Green Synthesis of Silver Nanoparticle from Anadenanthera colubrina Extract and Its Antimicrobial Action against ESKAPEE Group Bacteria
Abstract
1. Introduction
2. Results
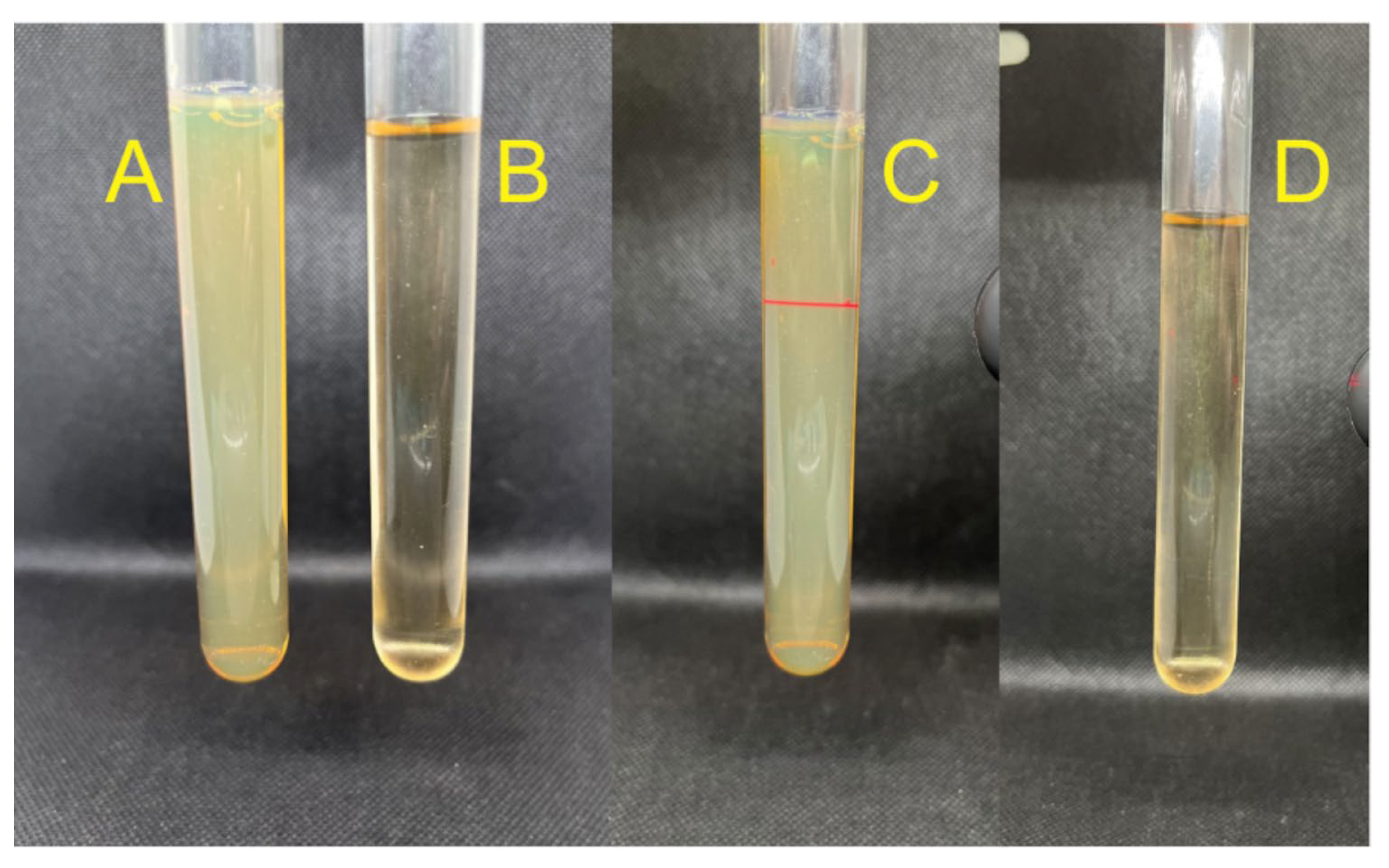
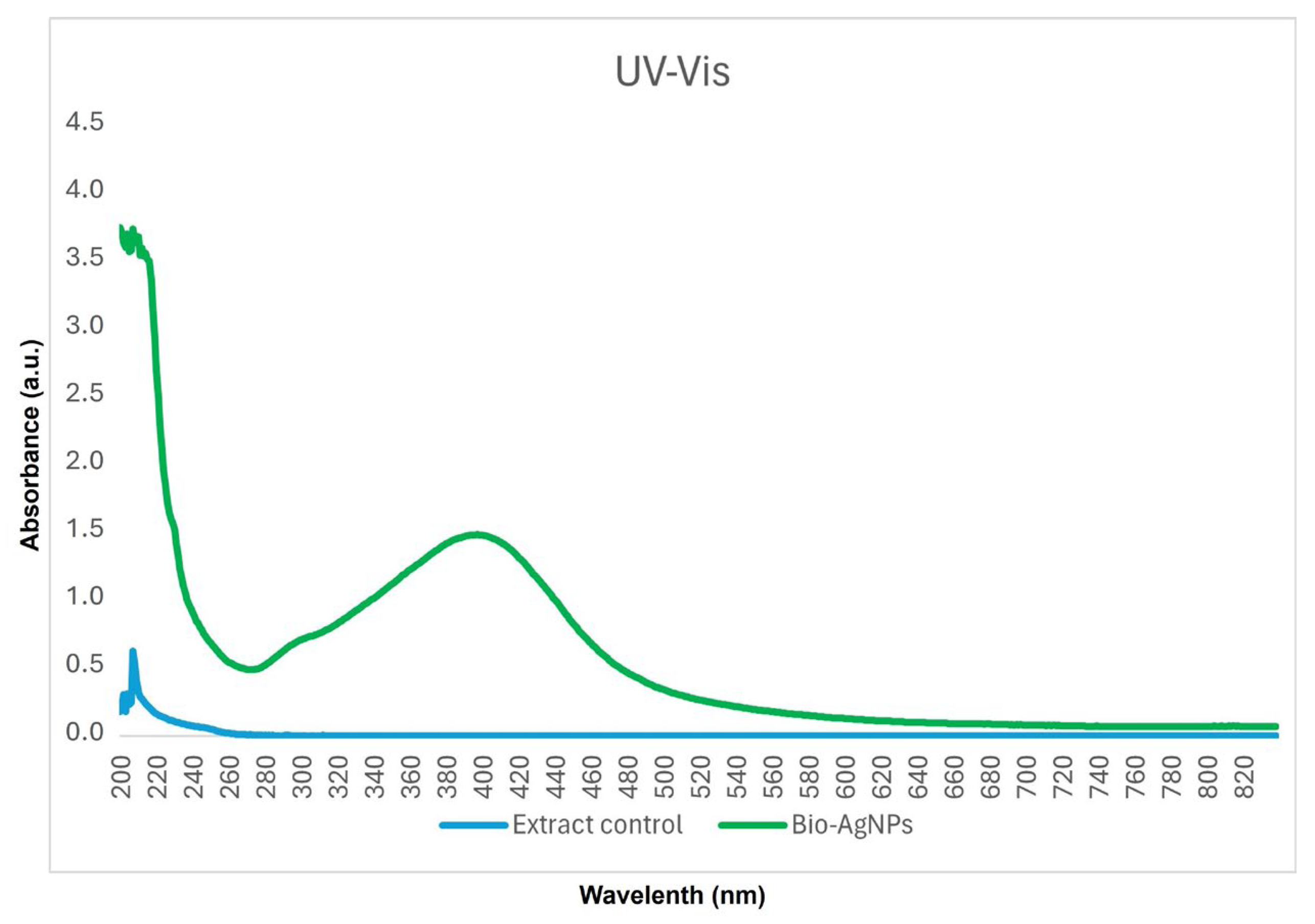
2.1. Synthesis of Biogenic Nanoparticles
2.2. Average Diameter Size, Zeta Potential and Polydispersity Index (PDI)
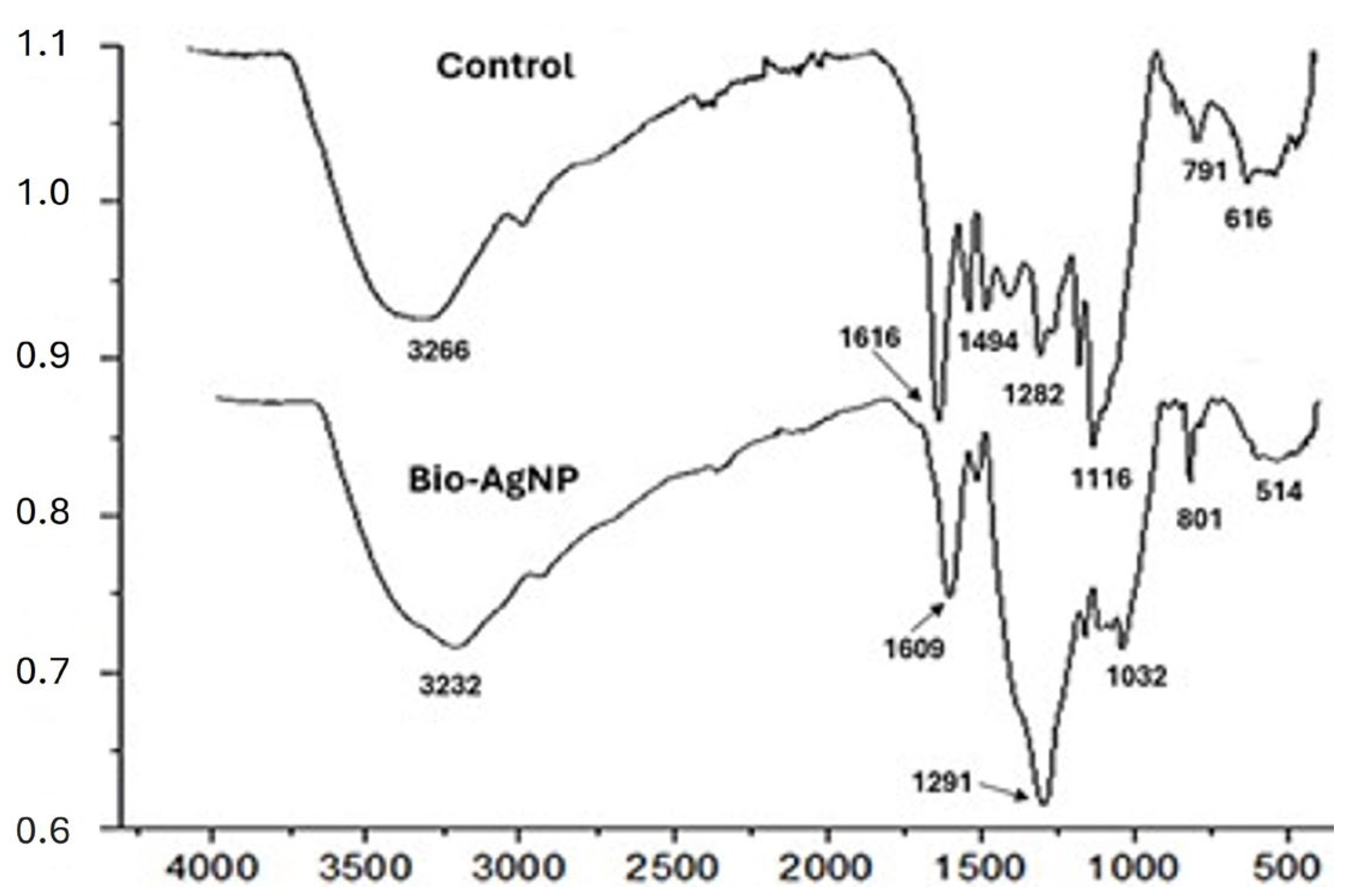
2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4. X-ray Diffraction (XRD)
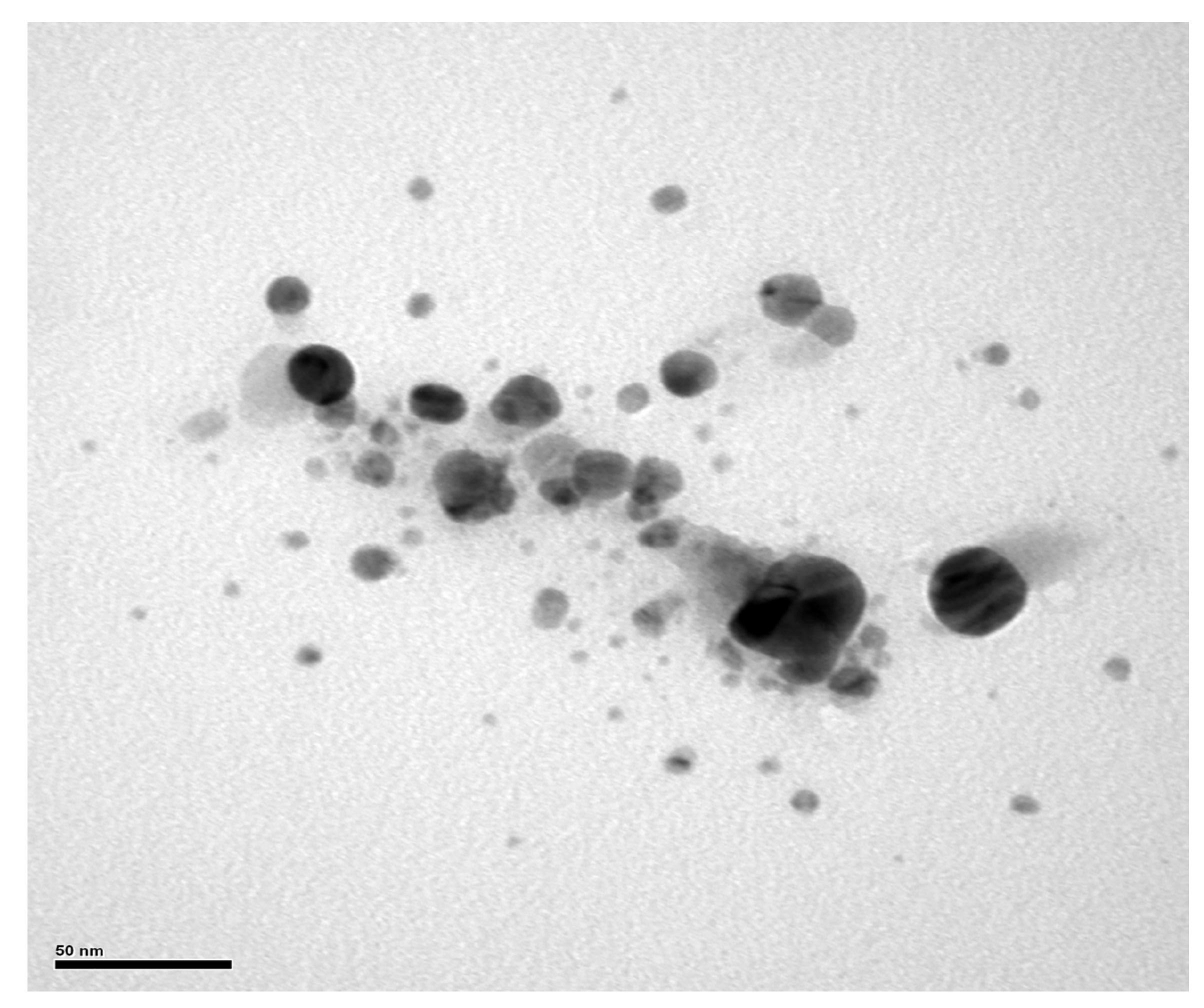
2.5. Electron Microscopy Images
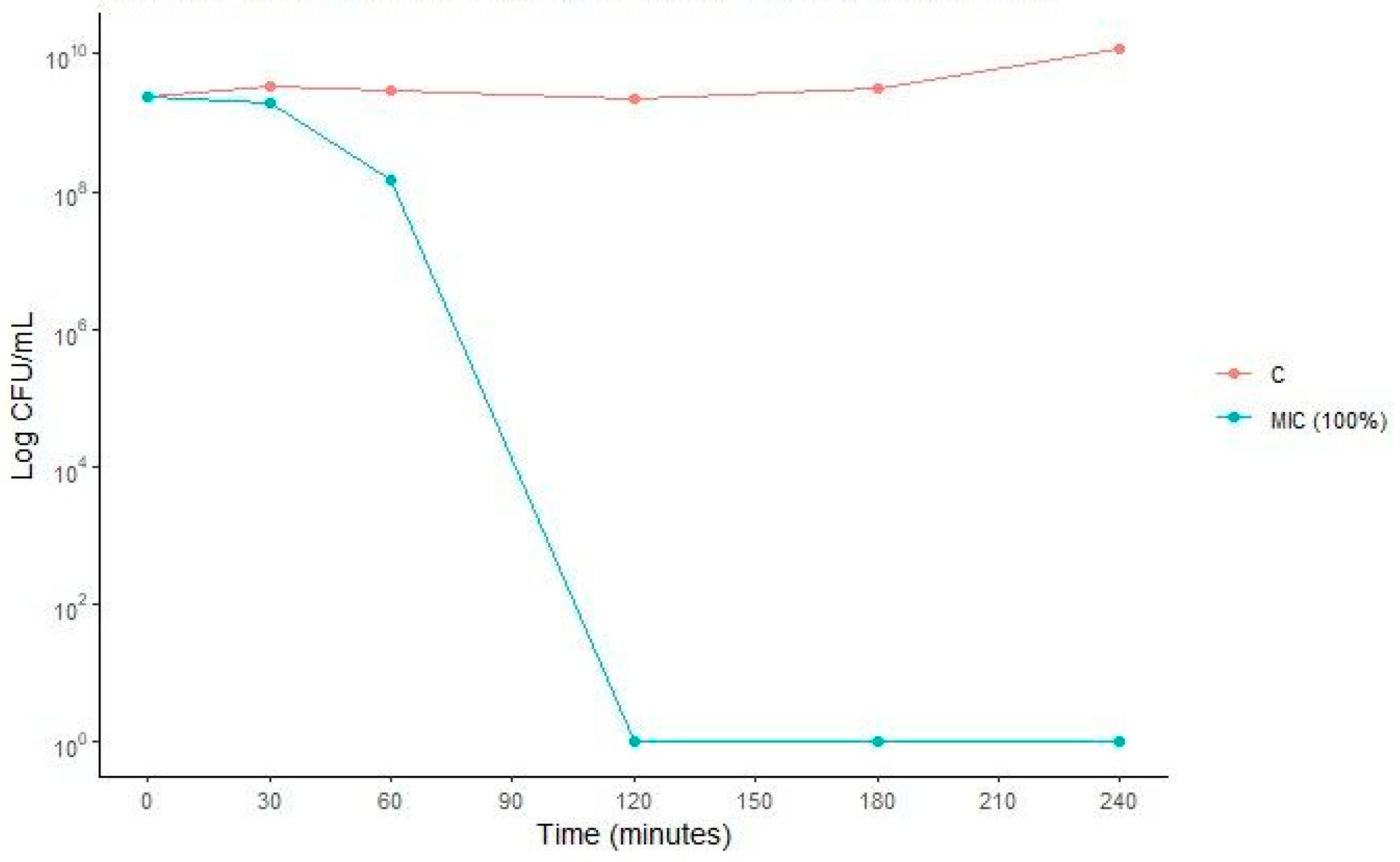
2.6. Antibacterial Activity
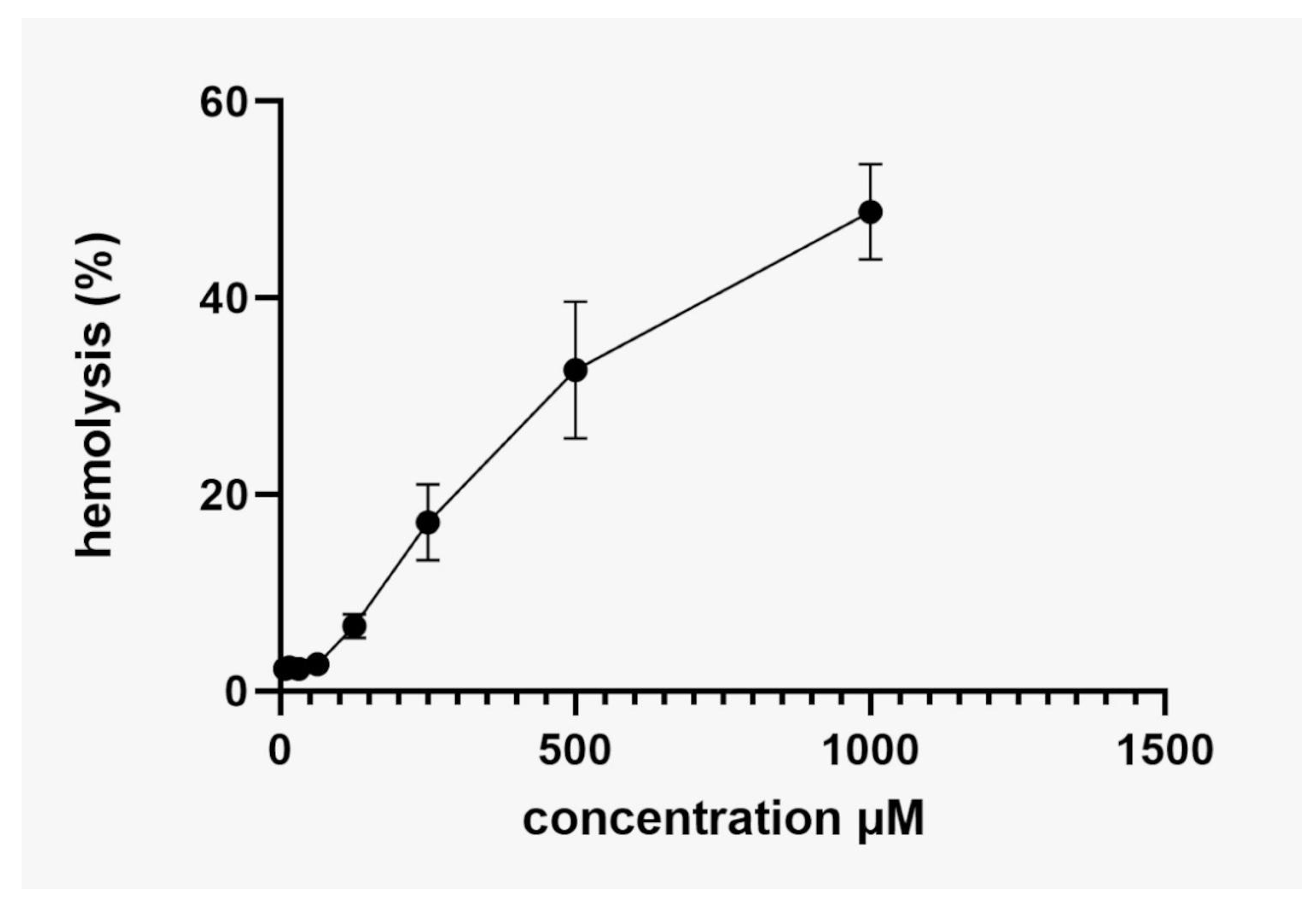
2.7. Hemolytic Activity
3. Discussion
4. Materials and Methods
4.1. Preparation of the Extract
4.2. Biosynthesis of Bio-AgNPs
4.3. Characterization of Bio-AgNPs
4.4. Evaluation of the Biological Activity of Bio-AgNPs
4.4.1. Tested Strains
4.4.2. Antibacterial Activity
4.4.3. Hemolytic Activity Assay of Bio-AgNPs
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuong, N.V.; Padungtod, P.; Thwaites, G.; Carrique-Mas, J.J. Antimicrobial usage in animal production: A review of the literature with a focus on low-and middle-income countries. Antibiotics 2018, 7, 75. [Google Scholar] [CrossRef]
- Franco, B.E.; Altagracia, M.M.; Sánchez, R.M.A.; Wertheimer, A.I. The determinants of the antibiotic resistance process. Infect. Drug Resist. 2009, 2, 1–11. [Google Scholar] [PubMed]
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics use in food animal production: Escalation of antimicrobial resistance: Where are we now in combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.J.; Gunn, A. The One Health Concept. Br. J. Biomed. Sci. 2024, 81, 12366. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 9 July 2024).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J.I.M. Tackling drug-resistant infections globally: Final report and recommendation. Rev. Antimicrob. Resist. 2014, 84. [Google Scholar] [CrossRef]
- Lin, Q.; Deslouches, B.; Montelaro, R.C.; Di, Y.P. Prevention of ESKAPE pathogen biofilm formation by antimicrobial peptides WLBU2 and LL37. Int. J. Antimicrob. Agents 2018, 52, 667–672. [Google Scholar] [CrossRef]
- Willyard, C. Drug-resistant bacteria ranked. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 9 July 2024).
- Gomes, T.A.T.; Elias, W.P.; Scaletsky, I.C.A.; Guth, B.E.C.; Rodrigues, J.F.; Piazza, R.M.F.; Ferreira, L.C.S.; Martinez, M.B. Diarrheagenic Escherichia coli. Brazilian J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.C.; Antunes, L.C.M.; Ferreira, R.B.R. Global priority pathogens: Virulence, antimicrobial resistance and prospective treatment options. Future Microbiol. 2020, 15, 649–677. [Google Scholar] [CrossRef] [PubMed]
- Hamers, V.; Huguet, C.; Bourjot, M.; Urbain, A. Antibacterial Compounds from Mushrooms: A Lead to Fight ESKAPEE Pathogenic Bacteria? Planta Med. 2021, 87, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Rahman, A.M.M.T.; Hassan, J.; Rahman, M.T. Extended-spectrum beta-lactamase in Escherichia coli isolated from humans, animals, and environments in Bangladesh: A One Health perspective systematic review and meta-analysis. One Health 2023, 16, 100526. [Google Scholar] [CrossRef] [PubMed]
- Brindangnanam, P.; Sawant, A.R.; Prashanth, K.; Coumar, M.S. Bacterial effluxome as a barrier against antimicrobial agents: Structural biology aspects and drug targeting. Tissue Barriers 2022, 10, 2013695. [Google Scholar] [CrossRef]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef]
- Scandorieiro, S.; Camargo, L.C.; Lancheros, C.A.; Yamada-Ogatta, S.F.; Nakamura, C.V.; Oliveira, A.G.; Andrade, C.G.; Duran, N.; Nakazato, G.; Kobayashi, R.K. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef]
- Figueiredo, E.P.; Ribeiro, J.M.; Nishio, E.K.; Scandorieiro, S.; Costa, A.F.; Cardozo, V.F.; Oliveira, A.G.; Durán, N.; Panagio, L.A.; Kobayashi, R.K.; et al. New approach for simvastatin as an antibacterial: Synergistic effect with bio-synthesized silver nanoparticles against multidrug-resistant bacteria. Int. J. Nanomedicine 2019, 14, 7975–7985. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Souza, L.M.S.; Miriam, D.; Sarmiento, J.J.P.; Seabra, A.B.; Medeiros, L.P.; Lourenço, I.M.; Kobayashi, R.K.; Nakazato, G. Biosynthesis of selenium nanoparticles using combinations of plant extracts and their antibacterial activity. Curr. Res. Green. Sustain. Chem. 2022, 5, 100303. [Google Scholar] [CrossRef]
- Fatima, M.; Zaidi, S.; Amraiz, D.; Afzal, F. In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 influenza A virus. J. Microbiol. Biotechnol. 2016, 26, 151–159. [Google Scholar] [CrossRef]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green nanotechnology: Plant-mediated nanoparticle synthesis and application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, N.; Jahan, N.; Khalil-Ur-Rahman; Anwar, T.; Qureshi, H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M. Plant-Based Green Synthesis of Silver Nanoparticles and Its Effective Role in Abiotic Stress Tolerance in Crop Plants. Saudi J. Biol. Sci. 2021, 28, 5631–5639. [Google Scholar] [CrossRef]
- de Almeida Maia, C.M.; Vasconcelos, P.G.S.; Pasetto, S.; Godwin, W.C.; Silva, J.P.R.; Tavares, J.F.; Pardi, V.; Costa, E.M.M. de B.; Murata, R.M. Anadenanthera Colubrina Regulated LPS-Induced Inflammation by Suppressing NF-ΚB and P38-MAPK Signaling Pathways. Sci. Rep. 2024, 14, 16028. [Google Scholar]
- Delices, M.; Muller, J.d.A.I.; Arunachalam, K.; Martins, D.T.O. Anadenanthera Colubrina (Vell) Brenan: Ethnobotanical, Phytochemical, Pharmacological and Toxicological Aspects. J. Ethnopharmacol. 2023, 300, 115745. [Google Scholar] [CrossRef]
- Nascimento, J.P.B.; Bispo, J.S.; Dantas, B.F. Angico Anadenanthera colubrina varo cebil (Vell.) Brenan. Nota Técnica 10 Angico. Inf. Abrates 2019, 29, 47–51. [Google Scholar]
- Mota, G.S.; Sartori, C.J.; Miranda, I.; Quilhó, T.; Mori, F.A.; Pereira, H. Bark anatomy, chemical composition and ethanol-water extract composition of Anadenanthera peregrina and Anadenanthera colubrina. PLoS ONE 2017, 12, e0189263. [Google Scholar] [CrossRef]
- Krysa, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef]
- Araújo, T.S.L.; de Oliveira, T.M.; de Sousa, N.A.; Souza, L.K.M.; Sousa, F.B.M.; de Oliveira, A.P.; Nicolau, L.A.D.; da Silva, A.A.V.; Araújo, A.R.; Magalhães, P.J.C.; et al. Biopolymer extracted from Anadenanthera colubrina (Red angico gum) exerts therapeutic potential in mice: Antidiarrheal activity and safety assessment. Pharmaceuticals 2020, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, J.; Stergiadis, S.; Koidis, A.; Smith, J.; Humphrey, C.; Whistance, L.; Theodoridou, K. Rapid tannin profiling of tree fodders using untargeted mid-infrared spectroscopy and partial least squares regression. Plant Methods 2021, 17, 14. [Google Scholar] [CrossRef]
- Omran, A.M.E. Green route synthesis of silver nanoparticles driven by Cassia fistula flower extract: Characterization, antioxidant, antibacterial, anticancer, and photocatalytic assessment. Biomass Conv. Bioref 2023. [Google Scholar] [CrossRef]
- Feria-Reyes, R.; Ramírez-Cruz, S.O.; Ruiz-Aquino, F.; Robledo-Taboada, L.H.; Sánchez-Medina, M.A.; Mijangos-Ricárdez, O.F.; Gabriel-Parra, R.; Suárez-Mota, M.E.; Puc-Kauil, R.; Porcallo-Vargas, J. Pine Bark as a Potential Source of Condensed Tannin: Analysis through Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), and Energy Dispersive X-ray (EDX). Forests 2023, 14, 1433. [Google Scholar] [CrossRef]
- Alzubaidi, A.K.; Al-Kaabi, W.J.; Al Ali, A.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Asiri, M.; Khane, Y. Green Synthesis and Characterization of Silver Nanoparticles Using Flaxseed Extract and Evaluation of Their Antibacterial and Antioxidant Activities. Appl. Sci. 2023, 13, 2182. [Google Scholar] [CrossRef]
- World Health Organization. Report of the International Conference on Primary Health Care, Jointly Sponsored by the World Health Organization and the United Nations Children’s Fund, Alma-Ata, URSS; World Health Organization: Geneva, Switzerland, 1978. [Google Scholar]
- Rodrigues, A.R.D.S.P.; Alencar, C.D.C. Ação antioxidante de espécies vegetais nativas do Brasil: Uma revisão integrativa. Revista Fitos 2023, 17, 551–560. [Google Scholar] [CrossRef]
- Silva, A.C.D. Fungos Endofíticos de Anadenanthera colubrina (Vell.) Brenan de área de Caatinga: Diversidade e Potencial Antifúngico Contra Espécies de Sporothrix. Master’s Thesis, Universidade Federal de Pernambuco, Recife, Brazil, 2023. [Google Scholar]
- Flora do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB126378 (accessed on 22 December 2023).
- Nakaoka Sakita, M.; Vallilo, M.I. Preliminary phytochemical studies of forest tree species of Morro do Diabo State Park. Rev. Do Inst. Florest. 1990, 2, 215226. [Google Scholar]
- Lorenzi, H.; Matos, F.J. Plantas Medicinais No Brasil: Nativas e Exóticas, 2nd ed.; Nova Odessa, S.P., Ed.; Instituto Plantarum: Nova Odessa, Brazil, 2008; p. 576. [Google Scholar]
- Luna, E.M.; Lopes, H.T.O.; Rodrigues, F.A.Á.; Coutinho, H.D.M.; de Oliveira, L.C.C. Antioxidant potential of the Caatinga flora. Phytomedicine Plus 2022, 2, 100240. [Google Scholar] [CrossRef]
- Duran, N.; Seabra, A.B. Biogenic synthesized Ag/Au nanoparticles: Production, characterization, and applications. Current Nanosci. 2018, 14, 82–94. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Aydin, G.; Altinsoy, B.; Altinkaynak, C.; Koşar, M.; Ocsoy, I. The Effect of Pelargonium endlicherianum Fenzl. root extracts on formation of nanoparticles and their antimicrobial activities. Enz. Microbial. Technolo. 2017, 97, 21–26. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Synthesis of biosurfactant stabilized silver nanoparticles, characterization and their potential application for bactericidal purposes. J. Hazard. Mater. 2020, 393, 122319. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Chapman, P.; Shah, M.; Fawcett, D. Green biosynthesis of silver nanocubes using the leaf extracts from Eucalyptus macrocarpa. Nano. Bull. 2013, 2, 1–7. [Google Scholar]
- Danaei, M.R.M.M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Bates, S.; Zografi, G.; Engers, D.; Morris, K.; Crowley, K.; Newman, A. Analysis of amorphous and nanocrystalline solids from their X-ray diffraction patterns. Pharm. Res. 2006, 23, 2333–2349. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Bieski, I.G.C.; Santos, F.R.; Oliveira, R.M.; Espinosa, M.M.; Macedo, M.; Albuquerque, U.P.; Martins, D.T.O. Ethnopharmacology of medicinal plants of the pantanal region (Mato Grosso, Brazil). Evi. Based Complement Altern. Med. 2012, 2012, 272749. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.J.L.; Pinheiro, A.A.V.; Oliveira Barreto, E. Medicinal plants used in the treatment of asthma in different regions of Brazil: A comprehensive review of ethnomedicinal evidence, preclinical pharmacology and clinical trials. Phytomedicine Plus 2022, 2, 100376. [Google Scholar] [CrossRef]
- Sena, A.E.C.; Ramos, A.L.; Faria, F.S.E.D.V. Avaliação da síntese de nanopartículas de prata sob diferentes concentrações do extrato de Copaíba multijuga (Heine). Sci. Nat. 2019, 1, 1. [Google Scholar]
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Ejobi, F.; Tweyongyere, R.; Lubwama, M.; Kirabira, J.B.; Wampande, E.M. Nanotechnological solutions for controlling transmission and emergence of antimicrobial-resistant bacteria, future prospects, and challenges: A systematic review. J. Nanoparticle Res. 2020, 22, 117. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Angwe, M.K.; Wampande, E.M.; Ejobi, F.; Nxumalo, E.; Maaza, M.; Sackey, J.; Kirabira, J.B. Phyto-mediated copper oxide nanoparticles for antibacterial, antioxidant and photocatalytic performances. Front. Bioeng Biotechnolo 2022, 10, 820218. [Google Scholar] [CrossRef]
- Filon, F.L.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.C.; Yadav, S.K. Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol. 2010, 85, 1301–1309. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Genebra, Suíça, 2017. [Google Scholar]
- Jiang, Y.; Ding, Y.; Wei, Y.; Jian, C.; Liu, J.; Zeng, Z. Carbapenem-resistant Acinetobacter baumannii: A challenge in the intensive care unit. Front. Microbiol. 2022, 13, 1045206. [Google Scholar]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The alarming antimicrobial resistance in ESKAPEE pathogens: Can essential oils come to the rescue. Fitoterapia 2020, 140, 104433. [Google Scholar] [CrossRef]
- World Health Organization. 2021 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Genebra, Suíça, 2022. [Google Scholar]
- Hashemi, A.; Tavafi, H.; Naseri, M.; Mojtabazadeh, H.; Abedi, M.; Tork, N. Structural and antibacterial properties of AgFe2O4 and Fe3O4 nanoparticles, and their nanocomposites. Prog. Phys. Appl. Mater. 2024, 4, 37–46. [Google Scholar]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Thomas, B.; Vithiya, B.; Prasad, T.; Mohamed, S.B.; Magdalane, C.M.; Kaviyarasu, K.; Maaza, M. Antioxidant and photocatalytic activity of aqueous leaf extract mediated green synthesis of silver nanoparticles using Passiflora edulis f. flavicarpa. J. Nanosci. Nanotechnol. 2019, 19, 2640–2648. [Google Scholar] [CrossRef]
- Lakkim, V.; Reddy, M.C.; Pallavali, R.R.; Reddy, K.R.; Reddy, C.V.; Inamuddin; Bilgrami, A.L.; Lomada, D. Green Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial Activity against Multidrug-Resistant Bacteria and Wound Healing Efficacy Using a Murine Model. Antibiotics 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.M.C.; da Silva, J.M.; Silva, R.B.; da Silva, H.C.H.; Silva, J.V.; Moura, F.D.B.P. Potencial alelopático de extratos aquosos de folhas de Anadenanthera colubrina (vell.) Brenan. Rev. Ouricuri. 2020, 10, 001–010. [Google Scholar] [CrossRef]
- Lima, A.R.N.; Macedo, R.G.; Batista, G.G.; Câmara, G.B.; de Freitas Lima, R.; de Oliveira, T.K.B. Atividade antimicrobiana e anti-inflamatória da Anadenanthera colubrina (Vell.) Brenan. Res. Soc. Dev. 2020, 9, e121911770. [Google Scholar] [CrossRef]
- Volpato, L.E.R.; Trigueiro, P.G.D.C.; Aranha, A.M.F.; Violante, I.M.P.; Silva, R.A.D.; Oliveira, R.C.D. Antimicrobial potential of plant extracts from the Brazilian Cerrado. Braz. Dent. J. 2022, 33, 96–104. [Google Scholar] [CrossRef]
- Gomes, D.M.D.; Durán, N.; Seabra, A.B.; de Paiva Silva, L.; Prado, F.B.; de Amorim Silva, T.; Teixeira, M.F.S. Síntese verde de nanopartículas de prata intermediada por fungo anamórfico e eficácia antibacteriana e antifúngica. Bol. Do Mus. Para. Emílio Goeldi-Ciências Nat. 2020, 15, 433–443. [Google Scholar] [CrossRef]
- Rocha, E.A.L.S.S.; de Medeiros, A.C.D.; de Castro, R.D.; Rosalen, P.L.; Saraiva, K.L.A.; Godoy, G.P.; da Silva, L.R.A.; Aleixo, C.S.S.; Silva, P.G.; de Brito Costa, E.M.M. Antifungal Activity, Phytochemical Characterization and Thermal Profile of Anadenanthera Colubrina (Vell.) Brenan. Pesqui. Bras. Odontopediatria Clin. Integr. 2017, 17, 1–14. [Google Scholar] [CrossRef]
- Vannini, A.B.; Gallassini Tonini, R.C.; Carles Sturn, T.; Onofre, S.B.; Caovilla Follador, F.A.; Arruda, G. Evaluation of the Antibacterial Activity of Bark Extracts from Anadenanthera Colubrine (Vell.) Brenan (Fabaceae Lindl). Int. J. New Technol. Res. 2019, 5, 21–24. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Wampande, E.M.; Ejobi, F.; Nakavuma, J.L.; Maaza, M.; Sackey, J.; Nxumalo, E.; Kirabira, J.B. Green strategy–based synthesis of silver nanoparticles for antibacterial applications. Front. Nanotechno 2021, 3, 697303. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the hemolysis assay for the assessment of cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Gorr, S.U.; Flory, C.M.; Schumacher, R.J. In vivo activity and low toxicity of the second-generation antimicrobial peptide DGL13K. PLoS ONE 2019, 14, e0216669. [Google Scholar] [CrossRef] [PubMed]
- M07-A10; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- M26-A; Methods for Determining Bactericidal Activity of Antimicrobial Agents. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999.
- Izumi, E.; Ueda-Nakamura, T.; Veiga, V.F., Jr.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 2012, 55, 2994–3001. [Google Scholar] [CrossRef] [PubMed]




| Tested Bacteria | MIC (µM) | MBC (µM) | AgNO3 MIC |
|---|---|---|---|
| Enterococcus faecium 700 | 39.06 | 156.25 | 31.25 |
| Staphylococcus aureus ATCC 25923 | 78.12 | 156.25 | 62.5 |
| Staphylococcus aureus BEC 9393 | 78.12 | 156.25 | 62.5 |
| Klebsiella pneumoniae 3978 | 39.06 | 39.06 | 31.25 |
| Acinetobacter baumannii 141 | ≤19.53 | ≤19.53 | 7.81 |
| Pseudomonas aeruginosa 3167 | ≤19.53 | ≤19.53 | 7.81 |
| Enterobacter cloacae 9434 | ≤19.53 | ≤19.53 | 31.25 |
| Escherichia coli ATCC 25922 | 78.12 | 78.12 | 15.62 |
| Escherichia coli ATCC 8739 | 78.12 | 156.25 | 15.62 |
| Escherichia coli 5616 | 39.06 | 78.12 | 15.62 |
| Bacterial Isolates | Resistance Profile |
|---|---|
| Enterococcus faecium 700 | Vancomycin-resistant |
| Staphylococcus aureus BEC 9393 | Methicillin-resistant |
| Klebsiella pneumoniae 3978 | Carbapenem-resistant * |
| Acinetobacter baumannii 141 | Carbapenem-resistant * |
| Pseudomonas aeruginosa 3167 | Carbapenem-resistant * |
| Enterobacter cloacae 9434 | Carbapenem-resistant * |
| Escherichia coli 5616 | Carbapenem-resistant * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deonas, A.N.; Souza, L.M.d.S.; Andrade, G.J.S.; Germiniani-Cardozo, J.; Dahmer, D.; de Oliveira, A.G.; Nakazato, G.; Torezan, J.M.D.; Kobayashi, R.K.T. Green Synthesis of Silver Nanoparticle from Anadenanthera colubrina Extract and Its Antimicrobial Action against ESKAPEE Group Bacteria. Antibiotics 2024, 13, 777. https://doi.org/10.3390/antibiotics13080777
Deonas AN, Souza LMdS, Andrade GJS, Germiniani-Cardozo J, Dahmer D, de Oliveira AG, Nakazato G, Torezan JMD, Kobayashi RKT. Green Synthesis of Silver Nanoparticle from Anadenanthera colubrina Extract and Its Antimicrobial Action against ESKAPEE Group Bacteria. Antibiotics. 2024; 13(8):777. https://doi.org/10.3390/antibiotics13080777
Chicago/Turabian StyleDeonas, Anastácia Nikolaos, Lucas Marcelino dos Santos Souza, Gabriel Jonathan Sousa Andrade, Jennifer Germiniani-Cardozo, Débora Dahmer, Admilton Gonçalves de Oliveira, Gerson Nakazato, José Marcelo Domingues Torezan, and Renata Katsuko Takayama Kobayashi. 2024. "Green Synthesis of Silver Nanoparticle from Anadenanthera colubrina Extract and Its Antimicrobial Action against ESKAPEE Group Bacteria" Antibiotics 13, no. 8: 777. https://doi.org/10.3390/antibiotics13080777
APA StyleDeonas, A. N., Souza, L. M. d. S., Andrade, G. J. S., Germiniani-Cardozo, J., Dahmer, D., de Oliveira, A. G., Nakazato, G., Torezan, J. M. D., & Kobayashi, R. K. T. (2024). Green Synthesis of Silver Nanoparticle from Anadenanthera colubrina Extract and Its Antimicrobial Action against ESKAPEE Group Bacteria. Antibiotics, 13(8), 777. https://doi.org/10.3390/antibiotics13080777







