Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance
Abstract
1. Introduction
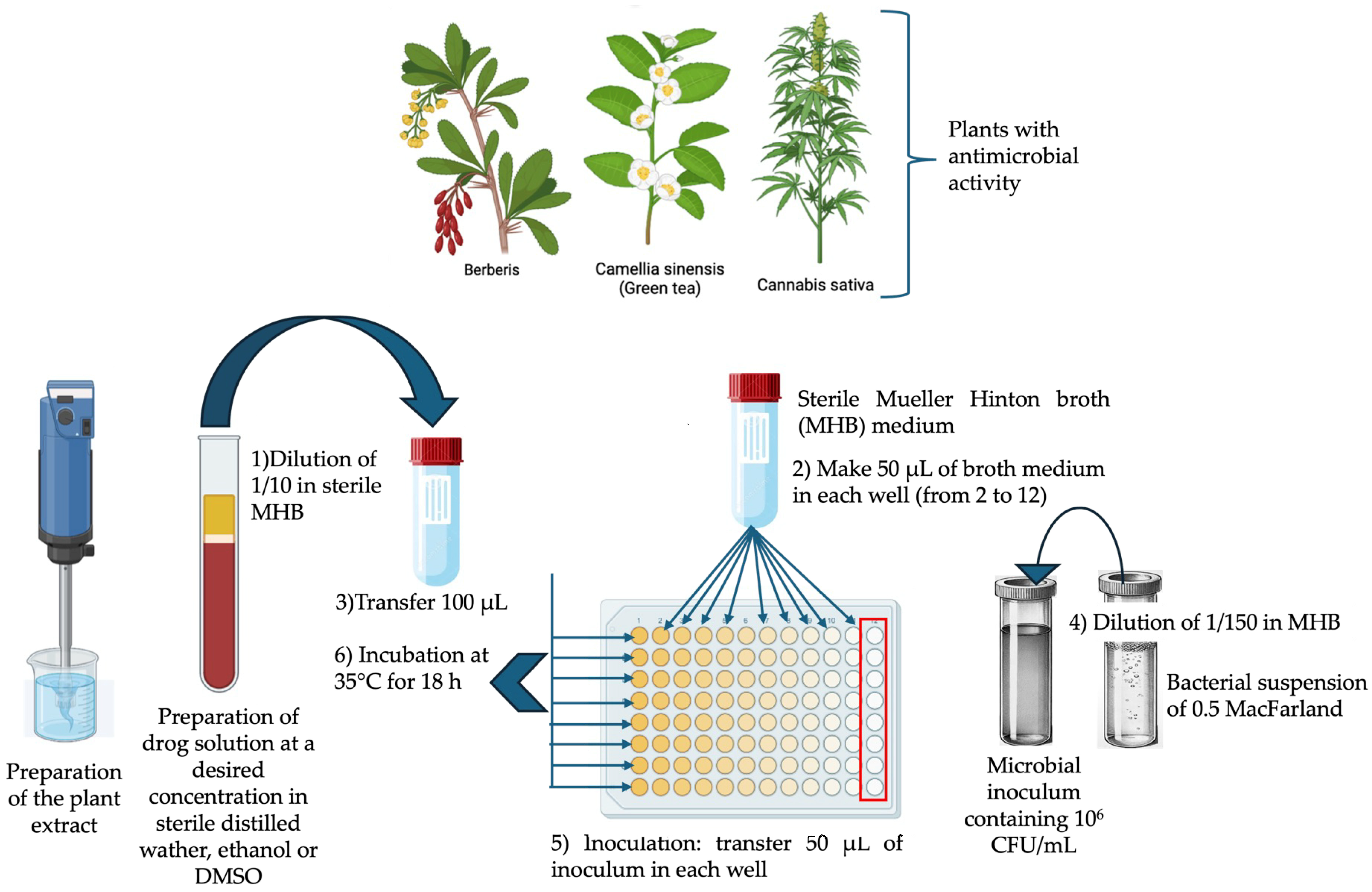
2. Methodology
3. Strategies for Overcoming Antibiotic Resistance
3.1. Antibiotic Modification
3.2. Modification of Antibiotic Target Sites
3.3. Antibiotic Resistence: Efflux Pump and Reduced Permeability
3.4. Enhancing Resistance through Target/Substrate Overproduction
3.5. Cell Wall Remodeling
4. Plant-Derived Antibiotics: A Solution to Multidrug-Resistant Microbes
| Family | Scientific Name (Common Name) | Compound | Effective in Combating | Drug Delivery System |
|---|---|---|---|---|
| Berberidaceae | Berberis vulgaris (Barberry) | Berberine | Bacteria, protozoa | Soft gel 1000 mg |
| Piperaceae | Piper nigrum (Black pepper) | Piperine | Fungi, Lactobacillus, Micrococcus | |
| Asteraceae | Arctium lappa (Burdock) | Bacteria, fungi, virus | Capsule 475 mg | |
| Apiaceae | Carum carvi (Caraway) | Bacteria, fungi, virus | Capsule 1000 mg | |
| Rhamnaceae | Rhamnus purshiana (Cascara sagrada) | Tannins | Bacteria, fungi, virus | Capsule 425, 450 mg |
| Asteraceae | Matricaria chamomilla (Chamomille) | Anthemic acid | M. tuberulosis, S. typhimurium, S. aureus | |
| Apiaceae | Syzygium aromaticum (Clove) | Eugenol | General | Capsule 500 mg |
| Ericaceae | Vaccinium spp. (Cranberry) | Fructose | Bacteria | Capsule 500 mg |
| Myrtaceae | Eucalyptus globulus (Eucalyptus) | Tannins | Bacteria, virus | Inhaler and tablet |
| Amaryllidaceae | Allium sativum (Garlic) | Allicin, ajoene | General | Tablet |
| Asteraceae | Hydrastis canadensis (Goldenseal) | Berberine, hydrastine | Bacteria, Giarda duodenale, Trypanosomes | Solution, 500 mg per dosage |
| Theaceae | Camellia sinensis (Green tea) | Catechin | General | |
| Fabaceae | Glycyrrhiza glabra (Licorice) | Glabrol | S. aureus, M. tuberculosis | Capsule 450 mg |
| Fagaceae | Quercus rubra (Oak) | Tannins, Quercetin | Capsule 500, 650 mg | |
| Amaryllidaceae | Allium cepa (Onion) | Allcin | Bacteria, Candida | |
| Berberidaceae | Mahonia aquifolia (Oregon grape) | Berberine | Plasmodium, Trypansomes, general | Capsule 500 mg |
| Hypericaceae | Hypericum perforatum (Senna St. John’s wort) | Hypericin, others | General | Capsule 450 mg |
| Lamiaceae | Thymus vulgaris (Thyme) | Caffeic acid, Thymol, Tannins | Viruses, bacteria, fungi | Capsule 450 mg |
| Zingiberaceae | Curcuma longa (Turmeric) | Curcumin, Turmeric oil | Bacteria, protozoa |
5. Plant Secondary Metabolites as Antimicrobial Agent
5.1. Alkaloids
5.2. Organosulfur Compounds
5.3. Phenolic Compounds
5.4. Coumarins
5.5. Terpenes
5.6. Antimicrobial Peptides from Plants
6. The Utility of Artificial Intelligence (AI)
7. Other Strategies to Overcome Antibiotic Resistance
8. Future Perspectives and Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleming, A. On the antibacterial action of cultures of a penicillium with special reference to their use in the isolation of B. influenza. Bull. World Health Org. 1929, 79, 780–790. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.I.; Khusro, A.; Matin Zidan, B.M.R.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, S.H. The Evolving Response to Antibiotic Resistance (1945–2018). Palgrave Commun. 2023, 12, 3077. [Google Scholar] [CrossRef]
- Parmar, A.; Lakshminarayanan, R.; Iyer, A.; Mayandi, V.; Leng Goh, E.T.; Lloyd, D.G.; Chalasani, M.L.S.; Verma, N.K.; Prior, S.H.; Beuerman, R.W.; et al. Design and Syntheses of Highly Potent Teixobactin Analogues against Staphylococcus aureus methicillin-resistant Staphylococcus aureus (MRSA), and Vancomycin-Resistant Enterococci (VRE) In Vitro and In Vivo. J. Med. Chem. 2018, 61, 2009–2017. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st century. Perspect. Medicin. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; HM Government and Wellcome Trust: London, UK, 2016; Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 15 June 2024).
- Dheda, K.; Mirzayev, F.; Cirillo, D.M.; Udwadia, Z.; Dooley, K.E.; Chang, K.C.; Omar, S.V.; Reuter, A.; Perumal, T.; Horsburgh, C.R., Jr.; et al. Multidrug-resistant tuberculosis. Nat. Rev. Dis. Primers 2024, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 10325, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Dreisbach, A.; Wang, M.; van der Kooi-Pol, M.M.; Reilman, E.; Koedijk, D.G.A.M.; Mars, R.A.T.; Duipmans, J.; Jonkman, M.; Benschop, J.J.; Bonarius, H.P.J.; et al. Tryptic Shaving of Staphylococcus aureus Unveils Immunodominant Epitopes on the Bacterial Cell Surface. J. Proteome Res. 2020, 19, 2997–3010. [Google Scholar] [CrossRef] [PubMed]
- Staley, J.T. The bacterial species dilemma and the genomic–phylogenetic species concept. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Soni, J.; Sinha, S.; Pandey, R. Understanding bacterial pathogenicity: A closer look at the journey of harmful microbes. Front. Microbiol. 2024, 15, 1370818. [Google Scholar] [CrossRef] [PubMed]
- Gidey, K.; Gidey, M.T.; Hailu, B.Y.; Gebreamlak, Z.B.; Niriayo, Y.L. Clinical and economic burden of healthcare-associated infections: A prospective cohort study. PLoS ONE 2023, 18, e0282141. [Google Scholar] [CrossRef] [PubMed]
- Clare, S.; Rowley, S. Implementing the Aseptic Non Touch Technique (ANTT) clinical practice framework for aseptic technique: A pragmatic evaluation using a mixed methods approach in two London hospitals. J. Infect. Prev. 2018, 19, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Tettey, F.; Parupelli, S.K.; Desai, S. A Review of Biomedical Devices: Classification Regulatory Guidelines Human Factors Software as a Medical Device and Cybersecurity. Biomed. Mater. Devices 2024, 2, 316–341. [Google Scholar] [CrossRef]
- Braun, J.; Eckes, S.; Rommens, P.M.; Schmitz, K.; Nickel, D.; Ritz, U. Toxic Effect of Vancomycin on Viability and Functionality of Different Cells Involved in Tissue Regeneration. Antibiotics 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a Global Threat to Humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef] [PubMed]
- Miklasinska-Majdanik, M.; Kepa, M.; Wojtyczka, R.D.; Idzil, D. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Low, L.Y.; Yap, P.S.X.; Yusoff, K.; Mai, C.W.; Lai, K.S.; Lim, S.H.E. Plant-Derived Antimicrobials: Insights into Mitigation of Antimicrobial Resistance. Rec. Nat. Prod. 2018, 12, 295–396. [Google Scholar] [CrossRef]
- Macheboeuf, P.; Di Guilmi, A.M.; Vernet, T.; Dideberg, O.; Dessen, A. Active site restructuring regulates ligand recognition in class A penicillin-binding proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Sacco, E.; Cortes, M.; Josseaume, N.; Rice, L.B.; Mainardi, J.L.; Arthur, M. Serine/threonine protein phosphatase-mediated control of the peptidoglycan cross-linking LD-transpeptidase pathway in Enterococcus faecium. MBio 2014, 5, e01446. [Google Scholar] [CrossRef] [PubMed]
- Morais-Cabral, J.H.; Jackson, A.P.; Smith, C.V.; Shikotra, A.; Maxwell, A.; Liddington, R.C. Crystal structure of the breakage-reunion domain of DNA gyrase. Nature 1997, 388, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Willmott, C.J.; Maxwell, A. A single point mutation in the DNA gyrase A protein greatly reduces binding of fluoroquinolones to the gyrase-DNA complex. Antimicrob. Agents Chemother. 1993, 37, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Grise-Miron, L.; Brakier-Gingras, L. Effect of neomycin and protein S1 on the binding of streptomycin to the ribosome. Eur. J. Biochem. 1982, 123, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Moazed, D.; Noller, H.F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature 1987, 327, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Ding, Q.; Ji, Z.; Liang, X.; Deng, C.C.; Wong, C.; Yi, L.; Zhang, S.; Xie, S.; Alvarez, L.M.; et al. Protein cysteine phosphorylation of SarA/MgrA family transcriptional regulators mediates bacterial virulence and antibiotic resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 15461–15466. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Subramanian, R.; Kumar, J.; Jass, J.; Jawali, N. Involvement of antibiotic efflux machinery in glutathione-mediated decreased ciprofloxacin activity in Escherichia coli. Antimicrob. Agents Chemother. 2016, 60, 4369–4374. [Google Scholar] [CrossRef] [PubMed]
- Kohler, T.; Michea-Hamzehpour, M.; Plesiat, P.L.; Kahr, A.; Pechere, J.C. Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1997, 41, 2540–2543. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Sakagawa, E.; Ohya, S.; Gotoh, H.; Tsujimoto, N.; Nishino, T. Substrate specificities of MexAB-OprM MexCD-OprJ and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2000, 44, 3322–3327. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Tomida, J.; Kawamura, Y. MexXY multidrug efflux system of Pseudomonas aeruginosa. Front. Microbiol. 2012, 3, 408. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Costa, M.; Viveiros, A.E.; Rosato, J.; Melo-Cristino, J.; Couto, I. Impact of efflux in the development of multidrug resistance phenotypes in Staphylococcus aureus. BMC Microbiol. 2015, 15, 232. [Google Scholar]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- De, E.; Basle, A.; Jaquinod, M.; Saint, N.; Mallea, G.; Molle, G.; Pages, J.M. A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin. Mol. Microbiol. 2001, 41, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gayet, S.; Chollet, R.; Molle, G.; Pages, J.M.; Chevalier, J. Modification of outer membrane protein profile and evidence suggesting an active drug pump in Enterobacter aerogenes clinical strains. Antimicrob. Agents Chemother. 2003, 47, 1555–1559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Melano, R.; Corso, A.; Petroni, D.; Centron, B.; Orman, A.; Pereyra, N.; Moreno, M.; Galas, M. Multiple antibiotic-resistance mechanisms including a novel combination of extended-spectrum betalactamases in a Klebsiella pneumoniae clinical strain isolated in Argentina. J. Antimicrob. Chemother. 2003, 52, 36–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gheorghiu, R.; Yuan, M.; Hall, L.M.; Livermore, D.M. Bases of variation in resistance to beta lactams in Klebsiella oxytoca isolates hyperproducing K1 beta-lactamase. J. Antimicrob. Chemother. 1997, 40, 533–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, P.J.; Shannon, K.; Phillips, I. Mechanisms of hyperproduction of TEM-1 beta-lactamase by clinical isolates of Escherichia coli. J. Antimicrob. Chemother. 1995, 36, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.V.; Bershtein, S.; Li, A.; Lozovsky, E.R.; Hartl, D.L.; Shakhnovich, E.I. Biophysical principles predict fitness landscapes of drug resistance. Proc. Natl. Acad. Sci. USA 2016, 113, 11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Rubin, E.J.; Bifani, P.; Mathys, V.; Lim, M.; Au, J.; Jang, J.; Nam, T.; Dick, J.R.; Walker, K.; et al. Para-Aminosalicylic acid is a prodrug targeting dihydrofolate reductase in Mycobacterium tuberculosis. J. Biol. Chem. 2013, 288, 23447–23456. [Google Scholar] [CrossRef] [PubMed]
- Frere, J.M.; Joris, B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit. Rev. Microbiol. 1985, 11, 299–396. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.; Molinas, C.; Depardieu, F.; Courvalin, P. Characterization of Tn1546 a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 1993, 175, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Meziane-Cherif, D.; Stogios, P.J.; Evdokimova, E.; Egorova, O.; Savchenko, A.; Courvalin, P. Structural and functional adaptation of vancomycin resistance VanT Serine racemases. MBio 2015, 6, e00806. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.; Wright, G.D.; Dutka-Malen, S.; Arthur, M.; Courvalin, P.; Walsh, C.T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: Biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 1991, 30, 10408–10415. [Google Scholar] [CrossRef] [PubMed]
- Bakal, S.N.; Bereswill, S.; Heimesaat, M.M. Finding novel antibiotic substances from medicinal plants—Antimicrobial properties of Nigella sativa directed against multidrug-resistant bacteria. Eur. J. Microbiol. Immunol. 2017, 7, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, L.Y.; Yoga, L. Extraction isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Valli, M.; Pivatto, M.; Danuello, A.; Castro-Gamboa, I.; Silva, D.H.S.; Cavalheiro, A.J.; Araújo, Â.R.; Furlan, M.; Lopes, M.N.; Bolzani, V.D.S. Tropical biodiversity: Has it been a potential source of secondary metabolites useful for medicinal chemistry? Trop. Biodivers. 2012, 35, 2278–2287. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests f or Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Parvin, S.; Kader, M.A.; Chouduri, A.U.; Rafshanjani, M.A.S.; Haque, M.E. Antibacterial antifungal and insecticidal activities of the n-hexane and ethyl-acetate fractions of methanolic extract of the leaves of Calotropis gigantea Linn. J. Pharmacogn. Phytochem. 2014, 2, 47–51. [Google Scholar]
- Rahman, M.S.; Anwar, M.N. Antimicrobial Activity of Crude Extract Obtained from the Root of Plumbago zeylanica. Bangladesh J. Microbiol. 2007, 24, 73–75. [Google Scholar] [CrossRef]
- Batool, K.; Sultana, S.; Akhtar, N.; Muhammad, H.; Naheed, A. Medicinal plants combating against human pathogens: A review. Int. J. Biotechnol. Food Sci. 2018, 6, 42–51. [Google Scholar]
- Chuah, E.; Zakaria, Z.; Suhaili, Z.; Abu Bakar Jamaludin, S.; Mohd Desa, M. Antimicrobial activities of plant extracts against methicillin-susceptible and methicillin-resistant Staphylococcus aureus. J. Microbiol. Res. 2014, 4, 6–13. [Google Scholar]
- Sharifi-Rad, J.; Mnayer, D.; Roointan, A.; Shahri, F.; Ayatollahi, S.A.M.; Sharifi-Rad, M.; Molaee, N. Antibacterial activities of essential oils from Iranian medicinal plants on extended-spectrum β-lactamase-producing Escherichia coli. Cell. Mol. Biol. 2016, 62, 75–82. [Google Scholar] [PubMed]
- Pacheco, A.G.; Alcântara, A.F.C.; Abreu, V.G.C.; Corrêa, G.M. Relationships Between Chemical Structure and Activity of Triterpenes against Gram-Positive and Gram-Negative Bacteria. In A Search for Antibact Agents; Bobbarala, V., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 1; pp. 1–25. [Google Scholar]
- Niranjan, P.S.; Kaushal, C.; Jain, S.K. Pharmacological Investigation of Leaves of Polypodium decumanum for Antidiabetic Activity. J. Drug Deliv. Ther. 2017, 7, 2685–2688. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Sissi, S.; Di Giacomo, S.; Ferrante, C.; Angelini, P.; Macone, A.; Giusti, A.M.; Toniolo, C.; Vitalone, A.; Abdellah, A.; Larhsini, M.; et al. Characterization of the Phytochemical Composition and Bioactivities of Anacyclus maroccanus Ball. and Anacyclus radiatus Loisel Aerial Parts: Preliminary Evidence for the Possible Development of Moroccan Plants. Molecules 2022, 27, 692. [Google Scholar] [CrossRef]
- Di Simone, S.C.; Angeles Flores, G.; Acquaviva, A.; Nilofar, M.; Libero, M.L.; Venanzoni, R.; Tirillini, B.; Orlando, G.; Zengin, G.; Lai, F.; et al. Phytochemical and biological properties of the water extract from roots and leaves of Lactuca longidentata an endemic phytoalimurgic (food) species of Central Sardinia (Italy). Plant Biosyst. 2023, 157, 594–604. [Google Scholar] [CrossRef]
- Serventi, L.; Flores, G.A.; Cusumano, G.; Barbaro, D.; Tirillini, B.; Venanzoni, R.; Angelini, P.; Acquaviva, A.; Di Simone, S.C.; Orlando, G.; et al. Comparative Investigation of Antimicrobial and Antioxidant Effects of the Extracts from the Inflorescences and Leaves of the Cannabis sativa L. cv. strawberry. Antioxidants 2023, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Adorisio, S.; Delfino, D.; Chiavaroli, A.; Brunetti, L.; Recinella, L.; Leone, S.; D’Antonio, M.; Zengin, G.; Acquaviva, A.; et al. Comparative Investigation of Composition Antifungal and Anti-Inflammatory Effects of the Essential Oil from Three Industrial Hemp Varieties from Italian Cultivation. Antibiotics 2021, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, A.C.; Castro, V.S. Phenolic Compounds in Bacterial Inactivation: A Perspective from Brazil. Antibiotics 2023, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Natural products from resurrection plants: Potential for medical applications. Biotechnol. Adv. 2014, 32, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Kim, H.K.; van Wezel, G.P.; Choi, Y.H. Expanding the chemical space for natural products by Aspergillus–Streptomyces co-cultivation and biotransformation. Sci. Rep. 2015, 5, 10868. [Google Scholar] [CrossRef] [PubMed]
- Youssef, S.; Hassan, M.; Ahmed, S. Lichens: Chemistry and biological activities. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 223–259. [Google Scholar]
- Deshmukh, S.K.; Verekar, S.A.; Bhave, S.V. Endophytic fungi: A reservoir of antibacterials. Front. Microbiol. 2015, 5, 715. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Cheung, R.C.F.; Wong, J.H.; Wang, Y.; Ip, D.T.M.; Wan, D.C.C.; Xia, J.; Chan, W.Y.; Fang, E.F. Antibacterial products of marine organisms. Appl. Microbiol. Biotechnol. 2015, 99, 4145–4173. [Google Scholar] [CrossRef] [PubMed]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.A.M.; Shin, H.J.; Rahman, M.A. Antibacterial and antiyeast compounds from marine-derived bacteria. Mar. Drugs 2014, 12, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. Marine pharmacology in 2009–2011: Marine compounds with antibacterial antidiabetic antifungal anti-inflammatory antiprotozoal antituberculosis and antiviral activities; affecting the immune and nervous systems and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Singh, A.; Arya, M.; Maheshwari, A.; Bhadouria, R.; Pandey, S.; Kharwar, R.N.; Stal, L.J.; Dubey, N.K. Antimicrobial compounds from seaweeds-associated bacteria and fungi. Appl. Microbiol. Biotechnol. 2015, 99, 1571–1586. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Park, H.C.; Park, S.W.; Park, S.C.; Seo, M.G.; Her, M.; Kang, J. Synergism of the Combination of Traditional Antibiotics and Novel Phenolic Compounds against Escherichia coli. Pathogens 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, Z.; Guo, L. Antibacterial Molecules from Marine Microorganisms against Aquatic Pathogens: A Concise Review. Mar. Drugs 2022, 20, 230. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zha, J.; Koffas, M.A.G. Microbial production of bioactive chemicals for human health. Curr. Opin. Food Sci. 2020, 32, 9–16. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Bribi, N. Pharmacological activity of alkaloids: A review. Asian J. Bot. 2018, 1, 1–6. [Google Scholar]
- Khameneh, B.; Iranshahi, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Jadimurthy, R.; Jagadish, S.; Nayak, S.C.; Kumar, S.; Mohan, C.D.; Rangappa, K.S. Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance. Life 2023, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Otmann, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic-Radic, Z.; Pejcic, M.; Dimitrijevic, M.; Aleksic, A.; Anil Kumar, V.N.; Salehi, B.; Cho, C.W.; Sharifi-Rad, J. Piperine-A major principle of black pepper: A review of its bioactivity and studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Zahin, M.; Bokhari, N.A.; Ahmad, I.; Husain, F.M.; Althubiani, A.S.; Alruways, M.W.; Perveen, K.; Shalawi, M. Antioxidant antibacterial and antimutagenic activity of Piper nigrum seeds extracts. Saudi J. Biol. Sci. 2021, 28, 5094–5105. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.B.; Yan, Y.; Liang, Y.Z.; Bao, Z. Evaluation of the antimicrobial mode of berberine by LC/ESI-MS combined with principal component analysis. J. Pharm. Biomed. Anal. 2007, 44, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.; Jannat, K.; Nissapatorn, V.; Rahmatullah, M.; Paul, A.K.; de Lourdes Pereira, M.; Rajagopal, M.; Suleiman, M.; Butler, M.S.; Break, M.K.B.; et al. Antibacterial and Antifungal Alkaloids from Asian Angiosperms: Distribution, Mechanisms of Action, Structure-Activity, and Clinical Potentials. Antibiotics 2022, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Casu, L.; Cottiglia, F.; Leonti, M.; De Logu, A.; Agus, E.; Tse-Dinh, Y.C.; Lombardo, V.; Sissi, C. Ungeremine effectively targets mammalian as well as bacterial type I and type II topoisomerases. Bioorg. Med. Chem. Lett. 2011, 23, 7041–7044. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Wansi, J.D.; Mbaveng, A.T.; Kana Sop, M.M.; Tadjong, A.T.; Beng, V.P. Antimicrobial activity of the methanolic extract and compounds from Teclea afzelii (Rutaceae). S. Afr. J. Bot. 2008, 74, 572–576. [Google Scholar] [CrossRef]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Camara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Parai, D.; Banerjee, M.; Dey, P.; Mukherjee, S.M. Reserpine attenuates biofilm formation and virulence of Staphylococcus aureus. Microb. Pathog. 2020, 138, 103790. [Google Scholar] [CrossRef] [PubMed]
- Abdelfatah, S.A.; Efferth, T. Cytotoxicity of the indole alkaloid reserpine from Rauwolfia serpentina against drug-resistant tumor cells. Phytomedicine 2015, 22, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, D.; Shankar, C.; Prakash, P.; Park, J.H.; Thamaraiselvi, K. Inhibitory effects of reserpine against efflux pump activity of antibiotic resistance bacteria. Chem. Biol. Lett. 2017, 4, 69–72. [Google Scholar]
- Jia, W.; Li, C.; Zhang, H.; Li, G.; Liu, X.; Wei, J. Prevalence of genes of OXA-23 carbapenemase and AdeABC efflux pump associated with multidrug resistance of Acinetobacter baumannii isolates in the ICU of a comprehensive hospital of northwestern China. Int. J. Environ. Res. Public Health 2015, 12, 10079–10092. [Google Scholar] [CrossRef] [PubMed]
- Obiang-Obounou, B.W.; Kang, O.H.; Choi, J.G.; Keum, J.H.; Kim, S.B.; Mun, S.H.; Shin, D.W.; Kim, K.W.; Park, C.B.; Kim, Y.G.; et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J. Toxicol. Sci. 2011, 36, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Newton, S.M.; Lau, C.; Gurcha, S.S.; Besra, G.S.; Wright, C.W. The evaluation of fortythree plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J. Ethnopharmacol. 2002, 79, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Lafrance, M.; Boulanger, S.; Séguin, D.L.; Guay, I.; Gattuso, M.; Marsault, E.; Bouarab, K.; Malouin, F. Tomatidine acts in synergy with aminoglycoside antibiotics against multiresistant Staphylococcus aureus and prevents virulence gene expression. J. Antimicrob. Chemother. 2012, 67, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.P.; Millette, G.; Guay, I.; Dubé-Duquette, A.; Chamberland, S.; Jacques, P.É.; Rodrigue, S.; Bouarab, K.; Marsault, É.; Malouin, F. Bactericidal Activity of the Bacterial ATP Synthase Inhibitor Tomatidine and the Combination of Tomatidine and Aminoglycoside against Persistent and Virulent Forms of Staphylococcus aureus. Front. Microbiol. 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, G.R.; Maurya, A.; Yadav, D.K.; Singh, V.; Khan, F.; Gupta, M.K.; Singh, M.; Darokar, M.P.; Srivastava, S.K. Synergy of clavine alkaloid “chanoclavine” with tetracycline against multi-drug-resistant E. coli. J. Biomol. Struct. Dyn. 2019, 37, 1307–1325. [Google Scholar] [CrossRef] [PubMed]
- Siriyong, T.; Srimanote, P.; Chusri, S.; Yingyongnarongkul, B.E.; Suaisom, C.; Tipmanee, V.; Voravuthikunchai, S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, B.; Bhandari, P.; Gupta, A.P.; Kaul, V.K. Steroidal alkaloids from Holarrhena antidysenterica (L.) WALL. Chem. Pharm. Bull. 2007, 55, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.N.; Ge, X.L.; Dong, T.T.; Gao, H.Y.; Sun, B.H. Antibacterial steroidal alkaloids from Holarrhena antidysenterica. Chin. J. Nat. Med. 2017, 15, 540–545. [Google Scholar] [PubMed]
- Alhanout, K.; Malesinki, S.; Vidal, N.; Peyrot, V.; Rolain, J.M.; Brunel, J.M. New insights into the antibacterial mechanism of action of squalamine. J. Antimicrob. Chemother. 2010, 65, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Miekus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Swiergiel, H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.R.; Shafaei, A.; Balmer, L.; Lewis, J.R.; Hodgson, J.M.; Millar, A.H.; Blekkenhorst, L.C. Sulfur compounds: From plants to humans and their role in chronic disease prevention. Crit. Rev. Food Sci. Nutr. 2022, 63, 8616–8638. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.V.; Pant, S.; Khan, M.A.H.; Shah, A.A.; Siddiqui, S.; Jeridi, M.; Alhamdi, H.W.S.; Ahmad, S. Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance. Pharmaceuticals 2023, 16, 881. [Google Scholar] [CrossRef] [PubMed]
- Boghrati, Z.; Iranshahi, M. Ferula species: A rich source of antimicrobial compounds. J. Herb. Med. 2019, 16, 100244. [Google Scholar] [CrossRef]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef] [PubMed]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Davis, S.R. An overview of the antifungal properties of allicin and its breakdown products—The possibility of a safe and effective antifungal prophylactic. Mycoses 2005, 48, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sen, D.; Bhattacharjee, C. Inhibition mechanism study for diallyl thiosulfinate (allicin) against crucial bacterial proteins through in silico molecular docking simulation. Process Biochem. 2022, 122, 110–119. [Google Scholar] [CrossRef]
- Rehman, F.; Mairaj, S. Antimicrobial studies of allicin and ajoene. Int. J. Pharm. Bio Sci. 2013, 4, 1095–1105. [Google Scholar]
- Nguyen, N.M.; Gonda, S.; Vasas, G. A Review on the Phytochemical Composition and Potential Medicinal Uses of Horseradish (Armoracia rusticana) Root. Food Rev. Int. 2013, 29, 261–275. [Google Scholar] [CrossRef]
- Kim, H.Y.; Pha-a-god, S.; Shin, I.S. Antibacterial Activities of Isothiocyanates Extracted from Horseradish (Armoracia rusticana) Root against Antibiotic-resistant Bacteria. Food Sci. Biotechnol. 2015, 24, 1029–1034. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Zieoelhofpt, A. “Lysine is the Lord”, thought some scientists in regard to the group interacting with fluorescein isothiocyanate in ATP-binding sites of P-type ATPases but, is it not cysteine? Gen. Physiol. Biophys. 2000, 19, 253–263. [Google Scholar] [PubMed]
- Benzekri, R.; Bouslama, L.; Papetti, A.; Snoussi, M.; Benslimene, I.; Hamami, M.; Limam, F. Isolation and identification of an antibacterial compound from Diplotaxis harra (Forssk.) Boiss. Ind. Crop. Prod. 2016, 80, 228–234. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Dockery, C.R.; Crosby, M.; Chavarria, K.; Patterson, B.; Giedd, M. Antibacterial activities of wasabi against Escherichia coli O157 and Staphylococcus aureus. Front. Microbiol. 2016, 7, 1403. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Massih, R.M.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F.M. Glucosinolates, a natural chemical arsenal: More to tell than the myrosinase story. Front. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Bogucka, K.; Szalewska-Pałasz, A.; Herman-Antosiewicz, A. Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria. Sci. Rep. 2019, 9, 13677. [Google Scholar] [CrossRef] [PubMed]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. Isothiocyanates in Food. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2021. [Google Scholar]
- Takahashi, H.; Nakamura, A.; Fujino, N.; Sawaguchi, Y.; Sato, M.; Kuda, T.; Kimura, B. Evaluation of the antibacterial activity of allyl isothiocyanate, clove oil, eugenol and carvacrol against spoilage lactic acid bacteria. LWT 2021, 145, 111263. [Google Scholar] [CrossRef]
- Cipollini, D.; Cipollini, K. A review of garlic mustard (Alliaria petiolata, Brassicaceae) as an allelopathic plant. J. Torrey Bot. Soc. 2016, 143, 339–348. [Google Scholar] [CrossRef]
- Dias, C.; Aires, A.; Saavedra, M.J. Antimicrobial activity of isothiocyanates from cruciferous plants against methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Mol. Sci. 2014, 15, 19552–19561. [Google Scholar] [CrossRef] [PubMed]
- Sofrata, A.; Santangelo, E.M.; Azeem, M.; Borg-Karlson, A.K.; Gustafsson, A.; Putsep, K. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against gram-negative bacteria. PLoS ONE 2011, 6, e23045. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Hong, E.; Kim, G.H. Evaluation of antibacterial activity of 3-butenyl, 4-pentenyl, 2-phenylethyl, and benzyl isothiocyanate in Brassica vegetables. J. Food Sci. 2010, 75, M412–M416. [Google Scholar] [CrossRef] [PubMed]
- Saladino, F.; Bordin, K.; Luciano, F.B.; Franzón, M.F.; Mañes, J.; Meca, G. Antimicrobial Activity of the Glucosinolates. In Glucosinolates. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Yu, H.; Jia, W.; Zhao, M.; Li, L.; Liu, J.; Chen, J.; Pan, H.; Zhang, X. Antifungal mechanism of isothiocyanates against Cochliobolus heterostrophus. Pest Manag. Sci. 2022, 12, 5133–5141. [Google Scholar] [CrossRef] [PubMed]
- Calmes, B.; N’Guyen, G.; Dumur, J.; Brisach, C.A.; Campion, C.; Iacomi, B.; Pigné, S.; Dias, E.; Macherel, D.; Guillemette, T.; et al. Glucosinolate-derived isothiocyanates impact mitochondrial function in fungal cells and elicit an oxidative stress response necessary for growth recovery. Front. Plant Sci. 2015, 6, 414. [Google Scholar] [CrossRef]
- Moon, J.K.; Kim, J.R.; Ahn, Y.J.; Shibamoto, T. Analysis and anti-helicobacter activity of sulforaphane and related compounds present in broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2010, 58, 6672–6677. [Google Scholar] [CrossRef] [PubMed]
- Sathianarayanan, S.; Ammanath, A.V.; Biswas, R.; Anita, B.; Sukumaran, S.; Venkidasamy, B. A new approach against Helicobacter pylory using plants and its constituents: A review study. Microb. Pathog. 2022, 168, 105594. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Hrytsyk, R.A.; Kutsyk, R.V.; Yurchyshyn, O.I.; Struk, O.A.; Kireev, I.V.; Grytsyk, A.R. The investigation of antimicrobial and antifungal activity of some Artemisia L. species. Pharmacia 2021, 68, 93–100. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Klancnik, A.; Sikic Pogacar, M.; Trost, K.; Tusek Znidaric, M.; Mozetic Vodopivec, B.; Smole, M.S. Anti-campylobacter activity of resveratrol and an extract from waste pinot noir grape skins and seeds, and resistance of camp. Jejuni planktonic and biofilm cells, mediated via the CmeABC efflux pump. J. Appl. Microbiol. 2017, 122, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Silva, F.; Queiroz, J.A.; Oleastro, M.; Domingues, F.C. Resveratrol against Arcobacter butzleri and Arcobacter cryaerophilus: Activity and effect on cellular functions. Int. J. Food Microbiol. 2014, 180, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lechner, D.; Gibbons, S.; Bucar, F. Plant phenolic compounds as ethidium bromide efflux inhibitors in mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 62, 345–348. [Google Scholar] [CrossRef]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef]
- Fujita, M.; Shiota, S.; Kuroda, T.; Hatano, T.; Yoshida, T.; Mizushima, T.; Tsuchiya, T. Remarkable synergies between baicalein and tetracycline, and baicalein and beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2005, 49, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Xie, K.; Wang, H.; Chen, Y.; Xie, M. Inhibitory effects of biochanin a on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA). Wei Sheng Wu Xue Bao 2014, 54, 1204–1211. [Google Scholar] [PubMed]
- Hanski, L.; Genina, N.; Uvell, H.; Malinovskaja, K.; Gylfe, Å.; Laaksonen, T.; Kolakovic, R.; Mäkilä, E.; Salonen, J.; Hirvonen, J.; et al. Inhibitory activity of the isoflavone biochanin A on intracellular bacteria of genus Chlamydia and initial development of a buccal formulation. PLoS ONE 2014, 9, e115115. [Google Scholar] [CrossRef] [PubMed]
- Cannalire, R.; Machado, D.; Felicetti, T.; Costa, S.S.; Massari, S.; Manfroni, G.; Barreca, M.L.; Tabarrini, O.; Couto, I.; Viveiros, M.; et al. Natural isoflavone biochanin a as a template for the design of new and potent 3-phenylquinolone efflux inhibitors against Mycobacterium avium. Eur. J. Med. Chem. 2017, 140, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Stermitz, F.R.; Cashman, K.K.; Halligan, K.M.; Morel, C.; Tegos, G.P.; Lewis, K. Polyacylated neohesperidosides from Geranium caespitosum: Bacterial multidrug resistance pump inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.; Stermitz, F.R.; Tegos, G.; Lewis, K. Isoflavones as potentiators of antibacterial activity. J. Agric. Food Chem. 2003, 51, 5677–5679. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Ettefagh, K.A.; Todd, D.A.; Cole, P.S.; Egan, J.M.; Foil, D.H.; Lacey, E.P.; Cech, N.B. Bacterial efflux inhibitors are widely distributed in land plants. J. Ethnopharmacol. 2021, 267, 113533. [Google Scholar] [CrossRef]
- Randhawa, H.K.; Hundal, K.K.; Ahirrao, P.N.; Jachak, S.M.; Nandanwar, H.S. Efflux pump inhibitory activity of flavonoids isolated from Alpinia calcarata against methicillin-resistant Staphylococcus aureus. Biologia 2016, 71, 484–493. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, M.; Wang, T.; Li, Y.; Wang, C. The roles of CDR1, CDR2, and MDR1 in kaempferol-induced suppression with fluconazole-resistant Candida albicans. Pharm. Biol. 2016, 54, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Ettefagh, K.A.; Todd, D.; Cole, P.S.; Egan, J.M.; Foil, D.H.; Graf, T.N.; Schindler, B.D.; Kaatz, G.W.; Cech, N.B. A mass spectrometry-based assay for improved quantitative measurements of efflux pump inhibition. PLoS ONE 2015, 10, e0124814. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.G.; Christensen, S.B.; Slotved, H.C.; Rasmussen, H.B.; Gúzman, A.; Olsen, C.E.; Petersen, B.; Mølgaard, P. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Holler, J.G.; Slotved, H.C.; Mølgaard, P.; Olsen, C.E.; Christensen, S.B. Chalcone inhibitors of the NorA efflux pump in Staphylococcus aureus whole cells and enriched everted membrane vesicles. Bioorg. Med. Chem. 2012, 20, 4514–4521. [Google Scholar] [CrossRef] [PubMed]
- Singhal, D.; Saxena, S. Catechin gallate a promising resistance modifying candidate to potentiate β-lactam antibiotics to overcome resistance in Staphylococcus aureus. Curr. Med. Res. Pract. 2017, 7, 224–228. [Google Scholar] [CrossRef]
- Anderle, C.; Stieger, M.; Burrell, M.; Reinelt, S.; Maxwell, A.; Page, M.; Heide, L. Biological activities of novel gyrase inhibitors of the aminocoumarin class. Antimicrob. Agents Chemother. 2008, 52, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Tyagi, C.; Goyal, S.; Jamal, S.; Wahi, D.; Jain, R.; Bharadvaja, N.; Grover, A. Identification of chebulinic acid as potent natural inhibitor of M. tuberculosis DNA gyrase and molecular insights into its binding mode of action. Comput. Biol. Chem. 2015, 59, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Li, X.; Cai, S.; Xin, G.; Wang, Y.; Du, D.; He, S.; Huang, B.; Guo, X.; Zhao, H.; et al. Haloemodin as novel antibacterial agent inhibiting DNA gyrase and bacterial topoisomerase I. J. Med. Chem. 2014, 57, 3707–3714. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial Activity and Membrane-Disruptive Mechanism of 3-p-trans-Coumaroyl-2-hydroxyquinic Acid, a Novel Phenolic Compound from Pine Needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Toth, I.V.; Rangel, A.O.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Dikdik, K.; Eti, A.; Cut, S.; Mieke, H.S. Antibacterial Flavonoids against Oral Bacteria of Enterococcus Faecalis ATCC 29212 from Sarang Semut (Myrmecodia pendans) and Its Inhibitor Activity against Enzyme MurA. Curr. Drug Discov. Technol. 2019, 16, 290–296. [Google Scholar]
- Li, B.H.; Zhang, R.; Du, Y.T.; Sun, Y.H.; Tian, W.X. Inactivation mechanism of the beta-ketoacyl-[acyl carrier protein] reductase of bacterial type-II fatty acid synthase by epigallocatechin gallate. Biochem. Cell Biol. 2006, 84, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Joung, D.K.; Kim, S.B.; Park, S.J.; Seo, Y.S.; Gong, R.; Choi, J.G.; Shin, D.W.; Rho, J.R.; Kang, O.H.; et al. The mechanism of antimicrobial activity of sophoraflavanone B against methicillin-resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2014, 11, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Maresso, A.W.; Schneewind, O. Sortase as a target of anti-infective therapy. Pharmacol. Rev. 2008, 60, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.P.; Peng, Z.Y.; Dong, J.J.; He, J.; Ouyang, H.; Feng, Y.T.; Lu, C.L.; Lin, W.Q.; Wang, J.X.; Xiang, Y.P.; et al. Synthesis, structure-activity relationship analysis and kinetics study of reductive derivatives of flavonoids as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem. 2013, 63, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Vidigal, P.G.; Müsken, M.; Becker, K.A.; Häussler, S.; Wingender, J.; Steinmann, E.; Kehrmann, J.; Gulbins, E.; Buer, J.; Rath, P.M.; et al. Effects of Green Tea Compound Epigallocatechin-3-Gallate against Stenotrophomonas maltophilia Infection and Biofilm. PLoS ONE 2014, 9, e92876. [Google Scholar] [CrossRef] [PubMed]
- Budhathoki, R.; Timilsina, A.P.; Regmi, B.P.; Sharma, K.R.; Aryal, N.; Parajuli, N. Metabolome Mining of Curcuma longa L. Using HPLC-MS/MS and Molecular Networking. Metabolites 2023, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Sun, L.; Gao, Y.; Niu, X.; Wang, H. Novel Inhibitor Discovery of Staphylococcus aureus Sortase B and the Mechanism Confirmation via Molecular Modeling. Molecules 2018, 23, 977. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural product coumarins: Biological and pharmacological perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Tsivileva, O.M.; Koftin, O.V. Fungal coumarins: Biotechnological and pharmaceutical aspects. Stud. Nat. Prod. Chem. 2023, 78, 441–479. [Google Scholar]
- Ismael, R.N.; Mustafa, Y.F.; Al-Qazaz, H.K. Coumarin-based products: Their biodiversity and pharmacology. Iraq J. Pharm. 2021, 18, 162–179. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.-S.; Cheng, M.; Xia, P.; Qian, K.; Wu, P.-C.; Lai, C.-Y.; Xia, Y.; Yang, Z.-Y.; Morris-Natschke, S.L.; et al. Antitumor agents 292. Design, synthesis and pharmacological study of S- and O-substituted 7-mercapto- or hydroxy-coumarins and chromones as potent cytotoxic agents. Eur. J. Med. Chem. 2012, 49, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Agrawal, R.; Ubaidullah, M.; Hassan, M.I.; Tarannum, N. Design, synthesis and validation of anti-microbial coumarin derivatives: An efficient green approach. Heliyon 2019, 5, e02615. [Google Scholar] [CrossRef] [PubMed]
- Cheke, R.S.; Patel, H.M.; Patil, V.M.; Ansari, I.A.; Ambhore, J.P.; Shinde, S.D.; Kadri, A.; Snoussi, M.; Adnan, M.; Kharkar, P.S.; et al. Molecular Insights into Coumarin Analogues as Antimicrobial Agents: Recent Developments in Drug Discovery. Antibiotics 2022, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Yazıcı-Tütüniş, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial Activities of Pyrenylated Coumarins from the Roots of Prangos hulusii. Molecules 2017, 22, 1098. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R. Antimicrobial arylcoumarins from Asphodelus microcarpus. J. Nat. Prod. 2007, 70, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.C.; Panda, S.S. DNA Gyrase as a Target for Quinolones. Biomedicines 2023, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Leyn, S.A.; Kent, J.E.; Zlamal, J.E.; Elane, M.L.; Vercruysse, M.; Osterman, A.L. Two classes of DNA gyrase inhibitors elicit distinct evolutionary trajectories toward resistance in gram-negative pathogens. NPJ Antimicrob. Resist. 2024, 2, 5. [Google Scholar] [CrossRef]
- Maxwell, A. The interaction between coumarin drugs and DNA gyrase. Mol. Microbiol. 1993, 9, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wen, Y.; Liang, C.G.; Liu, J.; Ding, Y.B.; Zhang, W.H. Design, Synthesis and Antifungal Activity of Psoralen Derivatives. Molecules 2017, 22, 1672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sass, A.; Van Acker, H.; Wille, J.; Verhasselt, B.; Van Nieuwerburgh, F.; Kaever, V.; Crabbé, A.; Coenye, T. Coumarin reduces virulence and biofilm formation in Pseudomonas aeruginosa by affecting quorum sensing, type III secretion and c-di-GMP levels. Front. Microbiol. 2018, 9, 1952. [Google Scholar]
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Parages, M.L. Coumarin: A novel player in microbial quorum sensing and biofilm formation inhibition. Appl. Microbiol. Biotechnol. 2018, 102, 2063–2073. [Google Scholar] [CrossRef]
- Sardari, S.; Mori, Y.; Horita, K.; Micetich, R.G.; Nishibe, S.; Daneshtalab, M. Synthesis and antifungal activity of coumarins and angular furanocoumarins. Bioorg Med Chem. 1999, 7, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- da SM Forezi, L.; Froes, T.Q.; Cardoso, M.F.; de Oliveira Maciel, C.A.; Nicastro, G.G.; Baldini, R.L.; Costa, D.; Ferreira, V.F.; Castilho, M.S.; de C da Silva, F. Synthesis and biological evaluation of Coumarins derivatives as potential inhibitors of the production of Pseudomonas aeruginosa virulence factor Pyocyanin. Curr. Top. Med. Chem. 2018, 18, 149–156. [Google Scholar]
- Mikulášová, M.; Chovanová, R.; Vaverková, Š. Synergism between antibiotics and plant extracts or essential oils with efflux pump inhibitory activity in coping with multidrug-resistant staphylococci. Phytochem. Rev. 2016, 15, 651–662. [Google Scholar] [CrossRef]
- Araújo-Neto, J.B.d.; Oliveira-Tintino, C.D.d.M.; de Araújo, G.A.; Alves, D.S.; Ribeiro, F.R.; Brancaglion, G.A.; Carvalho, D.T.; Lima, C.M.G.; Mohammed Ali, H.S.H.; Rather, I.A.; et al. 3-Substituted Coumarins Inhibit NorA and MepA Efflux Pumps of Staphylococcus aureus. Antibiotics 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, R.S.A.; Barbosa-Filho, J.M.; Scotti, M.T.; Scotti, L.; Cruz, R.M.D.D.; Falcão-Silva, V.D.S.; Siqueira-Júnior, J.P.D.; Mendonça-Junior, F.J.B. Modulation of drug resistance in Staphylococcus aureus with Coumarin derivatives. Scientifica 2016, 2016, 6894758. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.; Lin, F.Y. Terpene biosynthesis: Modularity rules. Angew. Chem. Int. Ed. 2012, 51, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food presenvatives. Food Chem. 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Toffolatti, S.L.; Maddalena, G.; Passera, A.; Casati, P.; Bianco, P.A.; Quaglino, F. Role of terpenes in plant defense to biotic stress. In Biocontrol Agents and Secondary Metabolites; Sudisha, J., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 401–417. [Google Scholar]
- Khameneh, B.; Eskin, N.A.M.; Iranshahi, M.; Fazly Bazzaz, B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Togashi, N.; Hamashima, H.; Shiraishi, A.; Inoue, Y.; Takano, A. Antibacterial activities against Staphylococcus aureus of terpene alcohols with aliphatic carbon chains. J. Essent. Oil Res. 2010, 22, 263–269. [Google Scholar] [CrossRef]
- Ephrem, E.; Najjar, A.; Charcosset, C.; Greige-Gerges, H. Selection of nerolidol among a series of terpenic and phenolic compounds for its potent activity against Lactobacillus fermentum ATCC 9338. Process Biochem. 2019, 80, 146–156. [Google Scholar] [CrossRef]
- El Fannassi, Y.; Gharsallaoui, A.; Khelissa, S.; El Amrani, M.A.; Suisse, I.; Sauthier, M.; Jama, C.; Boudra, S.; Chihib, N.E. Complexation of Terpenes for the Production of New Antimicrobial and Antibiofilm Molecules and Their Encapsulation in Order to Improve Their Activities. Appl. Sci. 2023, 13, 9854. [Google Scholar] [CrossRef]
- Catteau, L.; Zhu, L.; Van Bambeke, F.; Quetin-Leclercq, J. Natural and hemi-synthetic pentacyclic triterpenes as antimicrobials and resistance modifying agents against Staphylococcus aureus: A review. Phytochem. Rev. 2018, 17, 1129–1163. [Google Scholar] [CrossRef]
- Shakeri, A.; Akhtari, J.; Soheili, V.; Taghizadeh, S.F.; Sahebkar, A.; Shaddel, R.; Asili, J. Identification and biological activity of the volatile compounds of Glycyrrhiza triphylla Fisch. & C.A. Mey. Microb. Pathog. 2017, 109, 39–44. [Google Scholar] [PubMed]
- Tretyakova, E.V.; Salimova, E.V.; Parfenova, L.V. Synthesis and Antimicrobial and Antifungal Activity of Resin Acid Acetylene Derivatives. Russ. J. Bioorg. Chem. 2019, 45, 545–551. [Google Scholar] [CrossRef]
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; Omari, N.E. Health Benefits and Pharmacological Properties of Carvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, A.; Khosravi, A.R.; Shokri, H.; Shirzadi, H. Potential effect of 2-isopropyl-5-methylphenol (thymol) alone and in combination with fluconazole against clinical isolates of Candida albicans, C. glabrata and C. krusei. J. Mycol. Med. 2018, 28, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 333–359. [Google Scholar]
- Ben Miri, Y.; Nouasri, A.; Herrera, M.; Djenane, D.; Ariño, A. Antifungal Activity of Menthol, Eugenol and Their Combination against Aspergillus ochraceus and Aspergillus niger In Vitro and in Stored Cereals. Foods 2023, 12, 2108. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Qaralleh, H.; Ahmed Al-Dalin, S.Y.; Abboud, M.; Khleifat, K.; Majali, I.S.; Aldal’in, H.K.; Rayyan, W.A.; Jaafraa, A. Effect of thymol and carvacrol, the major components of Thymus capitatus on the growth of Pseudomonas aeruginosa. J. Pure Appl. Microbiol. 2016, 10, 367–374. [Google Scholar]
- Amaral, V.C.S.; Santos, P.R.; da Silva, A.F.; dos Santos, A.R.; Machinski, M.; Mikcha, J.M.G. Effect of carvacrol and thymol on Salmonella spp. biofilms on polypropylene. Int. J. Food Sci. Technol. 2015, 50, 2639–2643. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Luo, J.; Jie, J.; Deng, X.; Song, L. The Herbal Compound Thymol Targets Multiple Salmonella Typhimurium Virulence Factors for Lon Protease Degradation. Front. Pharmacol. 2021, 12, 674955. [Google Scholar] [CrossRef] [PubMed]
- Broniatowski, M.; Mastalerz, P.; Flasiński, M. Studies of the interactions of ursane-type bioactive terpenes with the model of Escherichia coli inner membrane—Langmuir monolayer approach. Biochim. Biophys. Acta Biomembr. 2015, 1848, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, S.M.; Edris, A.E.; Sadek, Z. Novel approach for the inhibition of Helicobacter pylori contamination in yogurt using selected probiotics combined with eugenol and cinnamaldehyde nanoemulsions. Food Chem. 2023, 417, 135877. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, P.; Vijay Kumar, H.S.; Viswanathan, P. Eugenol exhibits anti-virulence properties by competitively binding to quorum sensing receptors. Biofouling 2017, 33, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Liu, X.Y.; Jiang, P.P.; Li, W.D.; Wang, Y.F. Mechanism and antibacterial activity of cinnamaldehyde against Escherichia coli and Staphylococcus aureus. Mod. Food Sci. Technol. 2015, 31, 31–35. [Google Scholar]
- Copp, B.R. Anti-mycobacterial natural products and mechanisms of action. Nat. Prod. Rep. 2022, 39, 77–89. [Google Scholar]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant extracts—Importance in sustainable agriculture. Ital. J. Agron. 2021, 16, 1851. [Google Scholar] [CrossRef]
- Kulaeva, O.; Kliukova, M.; Afonin, A.; Sulima, A.; Zhukov, V.; Tikhonovich, I. The role of plant antimicrobial peptides (AMPs) in response to biotic and abiotic environmental factors. Bio. Comm. 2020, 65, 187–199. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, C. Antimicrobial peptides: Structure, mechanism, and modification. Eur. J. Med. Chem. 2023, 255, 115377. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Yang, P.; Lei, J.; Zhao, J. Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity. Antibiotics 2023, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos-Silva, C.A.; Zupin, L.; Oliveira-Lima, M.; Vilela, L.M.B.; Bezerra-Neto, J.P.; Ferreira-Neto, J.R.; Ferreira, J.D.C.; de Oliveira-Silva, R.L.; Pires, C.d.J.; Aburjaile, F.F.; et al. Plant Antimicrobial Peptides: State of the Art, In Silico Prediction and Perspectives in the Omics Era. Bioinform. Biol. Insights 2020, 14, 1177932220952739. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Rogozhin, E.A. Defense Peptides From the α-Hairpinin Family Are Components of Plant Innate Immunity. Front. Plant Sci. 2020, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Ryazantsev, D.Y.; Rogozhin, E.A.; Dimitrieva, T.V.; Drobyazina, P.E.; Khadeeva, N.V.; Egorov, T.A.; Grishin, E.V.; Zavriev, S.K. A novel hairpin-like antimicrobial peptide from barnyard grass (Echinochloa crusgalli L.) seeds: Structure–functional and molecular-genetics characterization. Biochimie 2014, 99, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.; Azevedo, M.I.G.; Sousa, L.M.; Oliveira, N.S.; Andrade, C.R.; Freitas, C.D.T.; Souza, P.F.N. Plant antimicrobial peptides: An overview about classification, toxicity and clinical applications. Int. J. Biol. Macromol. 2022, 214, 10–21. [Google Scholar] [CrossRef] [PubMed]
- de Souza Cândido, E.; Silva Cardoso, M.H.; Sousa, D.A.; Viana, J.C.; Gomes de Oliveira-Júnior, N.; Miranda, V.; Luiz Franco, O. The use of versatile plant antimicrobial peptides in agribusiness and human health. Peptides 2014, 55, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Panwar, S.; Thapliyal, M.; Kuriyal, V.; Tripathi, V.; Thapliyal, A. GEU-AMP50: Enhanced antimicrobial peptide prediction using a machine learning approach. Mater. Today Proc. 2023, 73, 81–87. [Google Scholar] [CrossRef]
- Barashkova, A.S.; Rogozhin, E.A. Isolation of antimicrobial peptides from different plant sources: Does a general extraction method exist? Plant Methods 2020, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Bakare, O.O.; Gokul, A.; Fadaka, A.O.; Wu, R.; Niekerk, L.A.; Barker, A.M.; Keyster, M.; Klein, A. Plant Antimicrobial Peptides (PAMPs): Features, Applications, Production, Expression, and Challenges. Molecules 2022, 27, 3703. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, S.; Zhang, C. Antimicrobial peptides with antiviral and anticancer properties and their modification and nanodelivery systems. Curr. Res. Biotechnol. 2023, 5, 100121. [Google Scholar] [CrossRef]
- Büyükkiraz, E.M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022, 132, 1573–1596. [Google Scholar] [CrossRef] [PubMed]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides—Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef] [PubMed]
- Koehbach, J.; Craik, D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Barashkova, A.S.; Sadykova, V.S.; Salo, V.A.; Zavriev, S.K.; Rogozhin, E.A. Nigellothionins from Black Cumin (Nigella sativa L.) Seeds Demonstrate Strong Antifungal and Cytotoxic Activity. Antibiotics 2021, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Culebras, P.V.; Gandía, M.; Garrigues, S.; Marcos, J.F.; Manzanares, P. Antifungal Peptides and Proteins to Control Toxigenic Fungi and Mycotoxin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 13261. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, W.; Fang, C.; Zheng, X.; Liu, C.; Huang, Q. Extraction and identification of new flavonoid compounds in dandelion Taraxacum mongolicum Hand.-Mazz. with evaluation of antioxidant activities. Sci. Rep. 2023, 13, 2166. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Korostyleva, T.V.; Odintsova, M.S.; Pukhalsky, V.A.; Grishin, E.V.; Egorov, T.A. Analysis of Triticum boeoticum and Triticum urartu seed defensins: To the problem of the origin of polyploid wheat genomes. Biochimie 2008, 90, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Chan, L.Y.; Gilding, E.K.; Henriques, S.T.; Condon, N.D.; Ravipati, A.S.; Kaas, Q.; Huang, Y.H.; Craik, D.J. Discovery and mechanistic studies of cytotoxic cyclotides from the medicinal herb Hybanthus enneaspermus. J. Biol. Chem. 2020, 295, 10911–10925. [Google Scholar] [CrossRef] [PubMed]
- Slazak, B.; Haugmo, T.; Badyra, B.; Göransson, U. The life cycle of cyclotides: Biosynthesis and turnover in plant cells. Plant Cell Rep. 2020, 3, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Shalovylo, Y.I.; Yusypovych, Y.M.; Hrunyk, N.I.; Roman, I.I.; Zaika, V.K.; Krynytskyy, H.T.; Nesmelova, I.V.; Kovaleva, V.A. Seed-derived defensins from Scots pine: Structural and functional features. Planta 2021, 254, 129. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, L.; Huang, J.; Serra, A.; Yang, H.; Tam, J.P. Potentides: New Cysteine-Rich Peptides with Unusual Disulfide Connectivity from Potentilla anserina. Chembiochem 2019, 20, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, D.K.; Kandaswamy, K. Antimicrobial peptides: A small molecule for sustainable healthcare applications. Med. Microecol. 2023, 18, 100090. [Google Scholar] [CrossRef]
- Pires, Á.S.; Rigueiras, P.O.; Dohms, S.M.; Porto, W.F.; Franco, O.L. Structure-guided identification of antimicrobial peptides in the spathe transcriptome of the non-model plant, arum lily (Zantedeschia aethiopica). Chem. Biol. Drug Des. 2019, 93, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Shelenkov, A.; Slavokhotova, A.; Odintsova, T. Predicting antimicrobial and other cysteine-rich peptides in 1267 plant transcriptomes. Antibiotics 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Tsekouras, V.; Mavrikou, S.; Vlachakis, D.; Makridakis, M.; Stroggilos, R.; Zoidakis, J.; Termentzi, A.; Moschopoulou, G.; Kintzios, S. Proteome analysis of leaf, stem and callus in Viscum album and identification of lectins and viscotoxins with bioactive properties. Plant Cell Tissue Organ. Cult. 2020, 141, 167–178. [Google Scholar] [CrossRef]
- Hayes, B.M.E.; Bleackley, M.R.; Anderson, M.A.; van der Weerden, N.L. The plant defensin NaD1 enters the cytoplasm of Candida albicans via endocytosis. J. Fungi 2018, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Arendt, E.K.; Thery, T.L.C. Isolation and characterisation of the antifungal activity of the cowpea defensin Cp-thionin II. Food Microbiol. 2019, 82, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Dancewicz, K.; Slazak, B.; Kiełkiewicz, M.; Kapusta, M. Behavioral and physiological effects of Viola spp. cyclotides on Myzus persicae (Sulz.). J. Insect Physiol. 2020, 122, 104025. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Catacutan, D.B.; Rathod, K.; Swanson, K.; Jin, W.; Mohammed, J.C.; Chiappino-Pepe, A.; Syed, S.A.; Fragis, M.; Rachwalski, K.; et al. Deep learning-guided discovery of an antibiotic targeting Acinetobacter baumannii. Nat. Chem. Biol. 2023, 19, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-L.; Akash, K.; Reid, T.; Jain, N. Computational modeling of the dynamics of human trust during human-machine interactions. IEEE Trans. Hum.-Mach. Syst. 2019, 49, 485–497. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Alhumaid, S.; Mutair, A.A.; Garout, M.; Abulhamayel, Y.; Halwani, M.A.; Alestad, J.H.; Bshabshe, A.A.; Sulaiman, T.; AlFonaisan, M.K.; et al. Application of artificial intelligence in combating high antimicrobial resistance rates. Antibiotics 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, D. Perspective: Limiting antimicrobial resistance with artificial intelligence/machine learning. BME Front. 2023, 4, 0033. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Ahmed, S.; Aslam, M. Artificial Intelligence for Antimicrobial Resistance Prediction: Challenges and Opportunities towards Practical Implementation. Antibiotics 2023, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Ayman, E.T.; Soliman, S.M.; Ahmed, A.E.; El-kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, S. Role of alternatives to antibiotics in mitigating the antimicrobial resistance crisis. Indian J. Med. Res. 2022, 156, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Lamut, A.; Peterlin Masic, L.; Kikelj, D.; Tomasic, T. Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med. Res. Rev. 2019, 39, 2460–2504. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.; González-Bello, C. Siderophores: Chemical tools for precise antibiotic delivery. Bioorganic Med. Chem. Lett. 2023, 87, 129282. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Nakashima, K.-I.; Nishino, K.; Kotani, K.; Tomida, J.; Inoue, M.; Kawamura, Y. Berberine Is a Novel Type Efflux Inhibitor Which Attenuates the MexXY-Mediated Aminoglycoside Resistance in Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 1223. [Google Scholar] [CrossRef] [PubMed]
- Seukep, A.J.; Mbuntcha, H.G.; Zeuko’o, E.M.; Woquan, L.S.; Nembu, N.E.; Bomba, F.T.; Watching, D.; Kuete, V. Chapter Five—Established Antibacterial Drugs from Plants; Kuete, V., Ed.; Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2023; Volume 106, pp. 81–149. [Google Scholar]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid. Based Complement. Alternat. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Li, J.; Sun, Z. The Combination of Antibiotic and Non-Antibiotic Compounds Improves Antibiotic Efficacy against Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2023, 24, 15493. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Liu, Y.; Jiang, F.; Ji, F.; Wang, H.; Han, X. Synergistic Antibacterial Activity of Designed Trp-Containing Antibacterial Peptides in Combination with Antibiotics against Multidrug-Resistant Staphylococcus epidermidis. Front. Microbiol. 2019, 25, 2719. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Pethe, K.; Chan-Park, M.B. Chemical Basis of Combination Therapy to Combat Antibiotic Resistance. JACS 2023, 3, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Siriphap, A.; Kiddee, A.; Duangjai, A.; Yosboonruang, A.; Pook-In, G.; Saokaew, S.; Sutheinkul, O.; Rawangkan, A. Antimicrobial Activity of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Multidrug-Resistant Vibrio cholerae. Antibiotics 2022, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Bonincontro, G.; Scuderi, S.A.; Marino, A.; Simonetti, G. Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp. Pharmaceuticals 2023, 16, 1531. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, R.; Obize, C.; Sibanda, T.; Abia, A.L.K.; Long, H. Evolution and Emergence of Antibiotic Resistance in Given Ecosystems: Possible Strategies for Addressing the Challenge of Antibiotic Resistance. Antibiotics 2023, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chauhan, A.; Ranjan, A.; Mathkor, D.M.; Haque, S.; Ramniwas, S.; Tuli, H.S.; Jindal, T.; Yadav, V. Emerging challenges in antimicrobial resistance: Implications for pathogenic microorganisms, novel antibiotics, and their impact on sustainability. Front. Microbiol. 2024, 15, 1403168. [Google Scholar] [CrossRef] [PubMed]
- Bobate, S.; Mahalle, S.; Dafale, N.D.; Bajaj, A. Emergence of environmental antibiotic resistance: Mechanism, monitoring and management. Environ. Adv. 2023, 13, 100409. [Google Scholar] [CrossRef]
- Samreen, A.I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar]
- Zhang, L.; Tian, X.; Sun, L.; Mi, K.; Wang, R.; Gong, F.; Huang, L. Bacterial Efflux Pump Inhibitors Reduce Antibiotic Resistance. Pharmaceutics 2024, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R. A one-health approach to antimicrobial resistance. Nat. Microbiol. 2018, 3, 854–855. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelini, P. Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance. Antibiotics 2024, 13, 746. https://doi.org/10.3390/antibiotics13080746
Angelini P. Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance. Antibiotics. 2024; 13(8):746. https://doi.org/10.3390/antibiotics13080746
Chicago/Turabian StyleAngelini, Paola. 2024. "Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance" Antibiotics 13, no. 8: 746. https://doi.org/10.3390/antibiotics13080746
APA StyleAngelini, P. (2024). Plant-Derived Antimicrobials and Their Crucial Role in Combating Antimicrobial Resistance. Antibiotics, 13(8), 746. https://doi.org/10.3390/antibiotics13080746








