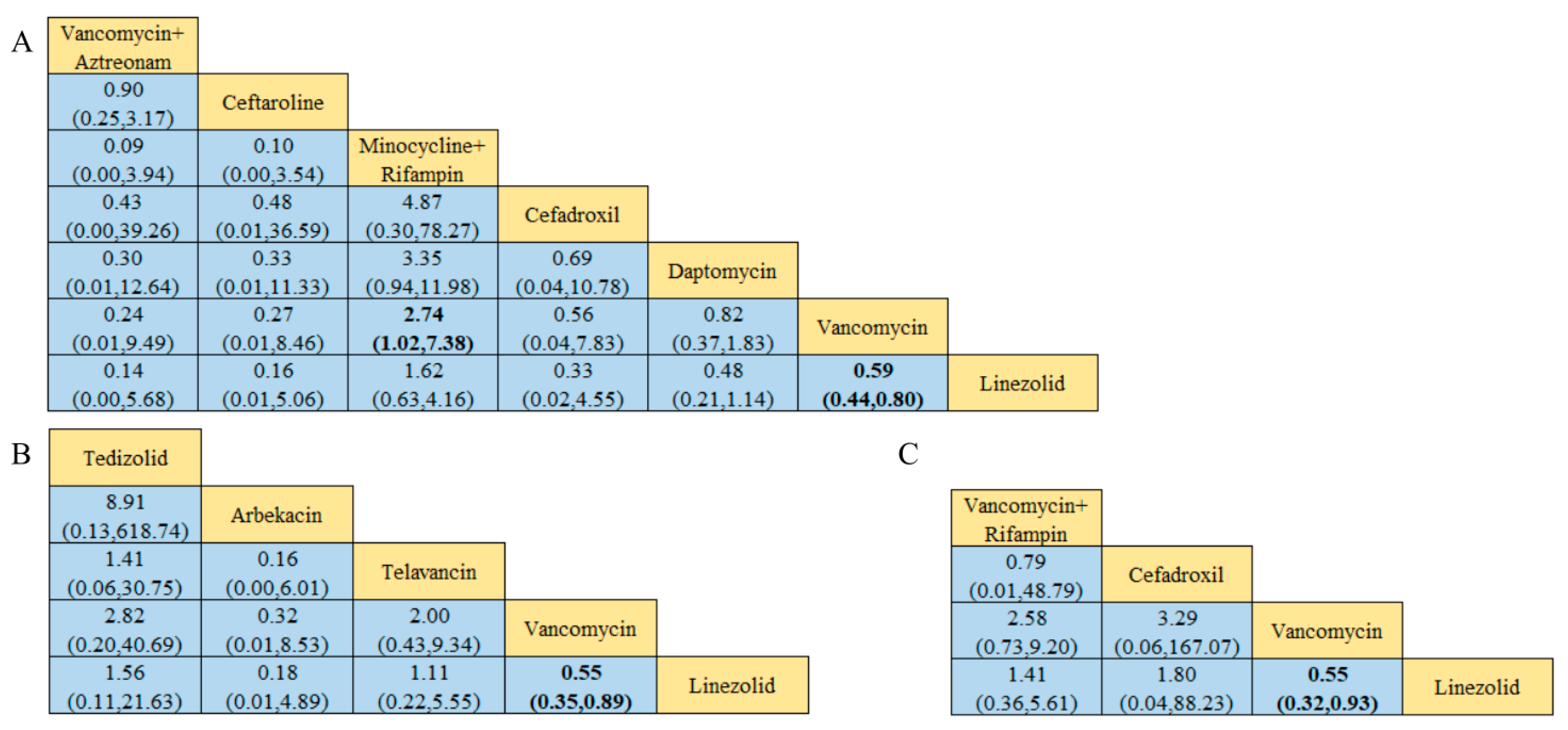
Of the 38 included articles, a total of 29 articles evaluated the clinical cure success of 4097 patients, 2825 of whom were treated successfully (Table 2). The direct meta-analysis results are shown in Figure S3. To further determine the direct and indirect comparative efficacy of these 13 antibiotics, we performed a network meta-analysis; the network evidence graph is shown in Figure 2A. The results showed that the clinical success rate of linezolid was better than that of vancomycin (RR 1.71; 95%-CI 1.45–2.02). The clinical success rate of vancomycin combined with rifampin was better than that of vancomycin alone (RR 2.46; 95%-CI 1.10–5.49). The clinical success rate of minocycline combined with rifampin was better than that of vancomycin (RR 2.77; 95%-CI 1.06 7.21), and the other antibiotics showed no statistical significance (see Figure 3 and Figure 4A).

Table 2.
Corresponding outcome indicators under different interventions and included references.

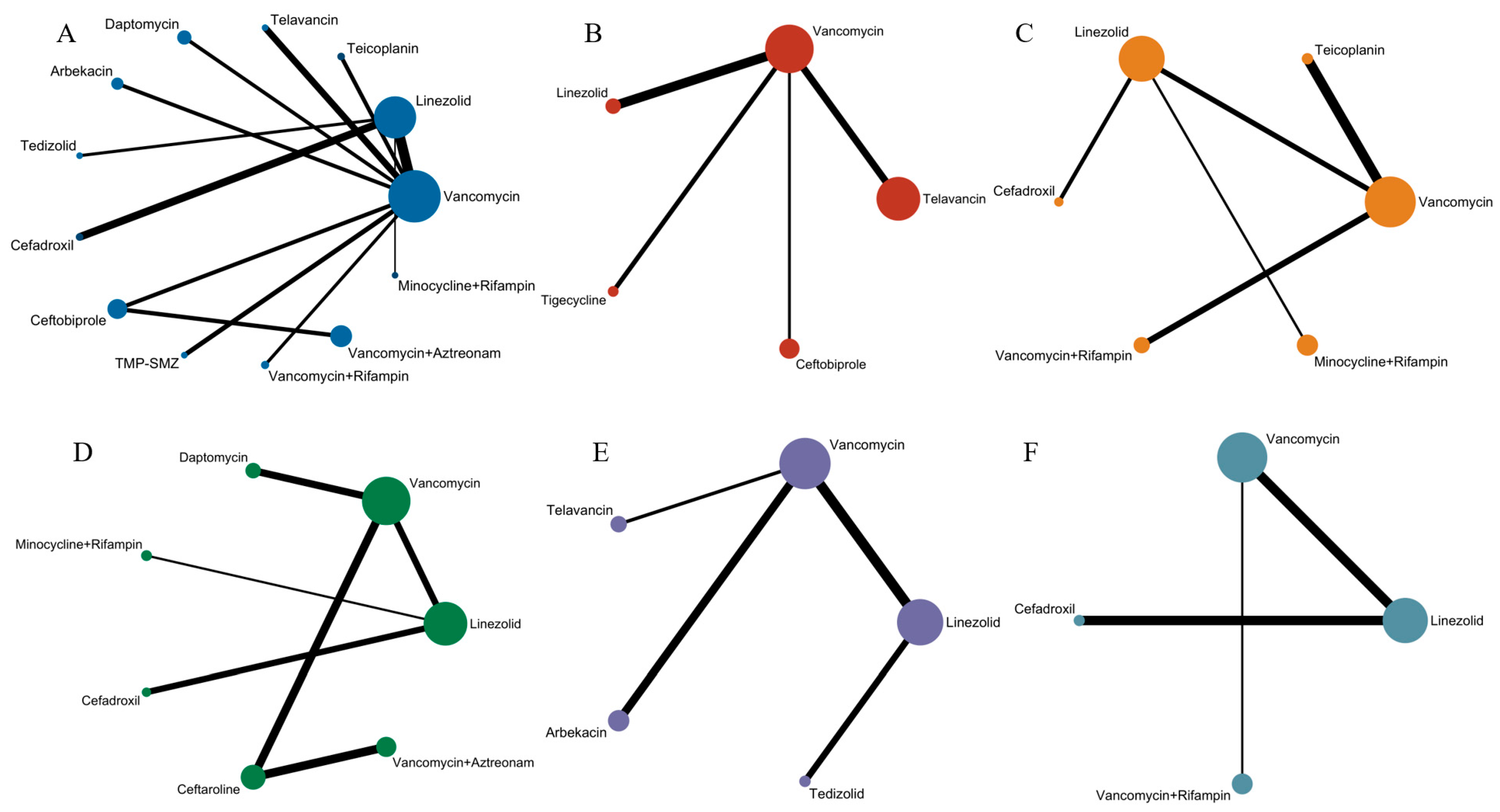
Figure 2.
Network evidence plot, where the size of nodes corresponds to the cumulative sample size for individual antibiotics, and the thickness of the lines is proportional to the number of studies for each treatment comparison: (A) Evidence chart of clinical cure rate of 13 treatment methods: minocycline and rifampin, vancomycin and rifampin, tedizolid, telavancin, linezolid, teicoplanin, cefadroxil, TMP-SMZ, vancomycin, daptomycin, arbekacin, ceftaroline, and vancomycin and aztreonam. (B) Evidence chart of clinical microbiological success rate of five treatment methods: linezolid, telavancin, ceftobiprole, tigecycline, and vancomycin. (C) Evidence chart of incidence of adverse effects of six treatment methods: linezolid, cefadroxil, minocycline and rifampin, vancomycin and rifampin, vancomycin, and teicoplanin. (D) Evidence chart of clinical cure rate of MRSA-induced cSSSIs of seven treatment methods: minocycline and rifampin, linezolid, vancomycin, daptomycin, cefadroxi, cefazolin, and vancomycin and aztreonam. (E) Evidence chart of clinical cure rate of MRSA-induced cSSTIs of five treatment methods: tedizolid, linezolid, telavancin, vancomycin, and arbekacin. (F) Evidence chart of clinical cure rate of patients with MRSA-induced pneumonia of four treatment methods: vancomycin and rifampin, cefadroxil, linezolid, and vancomycin.

Figure 3.
Forest plot of the network meta-analysis for clinical cure success. Includes 13 treatments: minocycline and rifampin, vancomycin and rifampin, tedizolid, telavancin, linezolid, teicoplanin, cefadroxil, TMP-SMZ, vancomycin, daptomycin, arbekacin, ceftaroline, and vancomycin and aztreonam.

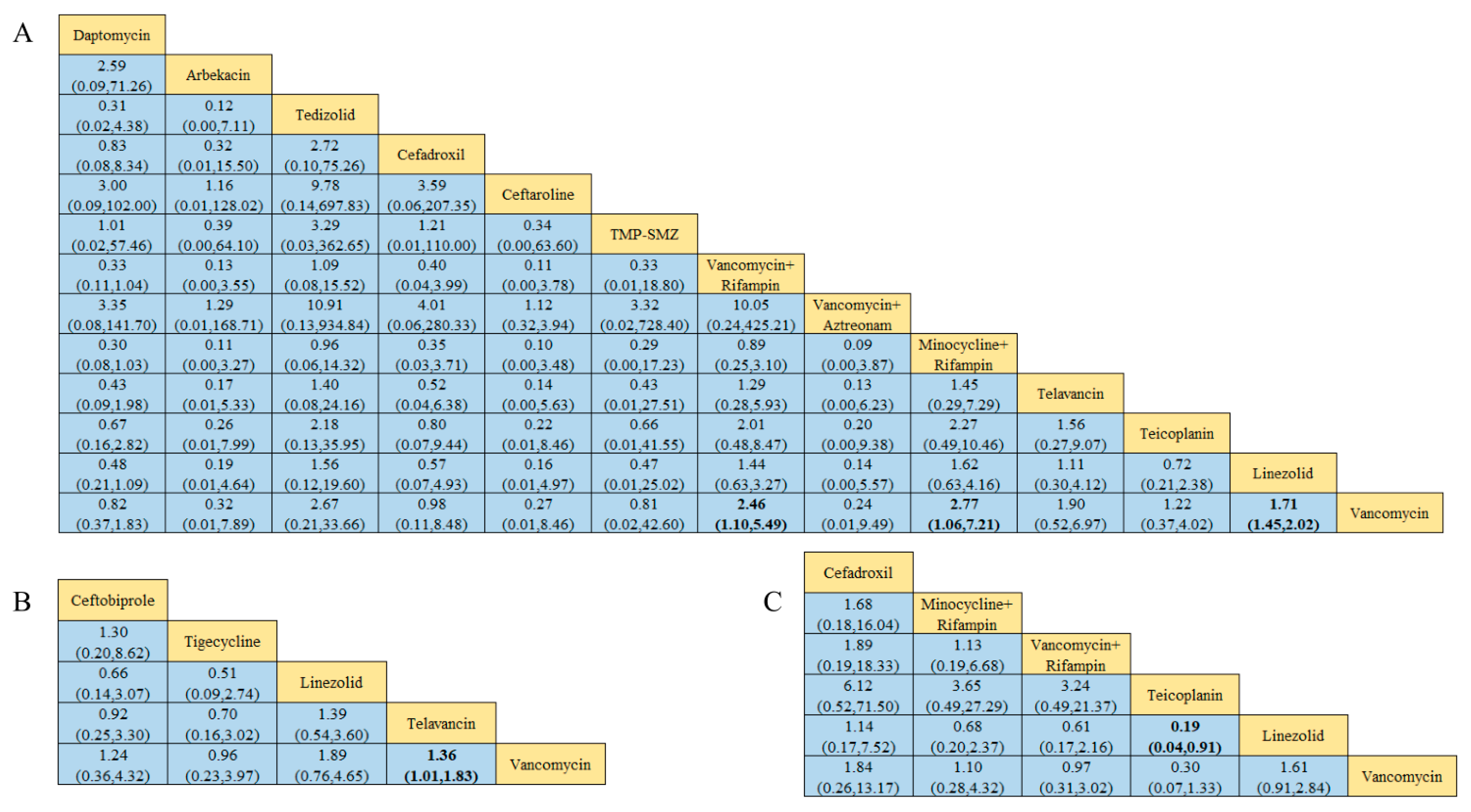
Figure 4.
Results of the network meta-analysis, with bold numbers indicating significant differences: (A) Clinical cure success rate. (B) Clinical microbiology success rate. (C) Incidence of adverse reactions.
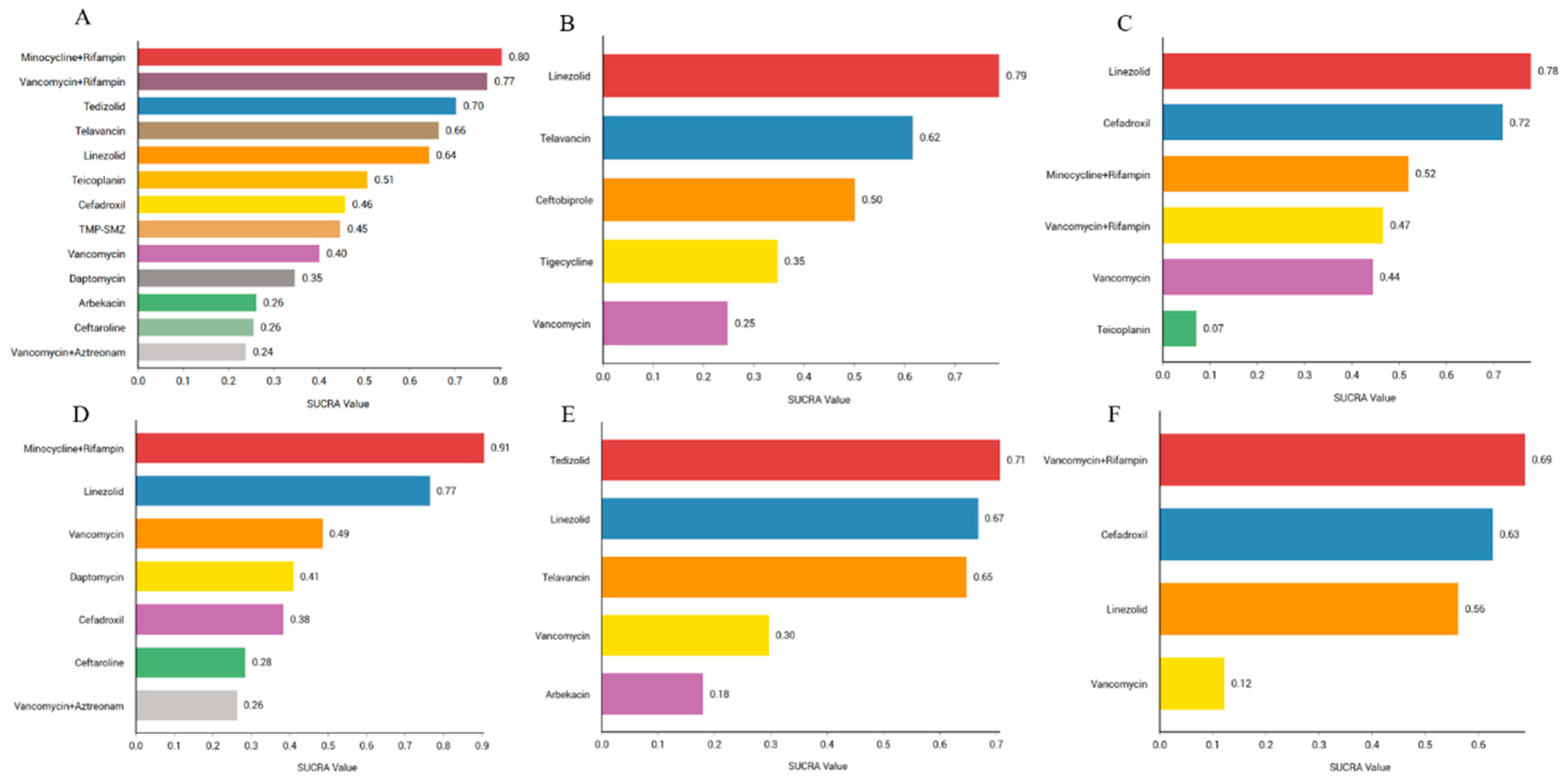
We sorted the antibiotics according to their probability of success in clinical cure. The results showed that the combination of minocycline and rifampin (80.4%) had the highest probability of being first, followed by the combination therapy of vancomycin and rifampin (77.2%), tedizolid (70.3%), telavancin (66.4%), linezolid (64.3%), teicoplanin (50.7%), cefadroxil (45.8%), TMP-SMZ (44.7%), vancomycin (40.1%), daptomycin (34. 7%), arbekacin (26.1%), ceftaroline (25.5%), and vancomycin and aztreonam (23.8%). Rank probability plots and rank cumulative probability plots for all antibiotics are shown in Figure 5A and Figure S14.
Figure 5.
Efficacy and safety rankings of drugs used to treat MRSA infections: (A) Ranking of clinical cure rates for 13 treatments: minocycline and rifampin, vancomycin and rifampin, tedizolid, telavancin, linezolid, teicoplanin, cefadroxil, TMP-SMZ, vancomycin, daptomycin, arbekacin, ceftaroline, and vancomycin and aztreonam. (B) Ranking of clinical microbiology success rates for five treatments: linezolid, telavancin, ceftobiprole, tigecycline, and vancomycin. (C) Ranking of incidence of adverse reactions for six treatments: linezolid, cefadroxil, minocycline and rifampin, vancomycin and rifampin, vancomycin, and teicoplanin. (D) Ranking of clinical cure rate of MRSA-induced cSSSIs for seven treatments: minocycline and rifampin, linezolid, vancomycin, daptomycin, cefadroxi, cefazolin, and vancomycin and aztreonam. (E) Ranking of clinical cure rate of MRSA-induced cSSTIs for five treatments: tedizolid, linezolid, telavancin, vancomycin, and arbekacin. (F) Ranking of clinical cure rate of patients with MRSA-induced pneumonia for four treatments: vancomycin and rifampin, cefadroxil, linezolid, and vancomycin.
3.5. Clinical Microbiology Success Rate
Of the 38 articles included, a total of nine articles, including 2289 cases, evaluated the clinical microbiological success, with 2001 cases of successful treatment (Table 2). The direct meta-analysis results are shown in Figure S4. To further compare the direct and indirect effects of five kinds of antibiotics, we conducted a network meta-analysis, and the evidence network diagram is shown in Figure 2B. The results showed that the success rate of clinical microbiological treatment with vancomycin was inferior to that with telavancin (RR 0.74; 95%-CI 0.55–0.99), which is consistent with the results of the direct meta-analysis, and no statistical significance was found for the remaining antibiotic comparisons (see Figure 4B and Figure S9).
We sorted the antibiotics according to their probability of success in clinical microbiology. The results showed that linezolid (78.8%) was the most likely to be ranked first, followed by telavancin (61.6%), ceftobiprole (50.1%), tigecycline (34.7%), and vancomycin (24.8%). Rank probability plots and rank cumulative probability plots for all antibiotics are shown in Figure 5B and Figure S15.
3.6. Incidence of Adverse Reactions
Among the 38 included articles, 10 articles evaluated the adverse reactions of 1536 patients, finding that they occurred in 1051 patients (Table 2). The results of the direct meta-analysis are shown in Figure S5. To further compare the direct and indirect efficacy of these six antibiotics, we performed a network meta-analysis; the network evidence graph is shown in Figure 2C. The evidence figure directly or indirectly compares the six kinds of antibiotic monotherapy or combination therapy. The results show that linezolid had a higher rate of adverse reactions than teicoplanin (RR 5.35; 95%-CI 1.10–25.98), and no statistical significance was found for the remaining antibiotics (see Figure 4C and Figure S10).
We ranked the antibiotics according to their probability of adverse effects. The results showed that linezolid (77.9%) had the highest probability, followed by cefadroxil (71.9%), minocycline combined with rifampin (52%), vancomycin combined with rifampin (46.6%), vancomycin (44.4%), and teicoplanin (7.1%). The rank probability diagram and the rank cumulative probability diagram for all antibiotics are shown in Figure 5C and Figure S16.
3.7. Clinical Cure Rate of Patients with MRSA-Induced cSSSIs
Of the 38 included articles, 10 reviewed the clinical cure rate of 1479 patients with MRSA-induced cSSSIs, of whom 1085 were successfully cured (Table 2). The results of the direct meta-analysis are shown in Figure S6. To further compare the direct and indirect efficacy of these seven antibiotics, we conducted a network meta-analysis, and the network evidence graph is shown in Figure 2D. The evidence map directly or indirectly compares monotherapy or combination therapy with seven antibiotics; the results show that the clinical success rate of vancomycin was worse than that of linezolid (RR 0.59; 95%-CI 0.44–0.80), while the clinical success rate of combined minocycline and rifampin treatment was superior to that of vancomycin (RR 2.74; 95%-CI 1.02–7.38), and no statistical significance was found for the remaining antibiotic comparisons (see Figure 6A and Figure S11).

Figure 6.
Graph of the results of the network meta-analysis, with bold numbers showing significant differences: (A) Clinical cure rate of MRSA-induced cSSSIs. (B) Clinical cure rate of MRSA-induced cSSTIs. (C) Clinical cure rate of patients with MRSA-induced pneumonia.
We ranked the antibiotics according to their probability of clinical cure. The results showed that the combination treatment with minocycline and rifampin (90.6%) ranked first, followed by linezolid (76.5%), vancomycin (48.6%), daptomycin (41%), cefadroxil (38.4%), ceftaroline (28.5%), and vancomycin and aztreonam (26.3%). The rank probability diagram and the rank cumulative probability diagram for all antibiotics are shown in Figure 5D and Figure S17.
3.8. Clinical Cure Rate of Patients with MRSA-Induced cSSTIs
Among the 38 included articles, a total of eight articles evaluated the clinical cure rate of 1397 patients with MRSA-induced cSSTIs, of whom 1055 were successfully cured (Table 2). The results of the direct meta-analysis are shown in Figure S7. To further compare the direct and indirect efficacy of these five antibiotics, we conducted a network meta-analysis, and the network evidence graph is shown in Figure 2E. The evidence map directly or indirectly compares the effects of monotherapy with five antibiotics; the results show that the clinical success rate of vancomycin was worse than that of linezolid (RR 0.55; 95%-CI 0.35–0.89), consistent with the results of the direct meta-analysis, while the remaining antibiotic comparisons were not found to be statistically significant (see Figure 6B and Figure S12).
We ranked the antibiotics according to their probability of clinical cure. The results showed that tedizolid (70.7%) was the most likely successful treatment, followed by linezolid (66.8%), telavancin (64.8%), vancomycin (29.7%), and arbekacin (18%). The rank probability diagram and the rank cumulative probability diagram for all antibiotics are shown in Figure 5E and Figure S18.
3.9. Clinical Cure Rate of MRSA-Induced Pneumonia Patients
Of the 38 included articles, seven evaluated the clinical cure rate in 955 patients with MRSA-induced pneumonia, of whom 449 were clinically cured (Table 2). The results of the direct meta-analysis are shown in Figure S8. To further compare the direct and indirect efficacy of these four antibiotics, we conducted a network meta-analysis, and the network evidence graph is shown in Figure 2F. The evidence map directly or indirectly compares the effects of the four antibiotics administered alone or in combination; the results show that the clinical success rate of vancomycin was worse than that of linezolid (RR 0.55; 95%-CI 0.32–0.93), consistent with the results of the direct meta-analysis, while the remaining antibiotic comparisons were not found to be statistically significant (see Figure 6C and Figure S13).
We ranked the antibiotics according to their probability of clinical cure. The results showed that the combination of vancomycin and rifampin (68.8%) ranked first, followed by cefadroxil (62.7%), linezolid (56.2%), and vancomycin (12.2%). The rank probability diagram and the rank cumulative probability diagram for all antibiotics are shown in Figure 5F and Figure S19.
3.10. Publication Bias and Inconsistency Evaluations
We observed the symmetry of the funnel plot to detect publication bias, finding that it had a generally symmetric distribution, and no significant publication bias was found in any of the results, while there were three points distributed outside the 95%-CI, indicating the possible influence of the small sample size (see Figures S20–S25). Heterogeneity or inconsistencies could not be assessed because no closed loops were present in any of the study networks.
4. Methods
4.1. Search Strategy
We systematically searched PubMed and EMBASE for potentially eligible studies (the database was established until April 2023) using Medical Subject Headings (MeSH) descriptors combined with free-text terms to retrieve two categories: MRSA and treatment drugs. In addition, the references of all included studies were manually searched, and a meta-analysis was performed on the same topic to identify additional eligible studies (see Tables S1–S3 in the Supplementary Materials).
4.2. Choice Criteria
This study included articles that complied with the following criteria: (1) RCTs or observational studies published in English. (2) Studies including patients diagnosed with MRSA infection (the diagnosis was confirmed by Gram staining, culture, and drug-sensitivity test), as well as patients with cSSSIs, cSSTIs, and pneumonia caused by MRSA infection, comparing antibiotics with anti-MRSA activity against one another. (3) The primary outcomes were the rate of clinical and microbiologic cure at the test-of-cure visit and the rate of adverse effects. In addition, we excluded relevant literature in which only antibiotic experiments were conducted.
4.3. Data Extraction
For each included study, data extraction was performed by two independent reviewers using a standardized data-extraction form, and the relevant study characteristics extracted included (1) the first author, (2) publication year, (3) research types, (4) disease types caused by MRSA infection, (5) the basic information of the patients, (6) medication information (i.e., medication method, dosage, and duration), (7) prognosis and efficacy (i.e., clinical and microbiological cure rates), and (8) adverse reactions.
4.4. Outcome Indicators
We considered clinical success, microbiological success, and adverse event (AE) outcomes in the network meta-analysis. First, the populations tested for clinical success included the intention-to-treat (ITT) population, the modified intention-to-treat (MITT) population, the clinically evaluable (CE) population, and some populations that were not specified in the study. The ITT population is defined as those who received at least one dose of the study drug, while all randomized and MITT patients were defined at baseline to confirm the presence of MRSA in all ITT patients. Clinical success was defined as cure or improvement to assess the status of the study population upon the test of cure (TOC), while TOC is assessed 7–14 days after the end of treatment. For microbiological success, we assessed the microbiologically evaluable (ME) population as patients who had at least one MRSA pathogen isolated from blood or infected tissue at baseline in the CE population, with microbiological success defined as the eradication of MRSA pathogens. Finally, safety, defined as the incidence of adverse events, was assessed. Some of the common adverse reactions include nausea, diarrhea, headache, and thrombocytopenia.
4.5. Quality Evaluation
The quality of the research was evaluated by two independent reviewers according to the Cochrane Handbook assessment tools; when necessary, disputes between the two independent reviewers were resolved through discussion with the third reviewer. Among the items evaluated in the manual were seven areas: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each indicator was evaluated separately using low, unclear, or high risk of bias. Finally, Review Manager 5.4 software was used to generate the risk-of-bias map.
4.6. Statistical Analysis
We evaluated three outcome measures, as well as subgroup analyses of three diseases in clinical success measures through a network meta-analysis, comparing the effectiveness and safety of various antibiotics for the treatment of MRSA infections. A meta-analysis of bicategorical variables was performed using the meta software package in R language. Relative risks (RRs) and 95% confidence intervals (95%-CIs) were calculated to compare the efficacy and safety of various antibiotics in a specified population. If 95%-CI of RR does not contain 1, it is considered that there is a statistically significant difference. The size of the heterogeneity was assessed using I2. If I2 < 50%, the effect size was combined using a fixed effect model, and if I2 ≥ 50%, the effect size was combined using a random effect model. Using Stata 14.0 software, a network meta-analysis was conducted based on the random effects model under the frequency framework, and the network evidence map was drawn. Subsequently, the cumulative ranking probability area under graph (SUCRA) was calculated by the “sucra prob” command to predict the efficacy ranking of each intervention. The larger the SUCRA value, the better the effectiveness of the intervention so as to facilitate the direct and indirect comparison of the efficacy of various antibiotics. Finally, the “netfunnel” command was used to plot funnel plots to evaluate publication bias in the included literature. This study’s PROSPERO registration number is CRD42023416788.
5. Discussion
This systematic review and the NMA evaluated the efficacy and safety of different antibiotics for the treatment of infections caused by MRSA. We classified the 38 included studies, and the NMA results showed that the clinical cure success rates of minocycline combined with rifampin, vancomycin combined with rifampin, and linezolid were superior to that of vancomycin. The SUCRA results indicated that combined treatment with minocycline and rifampin may have the highest clinical cure success rate. The efficacy of linezolid was higher than that of vancomycin in the evaluation of clinical microbial success rate and the incidence of adverse reactions. In addition, the results of the subgroup analysis showed that the clinical cure rates of linezolid combined with minocycline and rifampin were better than that of vancomycin in the treatment of cSSSI patients, and the clinical cure rate of linezolid in the treatment of cSSTI patients and pneumonia patients was better than that of vancomycin.
Three similar studies were conducted previously. In 2012, Bally et al. compared the efficacy of six antibiotics used to treat cSSTIs and HAP/VAP (hospital-acquired pneumonia/ventilator-associated pneumonia), finding that linezolid had the best efficacy against cSSTIs, while the efficacy of linezolid was better than that of vancomycin in treating pneumonia, consistent with the results of this study [52]. The Bayesian model emphasizes the introduction of subjective prior information, so different people may have had different prior distributions, resulting in different inferred results. In 2021, Feng et al. suggested that linezolid may be the antibiotic of choice for the treatment of skin and soft tissue infections (SSTIs) caused by MRSA, which is consistent with the findings of this study [53]. However, only 8 of the 20 trials included were blinded, and 16 of them were conducted in the United States, potentially limiting the results. In 2024, Ju et al. compared the efficacy of six antibiotics in the treatment of MRSA infections and found that linezolid was the most effective in the treatment of lung infections, as well as skin and soft tissue infections, which is generally consistent with the results of this study [54]. Compared with the previous three studies, the present study focused on the diagnosis of MRSA infections and conducted a reasonable classification analysis for different types of MRSA infections. We analyzed 13 antimicrobial agents, whether clinically used or not, to provide stronger and more comprehensive evidence for clinical use.
We found that the combination of minocycline and rifampicin in the treatment of MRSA infections had the best clinical cure rate, followed by linezolid, both of which were superior to vancomycin. The combination treatment with minocycline and rifampicin is mainly used due to the good absorption of these two drugs, along with their strong tissue permeability, long half life, and synergistic effect in fighting MRSA infection [55]. The incidence of adverse reactions is also relatively low, and the most common adverse reactions include metabolic and nutritional disorders [51]. The 2011 Infectious Diseases Society of America (IDSA) guidelines do not recommend the use of rifampicin alone for the treatment of MRSA-infection-related diseases [56]. Therefore, the combination of minocycline and rifampicin may be a better choice for the treatment of MRSA infections.
In the study of patients with MRSA-induced cSSSIs, we found that the combination of minocycline and rifampicin had the best effect and a low incidence of adverse reactions, but there have been few clinical trials of this combination, and further research is needed. In addition, in patients with MRSA-induced cSSTIs, we found that linezolid was more effective than vancomycin, which is consistent with previous results [52,57]. However, the SUCRA results of this study indicate that tedizolid may be the antibiotic with the highest success rate in the treatment of cSSTIs. Tedizolid, a new oxazolidone antibiotic, is no less effective than linezolid and has a lower incidence of drug-related adverse reactions [58,59]. At present, there have been few studies on the use of tedizolid in the treatment of cSSTIs, and the discovery of SUCRA may increase the research on tedizolid in the treatment of cSSTIs in the future. Finally, for patients with MRSA-induced pneumonia, we found a significant increase in the efficacy of linezolid, consistent with the results of previously published meta-analyses [60]. However, our SUCRA results showed that the combination of vancomycin and rifampicin was the most effective and had a lower incidence of adverse reactions. The 2011 IDSA guidelines recommend intravenous vancomycin, linezolid, or clindamycin for the treatment of MRSA-induced pneumonia [56]. The updated guidelines for 2021 recommend considering rifampicin as an adjunct in the treatment of MRSA-induced pneumonia [61]. In their study, Diekema et al. found no significant change in rifampicin resistance rates over 11 years [62]. Therefore, rifampicin may be a potential adjuvant treatment for MRSA infection in the future.
MRSA has become one of the major multidrug-resistant bacterial pathogens causing cSSSIs and hospital-acquired infections, especially associated pneumonia. This study found that the best treatment drugs for different types of diseases caused by MRSA infection are also different. cSSSIs are skin infections involving deep soft tissue, mainly including surgical sites, traumatic wound infections, and widespread cellulitis. Vancomycin, the drug of choice against MRSA, has poor penetration and struggles to work effectively in soft tissue and bone [63]. In addition, cSSSIs are not solely related to MRSA. Hospital-acquired skin infections are generally associated with Staphylococcus aureus, Gram-negative bacteria, and anaerobic bacteria, with diabetic foot infections typically involving three to five bacterial species [64]. cSSTIs are diseases caused by bacterial invasion of the epidermis, dermis, or subcutaneous tissue and can present with a variety of clinical symptoms. They are mostly caused by a single microbial infection caused by Gram-positive or -negative bacteria, and they often occur at sites of damaged tissue blood perfusion, which is conducive to the growth and reproduction of pathogenic bacteria [65]. MRSA is an important causative pathogen of pneumonia. Pneumonia is a respiratory infection, and its clinical symptoms depend on the continuous concentration of antibiotics at the infection site. Therefore, antibiotics need to achieve a certain level of biological activity in the relevant parts of the lung and not be inactivated by pulmonary surfactants. The three different types of MRSA infection diseases included in this study have different infection sites in the body, and the pathogens are also different, so different drug treatments can exert significant effects.
There are some limitations to this study. First, this study was limited to English-language literature, and some non-English literature may have been missed. Second, there were some differences in the drug-treatment methods included in this study. Thirdly, the population included was diverse in terms of clinical cure success rates, including EOS, ITT, MITT, CE, and those that stated only clinical cure rates. In addition, there are currently insufficient data to demonstrate significant differences in the efficacy of other antibiotics in treating MRSA infections, and more high-quality clinical studies are needed to support these antibiotics as alternatives to vancomycin.
6. Conclusions
This systematic review and NMA provide a new comparative framework for the clinical treatment of MRSA infections. The NMA suggests that linezolid may be the antibiotic of choice for the treatment of MRSA infections, and although it has disadvantages in terms of adverse effects, it can improve clinical and microbiological success rates. At the same time, combination treatment with minocycline and rifampicin can also improve the clinical cure success rate and clinical cure rate of cSSSI patients. However, the comparative results of other antibiotics were not statistically significant, so the search for an alternative to vancomycin—the first-line drug for the treatment of MRSA infections—remains urgent, and more high-quality clinical studies are still needed in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13090866/s1. Reference [66] is cited in Supplementary Materials.
Author Contributions
Q.L. and Y.S. initiated the project and were responsible for the design of the protocol. Q.L. performed the literature review, collected the data, and assessed the quality of the studies. Q.L., D.H., L.W., Y.W., X.L. and Y.Y. analyzed the data. Q.L., Z.C., Z.D. and Y.L. interpreted the data. Q.L. wrote the initial draft of the manuscript. Q.L. and Y.S. were responsible for the critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (grant No. 31860607).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the National Natural Science Foundation of China for funding support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barber, M. Methicillin-resistant staphylococci. J. Clin. Pathol. 1961, 14, 385. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 2001, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Daum, R.S. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 2007, 357, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B.; Talan, D.A. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kuti, J.L.; Nicolau, D.P. Antimicrobial management of complicated skin and skin structure infections in the era of emerging resistance. Surg. Infect. 2005, 6, 283–295. [Google Scholar] [CrossRef]
- Edelsberg, J.; Taneja, C.; Zervos, M.; Haque, N.; Moore, C.; Reyes, K.; Spalding, J.; Jiang, J.; Oster, G. Trends in US hospital admissions for skin and soft tissue infections. Emerg. Infect. Dis. 2009, 15, 1516. [Google Scholar] [CrossRef]
- Healthcare Cost Utilization Project. HCUP Facts and Figures. In HCUP Facts and Figures: Statistics on Hospital-Based Care in the United States, 2007; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2009. [Google Scholar]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef]
- Chalmers, S.J.; Wylam, M.E. Methicillin-Resistant Staphylococcus aureus Infection and Treatment Options; Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2069, pp. 229–251. [Google Scholar] [CrossRef]
- Samura, M.; Kitahiro, Y.; Tashiro, S.; Moriyama, H.; Hamamura, Y.; Takahata, I.; Kawabe, R.; Enoki, Y.; Taguchi, K.; Takesue, Y.; et al. Efficacy and Safety of Daptomycin versus Vancomycin for Bacteremia Caused by Methicillin-Resistant Staphylococcus aureus with Vancomycin Minimum Inhibitory Concentration > 1 µg/mL: A Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 714. [Google Scholar] [CrossRef]
- Matsumoto, K.; Samura, M.; Tashiro, S.; Shishido, S.; Saiki, R.; Takemura, W.; Misawa, K.; Liu, X.; Enoki, Y.; Taguchi, K. Target Therapeutic Ranges of Anti-MRSA Drugs, Linezolid, Tedizolid and Daptomycin, and the Necessity of TDM. Biol. Pharm. Bull. 2022, 45, 824–833. [Google Scholar] [CrossRef]
- Chastre, J.; Blasi, F.; Masterton, R.G.; Rello, J.; Torres, A.; Welte, T. European perspective and update on the management of nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin. Microbiol. Infect. 2014, 20, 19–36. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44, S27–S72. [Google Scholar] [CrossRef] [PubMed]
- Van Laethem, Y.; Hermans, P.; De Wit, S.; Goosens, H.; Clumeck, N. Teicoplanin compared with vancomycin in methicillin-resistant Staphylococcus aureus infections: Preliminary results. J. Antimicrob. Chemother. 1988, 21, 81–87. [Google Scholar] [CrossRef]
- Levine, D.P.; Fromm, B.S.; Reddy, B.R. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 1991, 115, 674–680. [Google Scholar] [CrossRef]
- Markowitz, N.; Quinn, E.L.; Saravolatz, L.D. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann. Intern. Med. 1992, 117, 390–398. [Google Scholar] [CrossRef]
- Liu, C.Y.; Lee, W.S.; Fung, C.P.; Cheng, N.C.; Liu, C.L.; Yang, S.P.; Chen, S.L. Comparative Study of Teicoplanin vs Vancomycin for the Treatment of Methicillin-Resistant Staphylococcus aureus Bacteraemia. Clin. Drug Investig. 1996, 12, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Cammarata, S.; Oliphant, T.; Wunderink, R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: A randomized, double-blind, multicenter study. Clin. Infect. Dis. 2001, 32, 402–412. [Google Scholar] [CrossRef]
- Stevens, D.L.; Herr, D.; Lampiris, H.; Hunt, J.L.; Batts, D.H.; Hafkin, B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin. Infect. Dis. 2002, 34, 1481–1490. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Rello, J.; Cammarata, S.K.; Croos-Dabrera, R.V.; Kollef, M.H. Linezolid vs vancomycin: Analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003, 124, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.L.; Afghani, B.; Lopez, P.; Wu, E.; Fleishaker, D.; Edge-Padbury, B.; Naberhuis-Stehouwer, S.; Bruss, J.B. Linezolid for the treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr. Infect. Dis. J. 2003, 22, S178–S185. [Google Scholar] [CrossRef]
- Kollef, M.H.; Rello, J.; Cammarata, S.K.; Croos-Dabrera, R.V.; Wunderink, R.G. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: Retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 2004, 30, 388–394. [Google Scholar] [CrossRef]
- Weigelt, J.; Kaafarani, H.M.; Itani, K.M.; Swanson, R.N. Linezolid eradicates MRSA better than vancomycin from surgical-site infections. Am. J. Surg. 2004, 188, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.N.; Shively, E.H.; Polk, H.C., Jr. Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA-complicated, lower-extremity skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. Am. J. Surg. 2005, 189, 425–428. [Google Scholar] [CrossRef]
- Stryjewski, M.E.; O’Riordan, W.D.; Lau, W.K.; Pien, F.D.; Dunbar, L.M.; Vallee, M.; Fowler, V.G., Jr.; Chu, V.H.; Spencer, E.; Barriere, S.L.; et al. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin. Infect. Dis. 2005, 40, 1601–1607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weigelt, J.; Itani, K.; Stevens, D.; Lau, W.; Dryden, M.; Knirsch, C. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob. Agents Chemother. 2005, 49, 2260–2266. [Google Scholar] [CrossRef]
- Stryjewski, M.E.; Chu, V.H.; O’Riordan, W.D.; Warren, B.L.; Dunbar, L.M.; Young, D.M.; Vallée, M.; Fowler, V.G., Jr.; Morganroth, J.; Barriere, S.L.; et al. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob. Agents Chemother. 2006, 50, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Talbot, G.H.; Thye, D.; Das, A.; Ge, Y. Phase 2 study of ceftaroline versus standard therapy in treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 2007, 51, 3612–3616. [Google Scholar] [CrossRef]
- Kohno, S.; Yamaguchi, K.; Aikawa, N.; Sumiyama, Y.; Odagiri, S.; Aoki, N.; Niki, Y.; Watanabe, S.; Furue, M.; Ito, T.; et al. Linezolid versus vancomycin for the treatment of infections caused by methicillin-resistant Staphylococcus aureus in Japan. J. Antimicrob. Chemother. 2007, 60, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.E.; Lindfield, K.C.; Steenbergen, J.N.; Benziger, D.P.; Blackerby, K.J.; Knapp, A.G.; Martone, W.J. A pilot study of high-dose short duration daptomycin for the treatment of patients with complicated skin and skin structure infections caused by gram-positive bacteria. Int. J. Clin. Pract. 2008, 62, 1455–1464. [Google Scholar] [CrossRef]
- Florescu, I.; Beuran, M.; Dimov, R.; Razbadauskas, A.; Bochan, M.; Fichev, G.; Dukart, G.; Babinchak, T.; Cooper, C.A.; Ellis-Grosse, E.J.; et al. Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: A Phase 3, multicentre, double-blind, randomized study. J. Antimicrob. Chemother. 2008, 62, i17–i28. [Google Scholar] [CrossRef]
- Noel, G.J.; Strauss, R.S.; Amsler, K.; Heep, M.; Pypstra, R.; Solomkin, J.S. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob. Agents Chemother. 2008, 52, 37–44. [Google Scholar] [CrossRef]
- Stryjewski, M.E.; Graham, D.R.; Wilson, S.E.; O’Riordan, W.; Young, D.; Lentnek, A.; Ross, D.P.; Fowler, V.G.; Hopkins, A.; Friedland, H.D.; et al. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 2008, 46, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H.; Tack, K.J.; Bouza, E.; Herr, D.L.; Ruf, B.R.; Ijzerman, M.M.; Croos-Dabrera, R.V.; Kunkel, M.J.; Knirsch, C. Complicated skin and skin-structure infections and catheter-related bloodstream infections: Noninferiority of linezolid in a phase 3 study. Clin. Infect. Dis. 2009, 48, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Itani, K.M.; Dryden, M.S.; Bhattacharyya, H.; Kunkel, M.J.; Baruch, A.M.; Weigelt, J.A. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am. J. Surg. 2010, 199, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Koh, Y.; Hong, S.B.; Chung, J.W.; Ho Choi, S.; Kim, N.J.; Kim, M.N.; Choi, I.S.; Han, S.Y.; Kim, W.D.; et al. Effect of vancomycin plus rifampicin in the treatment of nosocomial methicillin-resistant Staphylococcus aureus pneumonia. Crit. Care Med. 2010, 38, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Itani, K.M.; Weigelt, J.A.; Joseph, W.; Paap, C.M.; Reisman, A.; Myers, D.E.; Huang, D.B. The role of diabetes mellitus in the treatment of skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus: Results from three randomized controlled trials. Int. J. Infect. Dis. 2011, 15, e140–e146. [Google Scholar] [CrossRef] [PubMed]
- Corey, G.R.; Wilcox, M.H.; Talbot, G.H.; Thye, D.; Friedland, D.; Baculik, T. CANVAS 1: The first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 2010, 65, iv41–iv51. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Corey, G.R.; Talbot, G.H.; Thye, D.; Friedland, D.; Baculik, T. CANVAS 2: The second Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 2010, 65, iv53–iv65. [Google Scholar] [CrossRef] [PubMed]
- Corey, G.R.; Wilcox, M.; Talbot, G.H.; Friedland, H.D.; Baculik, T.; Witherell, G.W.; Critchley, I.; Das, A.F.; Thye, D. Integrated analysis of CANVAS 1 and 2: Phase 3, multicenter, randomized, double-blind studies to evaluate the safety and efficacy of ceftaroline versus vancomycin plus aztreonam in complicated skin and skin-structure infection. Clin. Infect. Dis. 2010, 51, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Barriere, S.L. ATLAS trials: Efficacy and safety of telavancin compared with vancomycin for the treatment of skin infections. Future Microbiol. 2010, 5, 1765–1773. [Google Scholar] [CrossRef]
- Duane, T.M.; Weigelt, J.A.; Puzniak, L.A.; Huang, D.B. Linezolid and vancomycin in treatment of lower-extremity complicated skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus in patients with and without vascular disease. Surg. Infect. 2012, 13, 147–153. [Google Scholar] [CrossRef]
- Itani, K.M.; Biswas, P.; Reisman, A.; Bhattacharyya, H.; Baruch, A.M. Clinical efficacy of oral linezolid compared with intravenous vancomycin for the treatment of methicillin-resistant Staphylococcus aureus-complicated skin and soft tissue infections: A retrospective, propensity score-matched, case-control analysis. Clin. Ther. 2012, 34, 1667–1673.e1. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Niederman, M.S.; Kollef, M.H.; Shorr, A.F.; Kunkel, M.J.; Baruch, A.; McGee, W.T.; Reisman, A.; Chastre, J. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A randomized, controlled study. Clin. Infect. Dis. 2012, 54, 621–629. [Google Scholar] [CrossRef]
- Stryjewski, M.E.; Barriere, S.L.; O’Riordan, W.; Dunbar, L.M.; Hopkins, A.; Genter, F.C.; Corey, G.R. Efficacy of telavancin in patients with specific types of complicated skin and skin structure infections. J. Antimicrob. Chemother. 2012, 67, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Stryjewski, M.E.; Lentnek, A.; O’Riordan, W.; Pullman, J.; Tambyah, P.A.; Miró, J.M.; Fowler, V.G., Jr.; Barriere, S.L.; Kitt, M.M.; Corey, G.R. A randomized Phase 2 trial of telavancin versus standard therapy in patients with uncomplicated Staphylococcus aureus bacteremia: The ASSURE study. BMC Infect. Dis. 2014, 14, 289. [Google Scholar] [CrossRef]
- Shaw, G.J.; Meunier, J.M.; Korfhagen, J.; Wayne, B.; Hart, K.; Lindsell, C.J.; Fermann, G. Randomized controlled noninferiority trial comparing daptomycin to vancomycin for the treatment of complicated skin and skin structure infections in an observation unit. J. Emerg. Med. 2015, 49, 928–936. [Google Scholar] [CrossRef]
- Equils, O.; da Costa, C.; Wible, M.; Lipsky, B.A. The effect of diabetes mellitus on outcomes of patients with nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus: Data from a prospective double-blind clinical trial comparing treatment with linezolid versus vancomycin. BMC Infect. Dis. 2016, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Deb, A.K.; Das, C.; Padhye, D.; Bhalla, H.; Basu, I.; Bs, M.; Srivastava, P.; Agarwal, R.; Agrawal, R.P.; et al. A Multicentre, Open label, Randomized, Comparative, Parallel Group, Active-controlled, Phase III Clinical Trial to Evaluate Safety and Efficacy of Arbekacin Sulphate Injection versus Vancomycin Injection in Patients Diagnosed with MRSA Infection. J. Assoc. Physicians India 2018, 66, 47–50. [Google Scholar] [PubMed]
- Mikamo, H.; Takesue, Y.; Iwamoto, Y.; Tanigawa, T.; Kato, M.; Tanimura, Y.; Kohno, S. Efficacy, safety and pharmacokinetics of tedizolid versus linezolid in patients with skin and soft tissue infections in Japan—Results of a randomised, multicentre phase 3 study. J. Infect. Chemother. 2018, 24, 434–442. [Google Scholar] [CrossRef]
- Kotsaki, A.; Tziolos, N.; Kontopoulou, T.; Koutelidakis, I.M.; Symbardi, S.; Reed, V.; O’Hare, M.; Alexiou, Z.; Sambatakou, H.; Toutouzas, K.; et al. Oral minocycline plus rifampicin versus oral linezolid for complicated skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus: The AIDA open label, randomized, controlled Phase 4 trial. EClinicalMedicine 2023, 56, 101790. [Google Scholar] [CrossRef]
- Bally, M.; Dendukuri, N.; Sinclair, A.; Ahern, S.P.; Poisson, M.; Brophy, J. A network meta-analysis of antibiotics for treatment of hospitalised patients with suspected or proven meticillin-resistant Staphylococcus aureus infection. Int. J. Antimicrob. Agents 2012, 40, 479–495. [Google Scholar] [CrossRef]
- Feng, J.; Xiang, F.; Cheng, J.; Gou, Y.; Li, J. Comparative Efficacy and Safety of Vancomycin, Linezolid, Tedizolid, and Daptomycin in Treating Patients with Suspected or Proven Complicated Skin and Soft Tissue Infections: An Updated Network Meta-Analysis. Infect. Dis. Ther. 2021, 10, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Ju, G.; Zhang, Y.; Ye, C.; Liu, Q.; Sun, H.; Zhang, Z.; Huang, X.; Jiang, Y.; Huang, Q. Comparative effectiveness and safety of six antibiotics in treating MRSA infections: A network meta-analysis. Int. J. Infect. Dis. 2024, 146, 107109. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. IDSA creates MRSA treatment guideline. JAMA 2011, 305, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Bounthavong, M.; Hsu, D.I. Efficacy and safety of linezolid in methicillin-resistant Staphylococcus aureus (MRSA) complicated skin and soft tissue infection (cSSTI): A meta-analysis. Curr. Med. Res. Opin. 2010, 26, 407–421. [Google Scholar] [CrossRef]
- Flanagan, S.; Fang, E.; Muñoz, K.A.; Minassian, S.L.; Prokocimer, P.G. Single- and multiple-dose pharmacokinetics and absolute bioavailability of tedizolid. Pharmacotherapy 2014, 34, 891–900. [Google Scholar] [CrossRef]
- Chen, R.; Shen, K.; Chang, X.; Tanaka, T.; Li, L.; Hu, P. Pharmacokinetics and Safety of Tedizolid after Single and Multiple Intravenous/Oral Sequential Administrations in Healthy Chinese Subjects. Clin. Ther. 2016, 38, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hagihara, M.; Asai, N.; Shibata, Y.; Koizumi, Y.; Yamagishi, Y.; Mikamo, H. Meta-analysis of vancomycin versus linezolid in pneumonia with proven methicillin-resistant Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2021, 24, 98–105. [Google Scholar] [CrossRef]
- Brown, N.M.; Goodman, A.L.; Horner, C.; Jenkins, A.; Brown, E.M. Treatment of methicillin-resistant Staphylococcus aureus (MRSA): Updated guidelines from the UK. JAC-Antimicrob. Resist. 2021, 3, dlaa114. [Google Scholar] [CrossRef]
- Diekema, D.J.; Pfaller, M.A.; Shortridge, D.; Zervos, M.; Jones, R.N. Twenty-Year Trends in Antimicrobial Susceptibilities Among Staphylococcus aureus From the SENTRY Antimicrobial Surveillance Program. Open Forum Infect. Dis. 2019, 6, S47–S53. [Google Scholar] [CrossRef]
- Graziani, A.; Lawson, L.; Gibson, G.; Steinberg, M.; MacGregor, R. Vancomycin concentrations in infected and noninfected human bone. Antimicrob. Agents Chemother. 1988, 32, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Rennie, R.P.; Jones, R.N.; Mutnick, A.H. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: Report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn. Microbiol. Infect. Dis. 2003, 45, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Baguneid, M.; Bouza, E.; Dryden, M.; Nathwani, D.; Wilcox, M. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin. Microbiol. Infect. 2014, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).