Antimicrobial Peptides and Their Biomedical Applications: A Review
Abstract
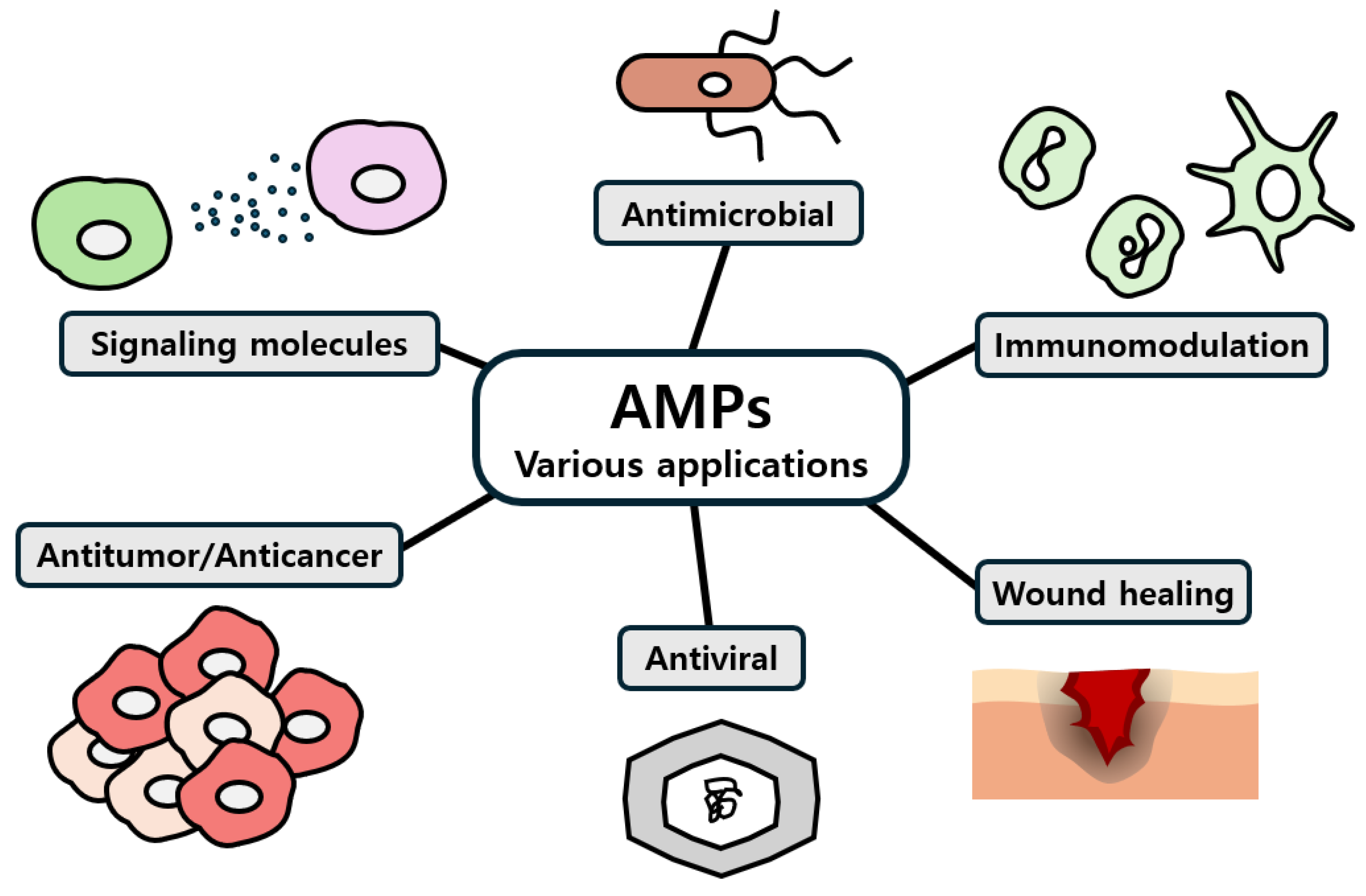
1. Introduction
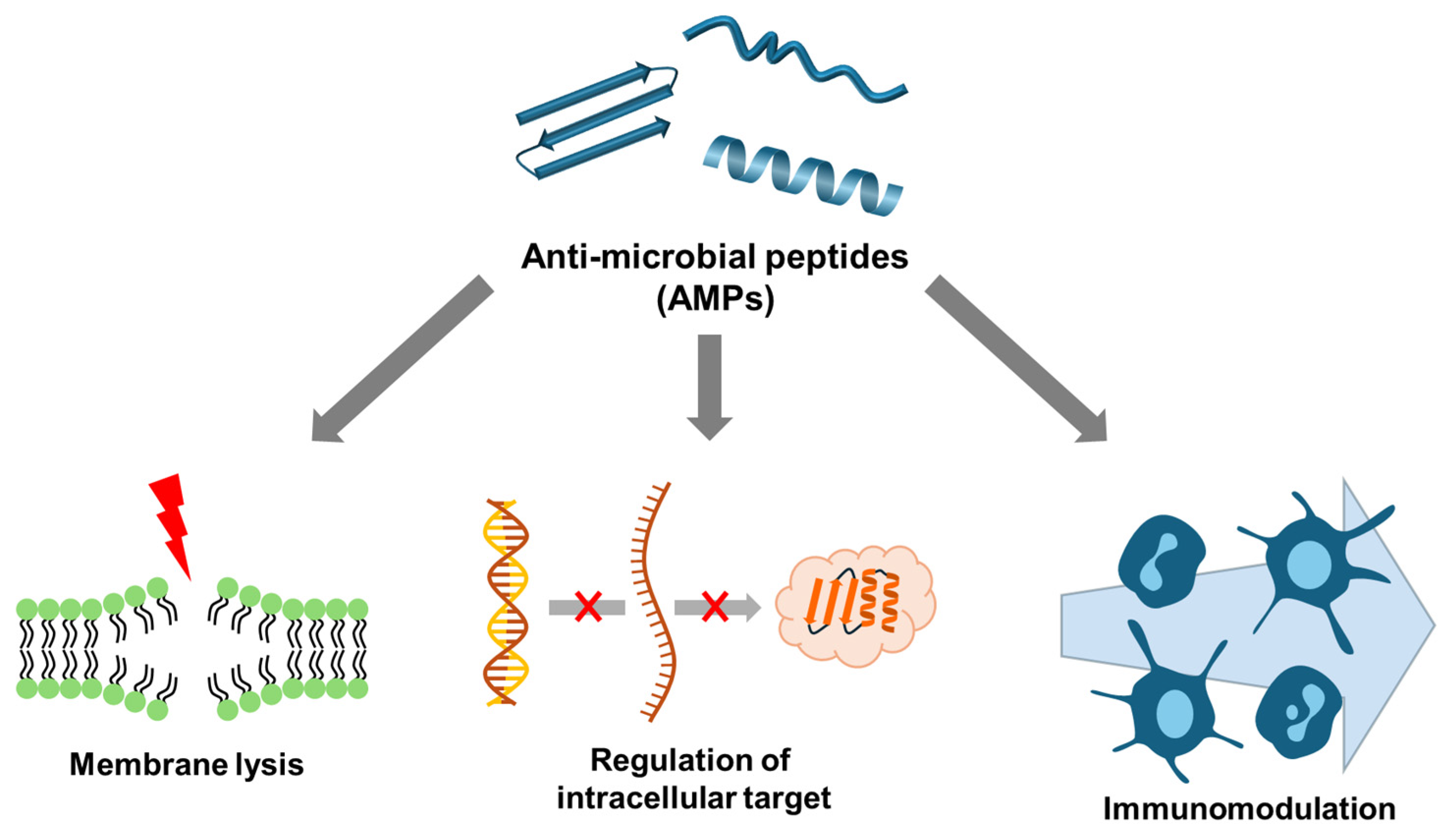
2. Modes of Action and Mechanisms of AMPs
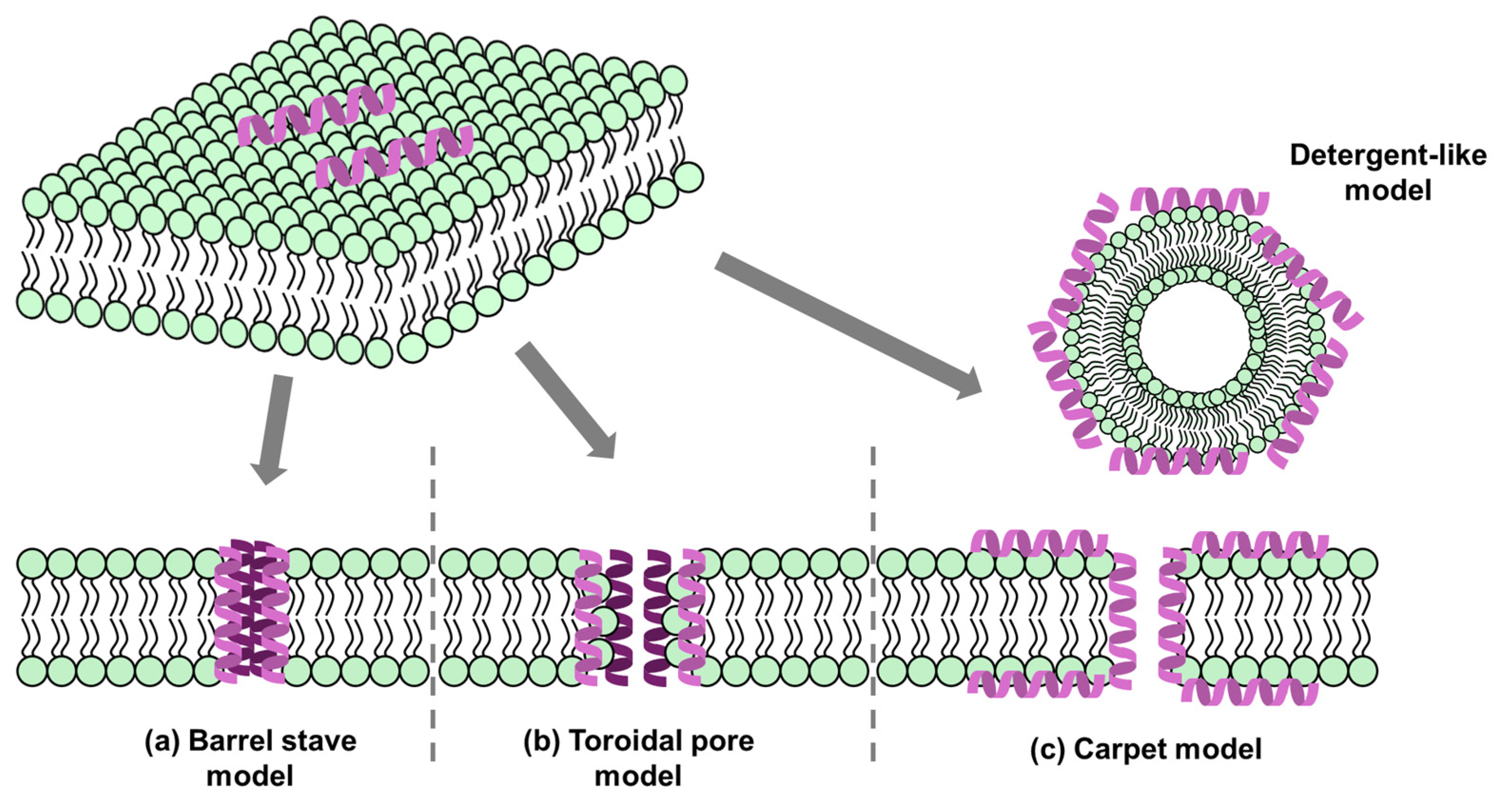
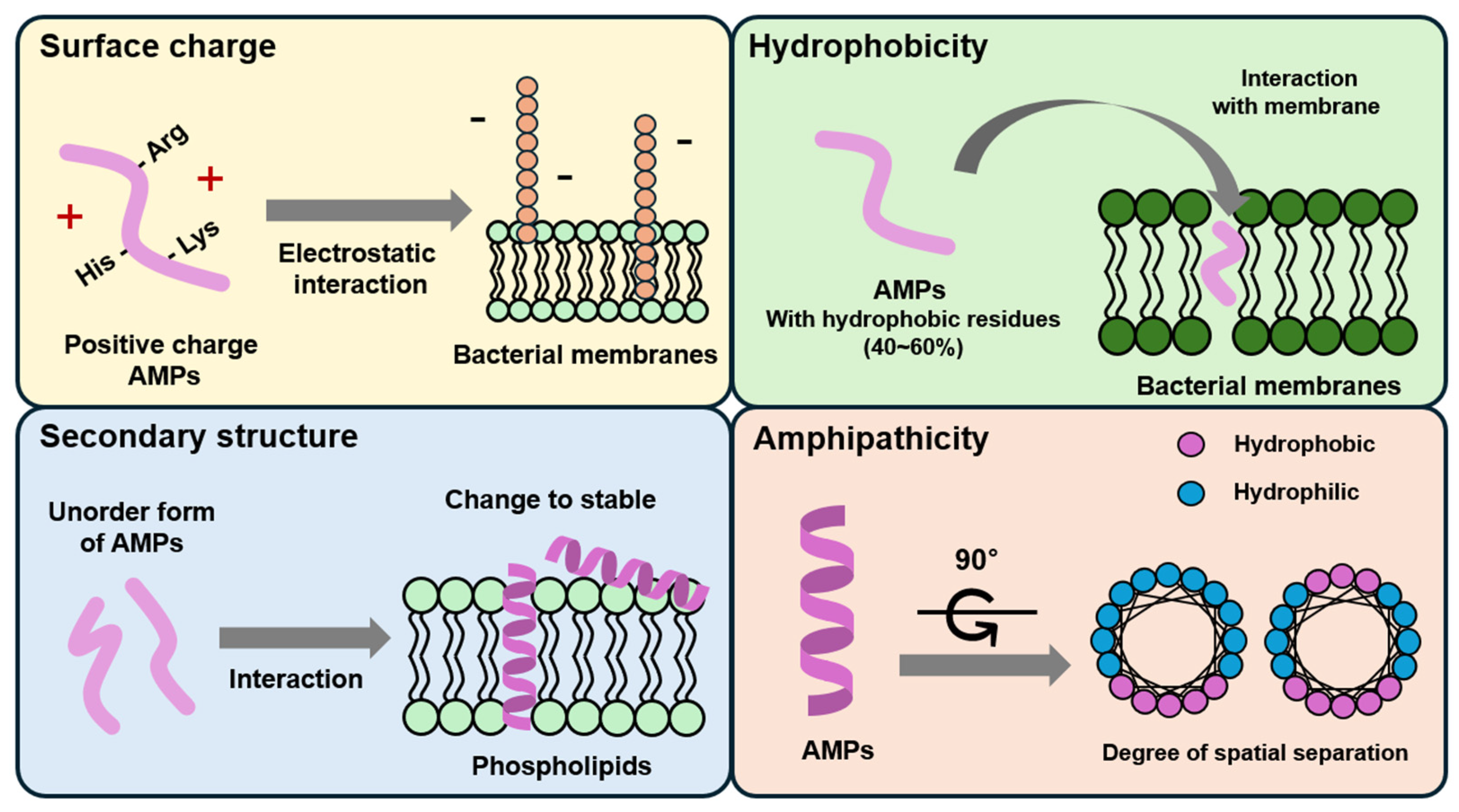
2.1. Membrane Targeting
2.2. Non-Membrane Targeting
3. AMP Modification to Enhance Antimicrobial Activity
| AMP | Materials | Biomedical Results | Refs. |
|---|---|---|---|
| T9W | Poly (ethylene glycol) | Anti-tripsin ability by self-assembled micelle structure Enhanced antibacterial effect 1.5–4 times against Gram-negative and Gram-positive bacteria Reduced lung injury and pro-inflammatory cytokines by P. aeruginosa PAO1 | [81] |
| Lysine and Valine | Poly (amidoamine) | Stable aerosolization, sustained release by nanoparticle formulation with tannic acid and iron ion Stability in intracellular environment Colocalization with endo/lysosome expected targeting bacteria in lung macrophage | [82] |
| KR-12 | Poly carprolactone Poly(ethylene glycol) methyl ether methacrylate | Specific binding effects by coating with macropharge membrane Antibacterial and antibiofilm effects against E. coli, S. aureus, and MRSA Increased adhesion and protection effects of main organ-involved sepsis | [85,86] |
| KRWWKWWRR | Hydoxyapatite binding peptide-1 | Providing implant with inhibited adhesion and antibacterial effects against E. coli and S. mutans | [87] |
| 9P2-2 | Ampicillin | Improved antibacterial activity against ampicillin-resistant A. baumannii Non-toxicity to mammalian cell | [88] |
| WRK | Acrylamide | High antibacterial activity by bacteria-mediated polymerization against E. coli and P. aeruginosa | [89] |
| G3KL | Chitosan | Boosting bacterial killing (P. aeruginosa) Decreased hemolytic activity by conjugation High biocomaptibility of dressing form | [84] |
| LWFYTMWH | Poly (ethylene glycol) | Antibacterial activity against E. coli and Bacillus sp. | [90] |
| Chimeric PR-39 | Cell penetrating peptide (R6) | Fast and non-resistant antibacterial effect High biosafety in vitro (64 uM of peptide) and in mice (~30 mg peptide/kg) | [91] |
4. AMP Application for Biomaterials
4.1. Surface-Based Applications
| AMP | Materials | Biomedical Results | Ref. |
|---|---|---|---|
| GL13K | Collagen membrane | Improved antimicrobial and antifouling activity, accelerated bone formation | [104] |
| GL13K | Mineralized collagen gel | Killing of Gram-negative E. coli and Gram-positive S. Gordonii, cytocompatible with human bone marrow-derived mesenchymal stromal cells | [107] |
| GL13K | Sandblasting and acid-etching-treated titanium | Sustained-release property, antibacterial property, oestoblast proliferation, and adhesion in vitro | [105] |
| QAGSNKGASQKGMS | Dopamine, 304 stainless steel | Antifouling capacity, antibacterial and antialgal properties, superior anticorrosion | [108] |
| ε-Polylysine hydrochloride (ε-PL), Nisin, | Starch/PBAT film | Higher moisture permeability and oxygen barrier property, synergistic antimicrobial effect | [106] |
| Lysozyme | Doapmine-modified graphene oxide | Antimicrobial activity, accelerated wound closure, reduced inflammation, improved angiogenesis, and accelerated re-epithelialization | [109] |
| Hs05 and Hs06 | Ureasil–polyether hybrid polymeric films | Antimicrobial activity | [110] |
| TCP-25 | Polyurethane | Anti-infective and anti-inflammatory effects in vitro and in vivo, reduced the concomitant inflammatory response | [111] |
| M2-DA | Stainless steel | Excellent antibacterial activity | [112] |
| HHC-36 | Pectolite nanorods on Ti implants | Antimicrobial activity while promoting cell adhesion, regulates the degradation of Ca- and Si-based ceramic | [113] |
| RRP9W4N | Mesoporous titania | Antibacterial activity, no negative effects on in vivo osseointegration | [114] |
| RRP9W4N | Surface-modified titanium implants with elastin-like polypeptide | Antibacterial activity, enabled mammalian osteogenic cell adhesion | [115] |
| HX-12C | Chitosan flim | Good antibacterial actibity, strong antibiofilm ability | [116] |
4.2. Particle-Based Applications
| AMP | Materials | Biomedical Results | Ref. |
|---|---|---|---|
| Ura56 | Gold nanoparticle | Peptide stability against protease Bacteria-killing effect against antibiotic-resistant bacteria by membrane attachment and lysis Antibiofilm activity | [117] |
| LL-37 | Titanium dioxide nanoparticle | Higher membrane attachment ability to anionic membrane compared to mammalian cell-like zwitterionic membrane | [121] |
| AS-48 | Biomimetic magnetic nanoparticle | Enhanced growth inhibition effects against E. coli compared to free peptide | [122] |
| Ib-M2 | Iron oxide nanoparticle coated with chitosan | Enhanced growth inhibiton effects against E. coli compared to free peptide | [123] |
| KYE21 and WWWKYE21 | Titanium dioxide nanoparticle | Bacteria- and lipopolysaccharide-like membrane attachment using peptide Enhanced antibacterial effects against E. coli Selective toxicity between bacteria and human cell | [119] |
| NGIVKAGPAIAVLGEAAL and JH8194 sequence | Silver nanoparticle with silk fidronin | Silver release at pH 5.0 Bacterial membrane permeability and bactericidal effect against MRSA In vitro and in vivo osteogenic activity | [124] |
| LL37 | Gold nanoparticle | Antibacterial effects against Gram-positive and Gram-negative bactria More or less cytotoxicity to endothelial cell Angiogenic activity | [125] |
| CCLLLLRRRRRR | Silver nanoclusters | Interaction with bacterial membrane targeting lipopolysaccharide 100-fold higher inhibition activity against E. coli compared to commercial silver nanoparticle | [126] |
| Thiol-terminated DDL90 BLG10 | Gold nanoparticle | Antibacterial activity against MRSA using over-production of reactive oxygen species In vitro and in vivo biocompatibility | [127] |
| Cathelicidin-BF | Nanoparticles composed of epigallocatechin-3-gallate and silk fibroin | Increased antibacterial effects against E. coli Lipopolysaccaride adsorption In vivo therapeutic effect against ulcerative colitis | [118] |
| SAAP-148 | Poly(lactic-co-glycolic) acid | Increased antibacterial activities (10–20 fold) and antibiofilm activities against antimicrobial-resistant S. aureus and Acinetobacter baumannii | [128] |
| PA-13 | Chitosan Dextran sulfate | Improved stability against protease Maintained antibaterial activites against Pseudomonas aeruginosa in trysine-challenged conditions | [129] |
| SET-M33 | poly(lactide-co-glycolide) conjugated with polyethylene glycol | Enhanced penetration of artifical mucus and bacterial alginate by PEGlyation Sustained release and persistent antibacterial activity against Pseudomonas aeruginosa | [130] |
| Trp-Arg-Trp-Arg-Trp-Tyr (WRWRWY) | Self-assembly after oxidization by tyrosinase | Positively increased surface charge Reactive oxygen stress scattering effects Stronger antibacterial efffect against E.coli and S. aureus compared to WRWRWY Boosted wound healing in mice skin | [120] |
| Gramicidin A‘ Alamethicin Melittin Indolicidin Pexiganan Cecropin A | lipid-based inverse bicontinuous cubic phase nanoparticles (cubosomes) | Enhanced antibacterial activity of indolicidin (against S. aureus and Bacillus cereus) and alamethicin (against Bacillus cereus) after cubosome formulation | [131] |
4.3. Three-Dimensional Printing-Based Applications
| AMP | Materials | Biomedical Results | Ref. |
|---|---|---|---|
| S100A12 | Mucoadhesive helatin methacryloyl/chisosan methacryloyl hedrogel | Strong antibacterial properties, reduced the bacterial load in vivo | [135] |
| Silk fibroin | Silica-silk fibroin-cecropin melittin-RGD aeregel | Potent bactericidal efficiency toward Gram-positive and Gram-negative bacteria, osteoconductivity of the scaffold. | [137] |
| Ponericin G1 | BMP-2, poly(L-lactide-co-glycolide), dopamine | Maintain long-term antibacterial activity, cell adhesion, proliferation, and differentiation | [138] |
| 3-poly-L-lysine (EPL) | polycaprolactone/ hydroxyapatite (PCL/HA) | Cytocompatible as well as capable of osteogenic differentiation and antimicrobial activity in vitro | [139] |
| Ponericin G1 | gelatin/nanohydroxyapatite, dopamine | Both Gram-positive and Gram-negative bacteria (E. coli and S. aureus) were effectively inhibited up to 24 h, and the inhibition zone could remain for 72 h. | [140] |
| P1 (poly(L-lysine)), P2 (poly(Lglutamic acid)) | N-carboxyanhydride (NCA) monomers | Antimicrobial displaying a significant 6–7-fold log10 reduction, with the built-in capacity to enhance the mechanical and biological properties | [141] |
| Mel4 | Polyaryl ether ketone (PAEK) | reducing the microbial count on PEEK surfaces, no growth-inhibiting effect on osteoblastic cells | [136] |
| RWRWRWA-(Bpa) | Ultrafiltration membranes | cell membrane disruption, antibacterial activity and reduced biofilm growth | [142] |
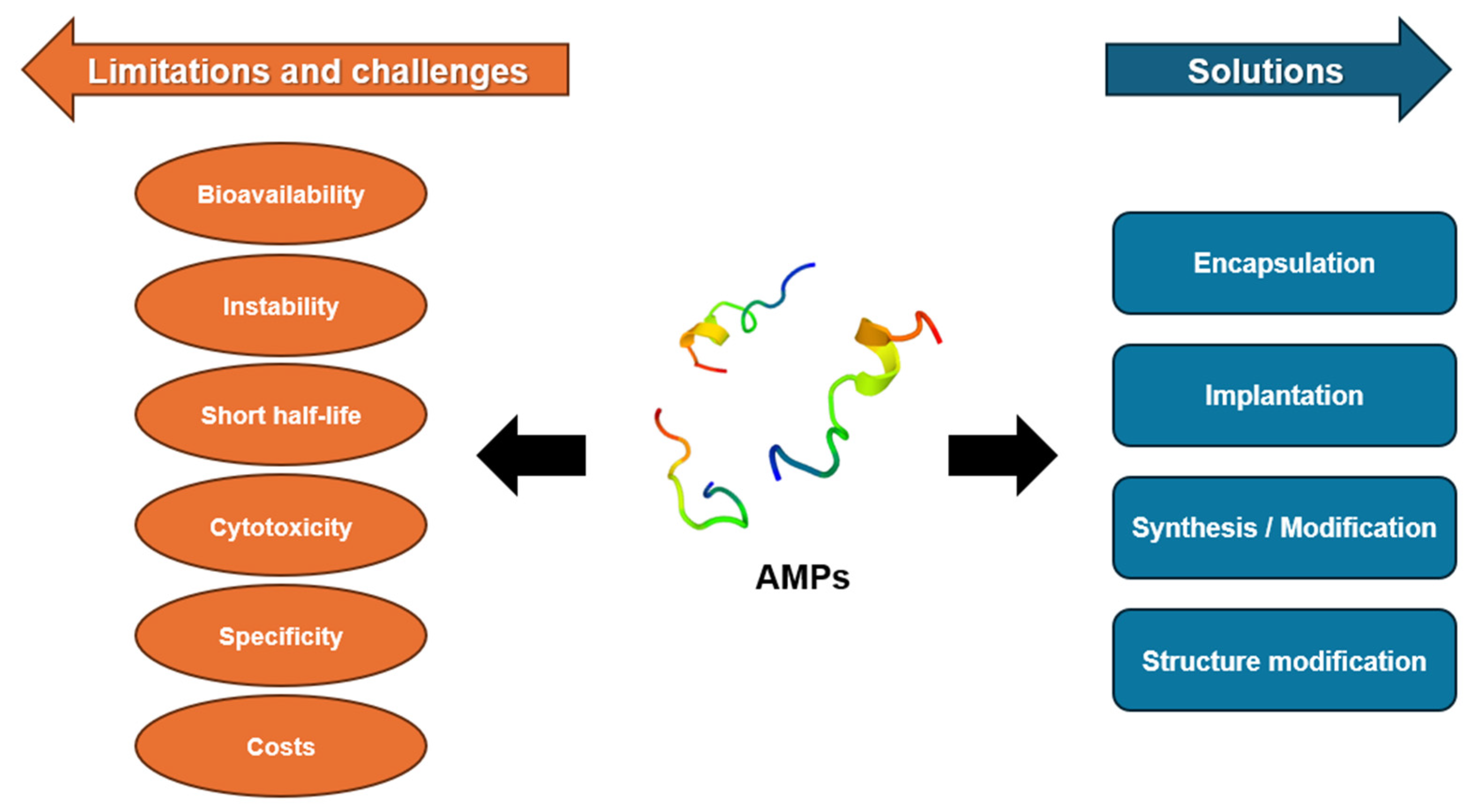
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, S.; Chauhan, A.; Ranjan, A.; Mathkor, D.M.; Haque, S.; Ramniwas, S.; Tuli, H.S.; Jindal, T.; Yadav, V. Emerging challenges in antimicrobial resistance: Implications for pathogenic microorganisms, novel antibiotics, and their impact on sustainability. Front. Microbiol. 2024, 15, 1403168. [Google Scholar] [CrossRef]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.C.; Huang, S.Y.; Zhu, C.H.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef]
- Mohammed, I.; Said, D.G.; Dua, H.S. Human antimicrobial peptides in ocular surface defense. Prog. Retin. Eye Res. 2017, 61, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Yang, P.; Lei, J.; Zhao, J. Biological function of antimicrobial peptides on suppressing pathogens and improving host immunity. Antibiotics 2023, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E.W. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial peptides: Old molecules with new ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef]
- Huan, Y.C.; Kong, Q.; Mou, H.J.; Yi, H.X. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Johansson, M.E.V.; Stabler, R.A.; Dalgakiran, F.; Hansson, G.C.; Wren, B.W.; Luzio, J.P.; Taylor, P.W. Altered innate defenses in the neonatal gastrointestinal tract in response to colonization by neuropathogenic. Infect. Immun. 2013, 81, 3264–3275. [Google Scholar] [CrossRef]
- Lyu, W.T.; Curtis, A.R.; Sunkara, L.T.; Zhang, G.L. Transcriptional regulation of antimicrobial host defense peptides. Curr. Protein Pept. Sci. 2015, 16, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Mangoni, M.L.; Pasquazzi, C.; Fiocco, D.; Marangi, M.; Miele, R.; Zechini, B.; Borro, M.; Versace, I.; Simmaco, M. α-defensin increase in peripheral blood mononuclear cells from patients with hepatitis C virus chronic infection. J. Viral Hepatitis 2006, 13, 821–827. [Google Scholar] [CrossRef]
- Wang, G.S.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, C.V.G.; Zhu, L.Y.; Oman, T.J.; van der Donk, W.A. Nmr structure of the S-linked glycopeptide sublancin 168. ACS Chem. Biol. 2014, 9, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, G.S. APD: The antimicrobial peptide database. Nucleic Acids Res. 2004, 32, D590–D592. [Google Scholar] [CrossRef]
- Lima, P.G.; Oliveira, J.T.A.; Amaral, J.L.; Freitas, C.D.T.; Souza, P.F.N. Synthetic antimicrobial peptides: Characteristics, design, and potential as alternative molecules to overcome microbial resistance. Life Sci. 2021, 278, 119647. [Google Scholar] [CrossRef]
- Tan, P.; Fu, H.Y.; Ma, X. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nano Today 2021, 39, 101229. [Google Scholar] [CrossRef]
- Maturana, P.; Gonçalves, S.; Martinez, M.; Espeche, J.C.; Santos, N.C.; Semorile, L.; Maffia, P.C.; Hollmann, A. Interactions of “de novo” designed peptides with bacterial membranes: Implications in the antimicrobial activity. Biochim. Biophys. Acta (BBA) Biomembr. 2020, 1862, 183443. [Google Scholar] [CrossRef]
- Takahashi, D.; Shukla, S.K.; Prakash, O.; Zhang, G.L. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 2010, 92, 1236–1241. [Google Scholar] [CrossRef]
- Yount, N.Y.; Bayer, A.S.; Xiong, Y.Q.; Yeaman, M.R. Advances in antimicrobial peptide immunobiology. Biopolymers 2006, 84, 435–458. [Google Scholar] [CrossRef]
- Frederiksen, N.; Hansen, P.R.; Zabicka, D.; Tomczak, M.; Urbas, M.; Domraceva, I.; Björkling, F.; Franzyk, H. Alternating cationic-hydrophobic peptide/peptoid hybrids: Influence of hydrophobicity on antibacterial activity and cell selectivity. ChemMedChem 2020, 15, 2544–2561. [Google Scholar] [CrossRef]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, α-helical antimicrobial peptides. Biopolymers 2000, 55, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog. Lipid Res. 2012, 51, 149–177. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Chen, Y.Y.; Song, Z.Y.; Tan, Z.Z.; Cheng, J.J. Recent advances in design of antimicrobial peptides and polypeptides toward clinical translation. Adv. Drug Deliver Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, D.; Nambiar, P.; Dutta, A.; Mitra, D. Rational design of antimicrobial peptides: An optimization approach. Mol. Syst. Des. Eng. 2024, 9, 311–322. [Google Scholar] [CrossRef]
- Datta, S.; Roy, A. Antimicrobial peptides as potential therapeutic agents: A review. Int. J. Pept. Res. Ther. 2021, 27, 555–577. [Google Scholar] [CrossRef]
- da Silva, A.M.B.; Silva-Gonçalves, L.C.; Oliveira, F.A.; Arcisio-Miranda, M. Pro-necrotic activity of cationic mastoparan peptides in human glioblastoma multiforme cells via membranolytic action. Mol. Neurobiol. 2018, 55, 5490–5504. [Google Scholar] [CrossRef] [PubMed]
- Alencar-Silva, T.; Braga, M.C.; Santana, G.O.S.; Saldanha-Araujo, F.; Pogue, R.; Dias, S.C.; Franco, O.L.; Carvalho, J.L. Breaking the frontiers of cosmetology with antimicrobial peptides. Biotechnol. Adv. 2018, 36, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Wu, R.L.; Li, X.J.; Ma, N.; Jin, X.F.; Yuan, X.F.; Qu, C.; Tang, H.M.; Liu, Z.G.; Zhang, Z.D. Bacterial quorum sensing molecules promote allergic airway inflammation by activating the retinoic acid response. iScience 2020, 23, 101288. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Sahl, H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.P.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Teixeira, C.; Gomes, P.; Martins, M.C.L. Clinical application of AMPs. Adv. Exp. Med. Biol. 2019, 1117, 281–298. [Google Scholar] [CrossRef]
- Di, L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- Fan, L.L.; Sun, J.; Zhou, M.F.; Zhou, J.; Lao, X.Z.; Zheng, H.; Xu, H.M. DRAMP: A comprehensive data repository of antimicrobial peptides. Sci. Rep. 2016, 6, 24482. [Google Scholar] [CrossRef]
- Liu, S.C.; Fan, L.L.; Sun, J.; Lao, X.Z.; Zheng, H. Computational resources and tools for antimicrobial peptides. J. Pept. Sci. 2017, 23, 4–12. [Google Scholar] [CrossRef]
- Liu, S.C.; Bao, J.X.; Lao, X.Z.; Zheng, H. Novel 3D structure based model for activity prediction and design of antimicrobial peptides. Sci. Rep. 2018, 8, 11189. [Google Scholar] [CrossRef]
- Kang, X.Y.; Dong, F.Y.; Shi, C.; Liu, S.C.; Sun, J.; Chen, J.X.; Li, H.Q.; Xu, H.M.; Lao, X.Z.; Zheng, H. DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci. Data 2019, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.K.; Kim, D.H.; Lee, B.J.; Kim, Y.W. Truncated and constrained helical analogs of antimicrobial esculentin-2EM. Bioorg. Med. Chem. Lett. 2013, 23, 6717–6720. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Lai, B.F.L.; Ding, C.F.; Kizhakkedathu, J.N.; Hancock, R.E.W.; Wang, R.Z. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 2013, 34, 5969–5977. [Google Scholar] [CrossRef]
- Nordström, R.; Malmsten, M. Delivery systems for antimicrobial peptides. Adv. Colloid Interface Sci. 2017, 242, 17–34. [Google Scholar] [CrossRef]
- Mourtada, R.; Herce, H.D.; Yin, D.J.; Moroco, J.A.; Wales, T.E.; Engen, J.R.; Walensky, L.D. Design of stapled antimicrobial peptides that are stable, nontoxic and kill antibiotic-resistant bacteria in mice. Nat. Biotechnol. 2019, 37, 1186–1197. [Google Scholar] [CrossRef]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-membrane permeabilizing modes of action of antimicrobial peptides on bacteria. Curr. Top. Med. Chem. 2016, 16, 76–88. [Google Scholar] [CrossRef]
- Haney, E.F.; Hancock, R.E.W. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 2013, 100, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Bin Hafeez, A.; Jiang, X.K.; Bergen, P.J.; Zhu, Y. Antimicrobial peptides: An update on classifications and databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.; Fan, H.M.; Tao, H.R.; Huang, J.D. Recent strategies to combat biofilms using antimicrobial agents and therapeutic approaches. Pathogens 2022, 11, 292. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y.Z. Mechanism of antimicrobial peptides: Antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial peptides: A potent alternative to antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Gergova, A. Diversity and mechanisms of action of plant, animal, and human antimicrobial peptides. Antibiotics 2024, 13, 202. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Military Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.D.; Chen, Y.; Chen, Z.Q.; Xue, Z.H.; Jia, Y.N.; Guo, Q.W.; Ma, Q.Q.; Zhang, M.; Chen, H.X. Identification and antimicrobial activity evaluation of three peptides from laba garlic and the related mechanism. Food Funct. 2019, 10, 4486–4496. [Google Scholar] [CrossRef] [PubMed]
- Kapil, S.; Sharma, V. D-Amino acids in antimicrobial peptides: A potential approach to treat and combat antimicrobial resistance. Can. J. Microbiol. 2021, 67, 119–137. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.Y.; Wang, B.; Zhang, K.Y.; Wang, Y. Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Kumari, S.; Booth, V. Antimicrobial peptide mechanisms studied by whole-cell deuterium nmr. Int. J. Mol. Sci. 2022, 23, 2740. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Jian, W.; Xie, C.; Yang, X. Plant antimicrobial peptides: Structures, functions, and applications. Bot. Stud. 2021, 62, 5. [Google Scholar] [CrossRef]
- Patocka, J.; Nepovimova, E.; Klimova, B.; Wu, Q.H.; Kuca, K. Antimicrobial peptides: Amphibian host defense peptides. Curr. Med. Chem. 2019, 26, 5924–5946. [Google Scholar] [CrossRef]
- Mardirossian, M.; Grzela, R.; Giglione, C.; Meinnel, T.; Gennaro, R.; Mergaert, P.; Scocchi, M. The host antimicrobial peptide Bac7 binds to bacterial ribosomal proteins and inhibits protein synthesis. Chem. Biol. 2014, 21, 1639–1647. [Google Scholar] [CrossRef]
- Mardirossian, M.; Pérébaskine, N.; Benincasa, M.; Gambato, S.; Hofmann, S.; Huter, P.; Müller, C.; Hilpert, K.; Innis, C.A.; Tossi, A.; et al. The dolphin proline-rich antimicrobial peptide Tur1A inhibits protein synthesis by targeting the bacterial ribosome. Cell Chem. Biol. 2018, 25, 530–539.e7. [Google Scholar] [CrossRef]
- Le, C.F.; Gudimella, R.; Razali, R.; Manikam, R.; Sekaran, S.D. Transcriptome analysis of treated with the designed antimicrobial peptides, DM3. Sci. Rep. 2016, 6, 26828. [Google Scholar] [CrossRef]
- Wronska, A.K.; Bogus, M.I. Heat shock proteins (HSP 90, 70, 60, and 27) in (Lepidoptera) hemolymph are affected by infection with (Entomophthorales). PLoS ONE 2020, 15, e0228556. [Google Scholar] [CrossRef] [PubMed]
- Kragol, G.; Lovas, S.; Varadi, G.; Condie, B.A.; Hoffmann, R.; Otvos, L. The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 2001, 40, 3016–3026. [Google Scholar] [CrossRef] [PubMed]
- Subbalakshmi, C.; Sitaram, N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96. [Google Scholar] [CrossRef]
- He, S.W.; Zhang, J.; Li, N.Q.; Zhou, S.; Yue, B.; Zhang, M. A TFPI-1 peptide that induces degradation of bacterial nucleic acids, and inhibits bacterial and viral infection in half-smooth tongue sole. Fish Shellfish. Immunol. 2017, 60, 466–473. [Google Scholar] [CrossRef]
- Shu, G.F.; Chen, Y.H.; Liu, T.D.; Ren, S.H.; Kong, Y. Antimicrobial peptide Cathelicidin-BF inhibits platelet aggregation by blocking protease-activated receptor 4. Int. J. Pept. Res. Ther. 2019, 25, 349–358. [Google Scholar] [CrossRef]
- Lutkenhaus, J. Regulation of cell division in E. coli. Trends Genet. 1990, 6, 22–25. [Google Scholar] [CrossRef]
- Cruz, G.F.; de Araujo, I.; Torres, M.D.T.; de la Fuente-Nunez, C.; Oliveira, V.X.; Ambrosio, F.N.; Lombello, C.B.; Almeida, D.V.; Silva, F.D.; Garcia, W. Photochemically-generated silver chloride nanoparticles stabilized by a peptide inhibitor of cell division and its antimicrobial properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2464–2474. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2001, 98, 14637–14642. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, R.; De Chiara, F.; Nocerino, N.; Montella, R.C.; Iannaccone, M.; Fulgione, A.; Romanelli, A.; Avitabile, C.; Blaiotta, G.; Capuano, F. New perspectives for natural antimicrobial peptides: Application as antinflammatory drugs in a murine model. Bmc Immunol. 2012, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Chow, L.N.Y.; Mookherjee, N. Cationic host defence peptides: Multifaceted role in immune modulation and inflammation. J. Innate Immun. 2012, 4, 361–370. [Google Scholar] [CrossRef]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Okumura, K.; Ogawa, H. The human β-defensins (-1,-2,-3,-4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 2005, 175, 1776–1784. [Google Scholar] [CrossRef]
- Smithrithee, R.; Niyonsaba, F.; Kiatsurayanon, C.; Ushio, H.; Ikeda, S.; Okumura, K.; Ogawa, H. Human β-defensin-3 increases the expression of interleukin-37 through CCR6 in human keratinocytes. J. Dermatol. Sci. 2015, 77, 46–53. [Google Scholar] [CrossRef]
- van der Does, A.M.; Joosten, S.A.; Vroomans, E.; Bogaards, S.J.P.; van Meijgaarden, K.E.; Ottenhoff, T.H.M.; van Dissel, J.T.; Nibbering, P.H. The antimicrobial peptide hLF1-11 drives monocyte-dendritic cell differentiation toward dendritic cells that promote antifungal responses and enhance Th17 polarization. J. Innate Immun. 2012, 4, 284–292. [Google Scholar] [CrossRef]
- Gupta, K.; Kotian, A.; Subramanian, H.; Daniell, H.; Ali, H. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget 2015, 6, 28573–28587. [Google Scholar] [CrossRef]
- Jayathilaka, E.H.T.T.; Nikapitiya, C.; De Zoysa, M.; Whang, I. Antimicrobial peptide octominin-encapsulated chitosan nanoparticles enhanced antifungal and antibacterial activities. Int. J. Mol. Sci. 2022, 23, 15882. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, B.; Yang, Y.; Ba, Z.; Zhang, J.; Zhang, T.; Chang, L.; Zhang, F.; Zhang, Y.; Liu, H.; et al. Improving the antimicrobial performance of amphiphilic cationic antimicrobial peptides using glutamic acid full-scan and positive charge compensation strategies. J. Med. Chem. 2022, 65, 13833–13851. [Google Scholar] [CrossRef]
- Yu, W.K.; Sun, Y.; Li, W.Y.; Guo, X.; Liu, X.S.; Wu, W.P.; Yu, W.Q.; Wang, J.J.; Shan, A.S. Self-assembly of antimicrobial peptide-based micelles breaks the limitation of trypsin. ACS Appl. Mater. Inter. 2023, 15, 494–510. [Google Scholar] [CrossRef]
- Song, J.Y.; Cortez-Jugo, C.; Shirbin, S.J.; Lin, Z.X.; Pan, S.J.; Qiao, G.G.; Caruso, F. Immobilization and intracellular delivery of structurally nanoengineered antimicrobial peptide polymers using polyphenol-based capsules. Adv. Funct. Mater. 2022, 32, 2107341. [Google Scholar] [CrossRef]
- Shirbin, S.J.; Insua, I.; Holden, J.A.; Lenzo, J.C.; Reynolds, E.C.; O’Brien-Simpson, N.M.; Qiao, G.G. Architectural effects of star-shaped “structurally nanoengineered antimicrobial peptide polymers” (SNAPPs) on their biological activity. Adv. Healthc. Mater. 2018, 7, e1800627. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Gan, B.-H.; Perron, K.; Cai, X.; Abdel-Sayed, P.; Sublet, E.; Ducret, V.; Nerhot, N.P.; Applegate, L.A.; Borchard, G. Synergistic effects of antimicrobial peptide dendrimer-chitosan polymer conjugates against Pseudomonas aeruginosa. Carbohydr. Polym. 2022, 280, 119025. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.Z.; Pan, L.Q.; Qian, S.Z.; Yang, X.; Pan, L.L.; Chi, R.R.; Chen, J.; Pan, J.Y.; Shi, C.C. Antimicrobial peptide nanoparticles coated with macrophage cell membrane for targeted antimicrobial therapy of sepsis. Mater. Des. 2023, 229, 111883. [Google Scholar] [CrossRef]
- Pan, L.L.; Jiang, D.W.; Pan, L.Q.; Meng, Z.Z.; Zhuang, Y.Y.; Huang, Y.Y.; Ye, F.R.; Shi, C.C.; Chen, J.; Pan, J.Y. ICAM-1-targeted and antibacterial peptide modified polymeric nanoparticles for specific combating sepsis. Mater. Des. 2022, 222, 111007. [Google Scholar] [CrossRef]
- Yazici, H.; Habib, G.; Boone, K.; Urgen, M.; Utku, F.S.; Tamerler, C. Self-assembling antimicrobial peptides on nanotubular titanium surfaces coated with calcium phosphate for local therapy. Mat. Sci. Eng. C-Mater. 2019, 94, 333–343. [Google Scholar] [CrossRef]
- Yamauchi, R.; Kawano, K.; Yamaoka, Y.; Taniguchi, A.; Yano, Y.; Takasu, K.; Matsuzaki, K. Development of antimicrobial peptide-antibiotic conjugates to improve the outer membrane permeability of antibiotics against gram-negative bacteria. ACS Infect. Dis. 2022, 8, 2339–2347. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, J.; Yu, W.; Bai, H.; Lv, F.; Huang, Y. Bacteria-mediated in situ polymerization of peptide-modified acrylamide for enhancing antimicrobial activity. Chem. Commun. 2022, 58, 9946–9949. [Google Scholar] [CrossRef]
- Lou, T.; Bai, X.Q.; He, X.Y.; Liu, W.C.; Yang, Z.C.; Yang, Y.; Yuan, C.Q. Enhanced antifouling properties of marine antimicrobial peptides by PEGylation. Front. Bioeng. Biotechnol. 2023, 11, 1124389. [Google Scholar] [CrossRef]
- Tang, Q.; Tan, P.; Dai, Z.; Wang, T.; Xu, S.; Ding, Y.; Jin, J.; Zhang, X.; Zhang, Y.; Zhou, C.; et al. Hydrophobic modification improves the delivery of cell-penetrating peptides to eliminate intracellular pathogens in animals. Acta Biomater. 2023, 157, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.R.; Kim, S.H.; Park, T.I.; Pack, S.P.; Monedeiro-Milanowski, M. Self-entrapment of antimicrobial peptides in silica particles for stable and effective antimicrobial peptide delivery system. Int. J. Mol. Sci. 2023, 24, 16423. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Yao, Q.; Chen, T.; Wu, H.; Liu, Y.; Wang, C.; Dong, A. Current status of development and biomedical applications of peptide-based antimicrobial hydrogels. Adv. Colloid. Interface Sci. 2024, 325, 103099. [Google Scholar] [CrossRef] [PubMed]
- Cresti, L.; Cappello, G.; Pini, A. Antimicrobial peptides towards clinical application-a long history to be concluded. Int. J. Mol. Sci. 2024, 25, 4870. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.; Kamau, P.M.; Thuku, R.C.; Lai, R. Design methods for antimicrobial peptides with improved performance. Zool. Res. 2023, 44, 1095–1114. [Google Scholar] [CrossRef]
- Sultana, A.; Luo, H.R.; Ramakrishna, S. Antimicrobial peptides and their applications in biomedical sector. Antibiotics 2021, 10, 1094. [Google Scholar] [CrossRef]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides—Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial peptides: A new hope in biomedical and pharmaceutical fields. Front. Cell Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Shin, J.W.; Ki, M.R.; Pack, S.P. Microalgae-derived peptide with dual-functionalities of silica deposition and antimicrobial activity for biosilica-based biomaterial design. Process Biochem. 2024, 146, 204–213. [Google Scholar] [CrossRef]
- Rai, A.; Ferrao, R.; Palma, P.; Patricio, T.; Parreira, P.; Anes, E.; Tonda-Turo, C.; Martins, M.C.L.; Alves, N.; Ferreira, L. Antimicrobial peptide-based materials: Opportunities and challenges. J. Mater. Chem. B 2022, 10, 2384–2429. [Google Scholar] [CrossRef]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef]
- Talapko, J.; Mestrovic, T.; Juzbasic, M.; Tomas, M.; Eric, S.; Aleksijevic, L.H.; Bekic, S.; Schwarz, D.; Matic, S.; Neuberg, M.; et al. Antimicrobial peptides-mechanisms of action, antimicrobial effects and clinical applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yang, M. Antimicrobial peptides: From design to clinical application. Antibiotics 2022, 11, 349. [Google Scholar] [CrossRef]
- Dai, J.H.; Fischer, N.G.; Rahimi, J.R.; Wang, H.N.; Hu, C.M.; Chen, W.E.; Lin, Y.F.; Sang, T.; Chew, H.P.; Kong, L.; et al. Interpenetrating nanofibrillar membrane of self-assembled collagen and antimicrobial peptides for enhanced bone regeneration. Int. J. Biol. Macromol. 2024, 267, 131480. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Chen, R.Y.; Wang, F.S.; Cai, X.J.; Wang, Y.N. Antimicrobial peptide GL13K immobilized onto SLA-treated titanium by silanization: Antibacterial effect against methicillin-resistant (MRSA). RSC Adv. 2022, 12, 6918–6929. [Google Scholar] [CrossRef]
- Gao, S.; Zhai, X.S.; Cheng, Y.; Zhang, R.; Wang, W.T.; Hou, H.X. Starch/PBAT blown antimicrobial films based on the synergistic effects of two commercial antimicrobial peptides. Int. J. Biol. Macromol. 2022, 204, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhu, X.; Mutreja, I.; Boda, S.K.; Fischer, N.G.; Zhang, A.Q.; Lui, C.; Qi, Y.P.; Aparicio, C. Biomimetic mineralized hybrid scaffolds with antimicrobial peptides. Bioact. Mater. 2021, 6, 2250–2260. [Google Scholar] [CrossRef]
- Cao, P.; Liu, D.; Zhang, Y.B.; Xiao, F.; Yuan, C.Q.; Liang, F.; Liu, X.D.; Zhang, C. Dopamine-assisted sustainable antimicrobial peptide coating with antifouling and anticorrosion properties. Appl. Surf. Sci. 2022, 589, 153019. [Google Scholar] [CrossRef]
- Fu, C.; Qi, Z.; Zhao, C.; Kong, W.; Li, H.; Guo, W.; Yang, X. Enhanced wound repair ability of arginine-chitosan nanocomposite membrane through the antimicrobial peptides-loaded polydopamine-modified graphene oxide. J. Biol. Eng. 2021, 15, 17. [Google Scholar] [CrossRef]
- Mariano, G.H.; Gomes de Sa, L.G.; Carmo da Silva, E.M.; Santos, M.A.; Cardozo Fh, J.L.; Lira, B.O.V.; Barbosa, E.A.; Araujo, A.R.; Leite, J.; Ramada, M.H.S.; et al. Characterization of novel human intragenic antimicrobial peptides, incorporation and release studies from ureasil-polyether hybrid matrix. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111581. [Google Scholar] [CrossRef] [PubMed]
- Stromdahl, A.C.; Ignatowicz, L.; Petruk, G.; Butrym, M.; Wasserstrom, S.; Schmidtchen, A.; Puthia, M. Peptide-coated polyurethane material reduces wound infection and inflammation. Acta Biomater. 2021, 128, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Du, C.W.; He, X.Y.; Zhang, C.; Yuan, C.Q. Modification of a derived antimicrobial peptide on steel surface for marine bacterial resistance. Appl. Surf. Sci. 2020, 510, 145512. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, Y.; Gopalakrishnan, S.; Li, K.; Han, Y.; Rotello, V.M. Antimicrobial peptide-loaded pectolite nanorods for enhancing wound-healing and biocidal activity of titanium. ACS Appl. Mater. Inter. 2021, 13, 28764–28773. [Google Scholar] [CrossRef]
- Pihl, M.; Galli, S.; Jimbo, R.; Andersson, M. Osseointegration and antibacterial effect of an antimicrobial peptide releasing mesoporous titania implant. J. Biomed. Mater. Res. B 2021, 109, 1787–1795. [Google Scholar] [CrossRef]
- Atefyekta, S.; Pihl, M.; Lindsay, C.; Heilshorn, S.C.; Andersson, M. Antibiofilm elastin-like polypeptide coatings: Functionality, stability, and selectivity. Acta Biomater. 2019, 83, 245–256. [Google Scholar] [CrossRef]
- Luo, X.F.; Peng, Y.F.; Qin, Z.D.; Tang, W.F.; Duns, G.J.; Dessie, W.; He, N.Y.; Tan, Y.M. Chitosan-based packaging films with an integrated antimicrobial peptide: Characterization, release and application to fresh pork preservation. Int. J. Biol. Macromol. 2023, 231, 123209. [Google Scholar] [CrossRef]
- Rajchakit, U.; Lamba, S.; Wang, K.; Lyons, N.; Lu, J.; Swift, S.; Pletzer, D.; Sarojini, V. Size-controlled synthesis of gold nanoparticles tethering antimicrobial peptides with potent broad-spectrum antimicrobial and antibiofilm activities. Mol. Pharm. 2024, 21, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Cao, Y.G.; Ma, L.L.; Sun, J.F.; Ramos-Mucci, L.; Ma, Y.; Yang, X.; Zhu, Z.H.; Zhang, J.X.; Xiao, B. Oral antimicrobial peptide-EGCG nanomedicines for synergistic treatment of ulcerative colitis. J. Control. Release 2022, 347, 544–560. [Google Scholar] [CrossRef]
- Caselli, L.; Traini, T.; Micciulla, S.; Sebastiani, F.; Koehler, S.; Nielsen, E.M.; Diedrichsen, R.G.; Skoda, M.W.A.; Malmsten, M. Antimicrobial peptide coating of TiO nanoparticles for boosted antimicrobial effects. Adv. Funct. Mater. 2024, 2405047. [Google Scholar] [CrossRef]
- Teng, R.X.; Yang, Y.Y.; Zhang, Z.; Yang, K.X.; Sun, M.; Li, C.; Fan, Z.; Du, J.Z. In situ enzyme-induced self-assembly of antimicrobial-antioxidative peptides to promote wound healing. Adv. Funct. Mater. 2023, 33, 2214454. [Google Scholar] [CrossRef]
- Caselli, L.; Parra-Ortiz, E.; Micciulla, S.; Skoda, M.W.A.; Häffner, S.M.; Nielsen, E.M.; van der Plas, M.J.A.; Malmsten, M. Boosting membrane interactions and antimicrobial effects of photocatalytic titanium dioxide nanoparticles by peptide coating. Small 2024, 20, e2309496. [Google Scholar] [CrossRef] [PubMed]
- Jabalera, Y.; Montalban-Lopez, M.; Vinuesa-Rodriguez, J.J.; Iglesias, G.R.; Maqueda, M.; Jimenez-Lopez, C. Antibacterial directed chemotherapy using AS-48 peptide immobilized on biomimetic magnetic nanoparticles combined with magnetic hyperthermia. Int. J. Biol. Macromol. 2021, 189, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Ropero-Vega, J.L.; Ardila-Rosas, N.; Hernández, I.P.; Flórez-Castillo, J.M. Immobilization of Ib-M2 peptide on core@shell nanostructures based on SPION nanoparticles and their antibacterial activity against. Appl. Surf. Sci. 2020, 515, 146045. [Google Scholar] [CrossRef]
- Zhou, W.H.; Bai, T.; Wang, L.; Cheng, Y.; Xia, D.D.; Yu, S.; Zheng, Y.F. Biomimetic AgNPs@antimicrobial peptide/silk fibroin coating for infection-trigger antibacterial capability and enhanced osseointegration. Bioact. Mater. 2023, 20, 64–80. [Google Scholar] [CrossRef]
- Comune, M.; Rai, A.; Palma, P.; TondaTuro, C.; Ferreira, L. Antimicrobial and pro-angiogenic properties of soluble and nanoparticle-immobilized LL37 peptides. Biomater. Sci. 2021, 9, 8153–8159. [Google Scholar] [CrossRef]
- Ye, Z.K.; Zhu, H.S.; Zhang, S.; Li, J.; Wang, J.; Wang, E.K. Highly efficient nanomedicine from cationic antimicrobial peptide-protected Ag nanoclusters. J. Mater. Chem. B 2021, 9, 307–313. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wu, Y.M.; Liu, L.Q.; Xiao, X.M.A.; Cong, Z.H.; Shao, N.; Qiao, Z.Q.; Chen, K.; Liu, S.Q.; Zhang, H.D.; et al. The membrane-targeting mechanism of host defense peptides inspiring the design of polypeptide-conjugated gold nanoparticles exhibiting effective antibacterial activity against methicillin-resistant. J. Mater. Chem. B 2021, 9, 5092–5101. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; van Gent, M.E.; de Waal, A.M.; van Doodewaerd, B.R.; Bos, E.; Koning, R.I.; Cordfunke, R.A.; Drijfhout, J.W.; Nibbering, P.H. Physical and functional characterization of PLGA nanoparticles containing the antimicrobial peptide SAAP-148. Int. J. Mol. Sci. 2023, 24, 2867. [Google Scholar] [CrossRef]
- Klubthawee, N.; Bovone, G.; Marco-Dufort, B.; Guzzi, E.A.; Aunpad, R.; Tibbitt, M.W. Biopolymer nano-network for antimicrobial peptide protection and local delivery. Adv. Healthc. Mater. 2022, 11, 2101426. [Google Scholar] [CrossRef] [PubMed]
- Cresti, L.; Conte, G.; Cappello, G.; Brunetti, J.; Falciani, C.; Bracci, L.; Quaglia, F.; Ungaro, F.; d’Angelo, I.; Pini, A. Inhalable polymeric nanoparticles for pulmonary delivery of antimicrobial peptide SET-M33: Antibacterial activity and toxicity in vitro and in vivo. Pharmaceutics 2023, 15, 3. [Google Scholar] [CrossRef]
- Meikle, T.G.; Dharmadana, D.; Hoffmann, S.V.; Jones, N.C.; Drummond, C.J.; Conn, C.E. Analysis of the structure, loading and activity of six antimicrobial peptides encapsulated in cubic phase lipid nanoparticles. J. Colloid Interface Sci. 2021, 587, 90–100. [Google Scholar] [CrossRef]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio 2019, 3, 100024. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, M.; Voelkel, A.; Sandomierski, M. Crystalline zeolite layers on the surface of titanium alloys in biomedical applications: Current knowledge and possible directions of development. Crystals 2022, 12, 1520. [Google Scholar] [CrossRef]
- Maher, S.; Linklater, D.; Rastin, H.; Liao, S.T.Y.; de Sousa, K.M.; Lima-Marques, L.; Kingshott, P.; Thissen, H.; Ivanova, E.P.; Losic, D. Advancing of 3D-printed titanium implants with combined antibacterial protection using ultrasharp nanostructured surface and gallium-releasing agents. ACS Biomater. Sci. Eng. 2022, 8, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Ch, S.; Mishra, P.; Padaga, S.G.; Ghosh, B.; Roy, S.; Biswas, S. 3D-printed inherently antibacterial contact lens-like patches carrying antimicrobial peptide payload for treating bacterial keratitis. Macromol. Biosci. 2024, 24, e2300418. [Google Scholar] [CrossRef]
- Kruse, H.V.; Chakraborty, S.; Chen, R.; Kumar, N.; Yasir, M.; Lewin, W.T.; Suchowerska, N.; Willcox, M.D.; McKenzie, D.R. Protecting orthopaedic implants from infection: Antimicrobial peptide Mel4 Is non-toxic to bone cells and reduces bacterial colonisation when bound to plasma Ion-implanted 3D-printed PAEK polymers. Cells 2024, 13, 656. [Google Scholar] [CrossRef]
- Karamat-Ullah, N.; Demidov, Y.; Schramm, M.; Grumme, D.; Auer, J.; Bohr, C.; Brachvogel, B.; Maleki, H. 3D printing of antibacterial, biocompatible, and biomimetic hybrid aerogel-based scaffolds with hierarchical porosities via integrating antibacterial peptide-modified silk fibroin with silica nanostructure. ACS Biomater. Sci. Eng. 2021, 7, 4545–4556. [Google Scholar] [CrossRef]
- Chen, L.; Shao, L.P.; Wang, F.P.; Huang, Y.F.; Gao, F.H. Enhancement in sustained release of antimicrobial peptide and BMP-2 from degradable three dimensional-printed PLGA scaffold for bone regeneration. RSC Adv. 2019, 9, 10494–10507. [Google Scholar] [CrossRef]
- Tian, L.J.; Zhang, Z.T.; Tian, B.; Zhang, X.; Wang, N. Study on antibacterial properties and cytocompatibility of EPL coated 3D printed PCL/HA composite scaffolds. RSC Adv. 2020, 10, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wang, Y.; Guo, M.; Wang, Z.L.; Shao, N.N.; Zhang, P.B.; Chen, X.S.; Huang, Y.B. Degradable three dimensional-printed polylactic acid scaffold with long-term antibacterial activity. ACS Sustain. Chem. Eng. 2018, 6, 2047–2054. [Google Scholar] [CrossRef]
- Murphy, R.; Kordbacheh, S.; Skoulas, D.; Ng, S.; Suthiwanich, K.; Kasko, A.M.; Cryan, S.A.; Fitzgerald-Hughes, D.; Khademhosseini, A.; Sheikhi, A.; et al. Three-dimensionally printable shear-thinning triblock copolypeptide hydrogels with antimicrobial potency. Biomater. Sci. 2021, 9, 5144–5149. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, G.; Mao, C.W.; Armine, A.; Kasher, R.; Arnusch, C.J. Ink-jet printing-assisted modification on polyethersulfone membranes using a UV-reactive antimicrobial peptide for fouling-resistant surfaces. ACS Omega 2018, 3, 8752–8759. [Google Scholar] [CrossRef]
- Asif, F.; Zaman, S.U.; Arnab, M.K.H.; Hasan, M.; Islam, M.M. Antimicrobial peptides as therapeutics: Confronting delivery challenges to optimize efficacy. Microbe 2024, 2, 100051. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef]
- Molhoek, E.M.; van Dijk, A.; Veldhuizen, E.J.A.; Haagsman, H.P.; Bikker, F.J. Improved proteolytic stability of chicken cathelicidin-2 derived peptides by D-amino acid substitutions and cyclization. Peptides 2011, 32, 875–880. [Google Scholar] [CrossRef]
- Giuliani, A.; Rinaldi, A.C. Beyond natural antimicrobial peptides: Multimeric peptides and other peptidomimetic approaches. Cell Mol. Life Sci. 2011, 68, 2255–2266. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; De Biasi, M.G.; Mancusi, A. An overview of the potentialities of antimicrobial peptides derived from natural sources. Antibiotics 2022, 11, 1483. [Google Scholar] [CrossRef]
- He, S.Q.; Yang, Z.Y.; Li, X.F.; Wu, H.; Zhang, L.C.; Shan, A.S.; Wang, J.J. Boosting stability and therapeutic potential of proteolysis-resistant antimicrobial peptides by end-tagging β-naphthylalanine. Acta Biomater. 2023, 164, 175–194. [Google Scholar] [CrossRef]
- Boda, S.K.; Fischer, N.G.; Ye, Z.; Aparicio, C. Dual oral tissue adhesive nanofiber membranes for pH-responsive delivery of antimicrobial peptides. Biomacromolecules 2020, 21, 4945–4961. [Google Scholar] [CrossRef]
- Dubey, A.; Vahabi, H.; Kumaravel, V. Antimicrobial and biodegradable 3D printed scaffolds for orthopedic infections. Acs Biomater. Sci. Eng. 2023, 9, 4020–4044. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Xuan, J.Q.; Feng, W.G.; Wang, J.Y.; Wang, R.C.; Zhang, B.W.; Bo, L.T.; Chen, Z.S.; Yang, H.; Sun, L.M. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Update 2023, 68, 100954. [Google Scholar] [CrossRef]
- Ciulla, M.G.; Civera, M.; Sattin, S.; Kumar, K. Nature-inspired and medicinally relevant short peptides. Explor. Drug Sci. 2023, 1, 140–171. [Google Scholar] [CrossRef]
- Su, M.; Su, Y.X. Recent advances in amphipathic peptidomimetics as antimicrobial agents to combat drug resistance. Molecules 2024, 29, 2492. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.H.; Yuan, X.J.; Chen, H.Y.; Zhu, Y.H.; Dong, N.; Shan, A.S. Strategies employed in the design of antimicrobial peptides with enhanced proteolytic stability. Biotechnol. Adv. 2022, 59, 107962. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Shamseddine, L.; Roblin, C.; Veyrier, I.; Basset, C.; De Macedo, L.; Boyeldieu, A.; Maresca, M.; Nicoletti, C.; Brasseur, G.; Kieffer-Jaquinod, S. Mechanistic and functional aspects of the Ruminococcin C sactipeptide isoforms. iScience 2023, 26, 107563. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial peptides targeting Gram-positive bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- Chaudhary, S.; Ali, Z.; Mahfouz, M. Molecular farming for sustainable production of clinical-grade antimicrobial peptides. Plant Biotechnol. J. 2024, 22, 2282–2300. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.H.; Kim, K.H.; Ki, M.-R.; Pack, S.P. Antimicrobial Peptides and Their Biomedical Applications: A Review. Antibiotics 2024, 13, 794. https://doi.org/10.3390/antibiotics13090794
Min KH, Kim KH, Ki M-R, Pack SP. Antimicrobial Peptides and Their Biomedical Applications: A Review. Antibiotics. 2024; 13(9):794. https://doi.org/10.3390/antibiotics13090794
Chicago/Turabian StyleMin, Ki Ha, Koung Hee Kim, Mi-Ran Ki, and Seung Pil Pack. 2024. "Antimicrobial Peptides and Their Biomedical Applications: A Review" Antibiotics 13, no. 9: 794. https://doi.org/10.3390/antibiotics13090794
APA StyleMin, K. H., Kim, K. H., Ki, M.-R., & Pack, S. P. (2024). Antimicrobial Peptides and Their Biomedical Applications: A Review. Antibiotics, 13(9), 794. https://doi.org/10.3390/antibiotics13090794