Peripheral Intravenous Therapy in Internal Medicine Department—Antibiotics and Other Drugs’ Consumption and Characteristics of Vascular Access Devices in 2-Year Observation Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waitt, C.; Waitt, P.; Pirmohamed, M. Intravenous therapy. Postgrad. Med. J. 2004, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Regina, M.L.; Vecchié, A.; Bonaventura, A.; Prisco, D. Patient Safety in Internal Medicine. In Textbook of Patient Safety and Clinical Risk Management; Donaldson, L., Ricciardi, W., Sheridan, S., Tartaglia, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 213–252. [Google Scholar]
- Niederman, M.S.; Baron, R.M.; Bouadma, L.; Calandra, T.; Daneman, N.; DeWaele, J.; Kollef, M.H.; Lipman, J.; Nair, G.B. Initial antimicrobial management of sepsis. Crit. Care 2021, 25, 307. [Google Scholar] [CrossRef] [PubMed]
- Pittiruti, M.; Van Boxtel, T.; Scoppettuolo, G.; Carr, P.; Konstantinou, E.; Ortiz Miluy, G.; Simcock, L.; Dupont, C.; Inwood, S.; Pepe, G.; et al. European recommendations on the proper indication and use of peripheral venous access devices: A WoCoVA project. J. Vasc. Access 2023, 24, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.G.; Ullman, A.J.; Rickard, C.M.; Johnston, A. Factors impacting emergency department clinicians’ peripheral intravenous catheter practice: A qualitative analysis. Int. Emerg. Nurs. 2023, 71, 101366. [Google Scholar] [CrossRef] [PubMed]
- Simin, D.; Milutinović, D.; Turkulov, V.; Brkić, S. Incidence, severity and risk factors of peripheral intravenous cannula-induced complications: An observational prospective study. J. Clin. Nurs. 2019, 28, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Helm, R.E.; Klausner, J.D.; Klemperer, J.D.; Flint, L.M.; Huang, E. Accepted but Unacceptable: Peripheral IV Catheter Failure. J. Infus. Nurs. 2019, 42, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.N.; Marsh, N.; Mihala, G.; King, M.; Zunk, M.; Ullman, A.J.; Keogh, S.; Kleidon, T.M.; Rickard, C.M. Intravenous antimicrobial administration through peripheral venous catheters—Establishing risk profiles from an analysis of 5252 devices. Int. J. Antimicrob. Agents 2022, 59, 106552. [Google Scholar] [CrossRef] [PubMed]
- Duława, J. Reflections on internal medicine in Poland on the occasion of the 100th anniversary of Polish Archives of Internal Medicine. Pol. Arch. Intern. Med. 2023, 133, 16482. [Google Scholar] [CrossRef] [PubMed]
- Ray-Barruel, G.; Xu, H.; Marsh, N.; Cooke, M.; Rickard, C.M. Effectiveness of insertion and maintenance bundles in preventing peripheral intravenous catheter-related complications and bloodstream infection in hospital patients: A systematic review. Infect. Dis. Health 2019, 24, 152–168. [Google Scholar] [CrossRef]
- Bayram, A.; Chiappinotto, S.; Palese, A. Unfinished nursing care in healthcare settings during the COVID-19 pandemic: A systematic review. BMC Health Serv. Res. 2024, 24, 352. [Google Scholar] [CrossRef]
- Guidelines for the Prevention of Bloodstream Infections and Other Infections Associated with the Use of Intravascular Catheters. Part I: Peripheral Catheters; World Health Organization: Geneva, Switzerland, 2024.
- Moureau, N.; Chopra, V. Indications for peripheral, midline and central catheters: Summary of the MAGIC recommendations. Br. J. Nurs. 2016, 25, S15–S24. [Google Scholar] [CrossRef] [PubMed]
- Dutta, C.; Pasha, K.; Paul, S.; Abbas, M.S.; Nassar, S.T.; Tasha, T.; Mohammed, L. Urinary Tract Infection Induced Delirium in Elderly Patients: A Systematic Review. Cureus 2022, 14, e32321. [Google Scholar] [CrossRef] [PubMed]
- Moureau, N. Vessel Health and Preservation: The Right Approach for Vascular Access; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Tripathi, S.; Gladfelter, T. Peripheral intravenous catheters in hospitalized patients: Practice, Dwell times, and factors impacting the dwell times: A single center retrospective study. J. Vasc. Access 2022, 23, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Gorski, L.A.M.; Hadaway, L.M.; Hagle, M.E.P.; Broadhurst, D.M.; Clare, S.M.; Kleidon, T.M.P.; Meyer, B.M.P.; Nickel, B.A.-C.; Rowley, S.M.; Sharpe, E.D.; et al. Infusion Therapy Standards of Practice, 8th Edition. J. Infus. Nurs. 2021, 44 (Suppl. 1), S1–S224. [Google Scholar] [CrossRef]
- Marsh, N.; Webster, J.; Ullman, A.J.; Mihala, G.; Cooke, M.; Chopra, V.; Rickard, C.M. Peripheral intravenous catheter non-infectious complications in adults: A systematic review and meta-analysis. J. Adv. Nurs. 2020, 76, 3346–3362. [Google Scholar] [CrossRef]
- Guanche-Sicilia, A.; Sánchez-Gómez, M.B.; Castro-Peraza, M.E.; Rodríguez-Gómez, J.Á.; Gómez-Salgado, J.; Duarte-Clíments, G. Prevention and Treatment of Phlebitis Secondary to the Insertion of a Peripheral Venous Catheter: A Scoping Review from a Nursing Perspective. Healthcare 2021, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Wallis, M.C.; McGrail, M.; Webster, J.; Marsh, N.; Gowardman, J.; Playford, E.G.; Rickard, C.M. Risk factors for peripheral intravenous catheter failure: A multivariate analysis of data from a randomized controlled trial. Infect. Control Hosp. Epidemiol. 2014, 35, 63–68. [Google Scholar] [CrossRef]
- OECD; European Observatory on Health Systems and Policies. Poland: Country Health Profile 2023; State of Health in the EU; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Maymon, S.L.; Moravsky, G.; Marcus, G.; Shuvy, M.; Pereg, D.; Epstein, D.; Litovchik, I.; Fuchs, S.; Minha, S. Disparities in the characteristics and outcomes of patients hospitalized with acute decompensated heart failure admitted to internal medicine and cardiology departments: A single-centre, retrospective cohort study. ESC Heart Fail. 2021, 8, 390–398. [Google Scholar] [CrossRef]
- Vazir, A.; Cowie, M.R. Decongestion: Diuretics and other therapies for hospitalized heart failure. Indian Heart J. 2016, 68 (Suppl. S1), S61–S68. [Google Scholar] [CrossRef]
- Bitterman, R.; Hussein, K.; Leibovici, L.; Carmeli, Y.; Paul, M. Systematic review of antibiotic consumption in acute care hospitals. Clin. Microbiol. Infect. 2016, 22, 561.e7–561.e19. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influenza Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Siewierska, M.; Gajda, M.; Opalska, A.; Brudło, M.; Krzyściak, P.; Gryglewska, B.; Różańska, A.; Wójkowska-Mach, J. Hospital antibiotic consumption—An interrupted time series analysis of the early and late phases of the COVID-19 pandemic in Poland, a retrospective study. Pharmacol. Rep. 2023, 75, 715–725. [Google Scholar] [CrossRef]
- Eyler, R.F.; Shvets, K. Clinical Pharmacology of Antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef]
- Masot, O.; Miranda, J.; Santamaría, A.L.; Paraiso Pueyo, E.; Pascual, A.; Botigué, T. Fluid Intake Recommendation Considering the Physiological Adaptations of Adults Over 65 Years: A Critical Review. Nutrients 2020, 12, 3383. [Google Scholar] [CrossRef] [PubMed]
- Gawronska, J.; Koyanagi, A.; Sánchez, G.F.L.; Veronese, N.; Ilie, P.C.; Carrie, A.; Smith, L.; Soysal, P. The Prevalence and Indications of Intravenous Rehydration Therapy in Hospital Settings: A Systematic Review. Pidemiologia 2022, 4, 18–32. [Google Scholar] [CrossRef]
- Jackson, A. Infection control-a battle in vein: Infusion phlebitis. Nurs. Times 1998, 94, 68–71. [Google Scholar]
- CDC. Determining Patient Days for Summary Data Collection: Observation vs. Inpatients. Available online: https://www.cdc.gov/nhsn/pdfs/commup/patientday_sumdata_guide.pdf (accessed on 1 December 2023).
- WHO. ATC/DDD Index 2024. Available online: https://www.whocc.no (accessed on 1 December 2023).
| Study Patient Population | |||
|---|---|---|---|
| Total number of admissions (n) | 1406 | ||
| Patients with PIVC * (n, %) | 1176 | 83.6% | |
| PIVC utilization ratio ** | 0.8 | ||
| Sex (n, %) | Female | 578 | 49.1% |
| Male | 598 | 50.9% | |
| Age (years) | Median (IQR) | 74 (66–83) | |
| Hospital admission mode (n, %) | Emergency | 1028 | 87.4% |
| Planned | 148 | 12.6% | |
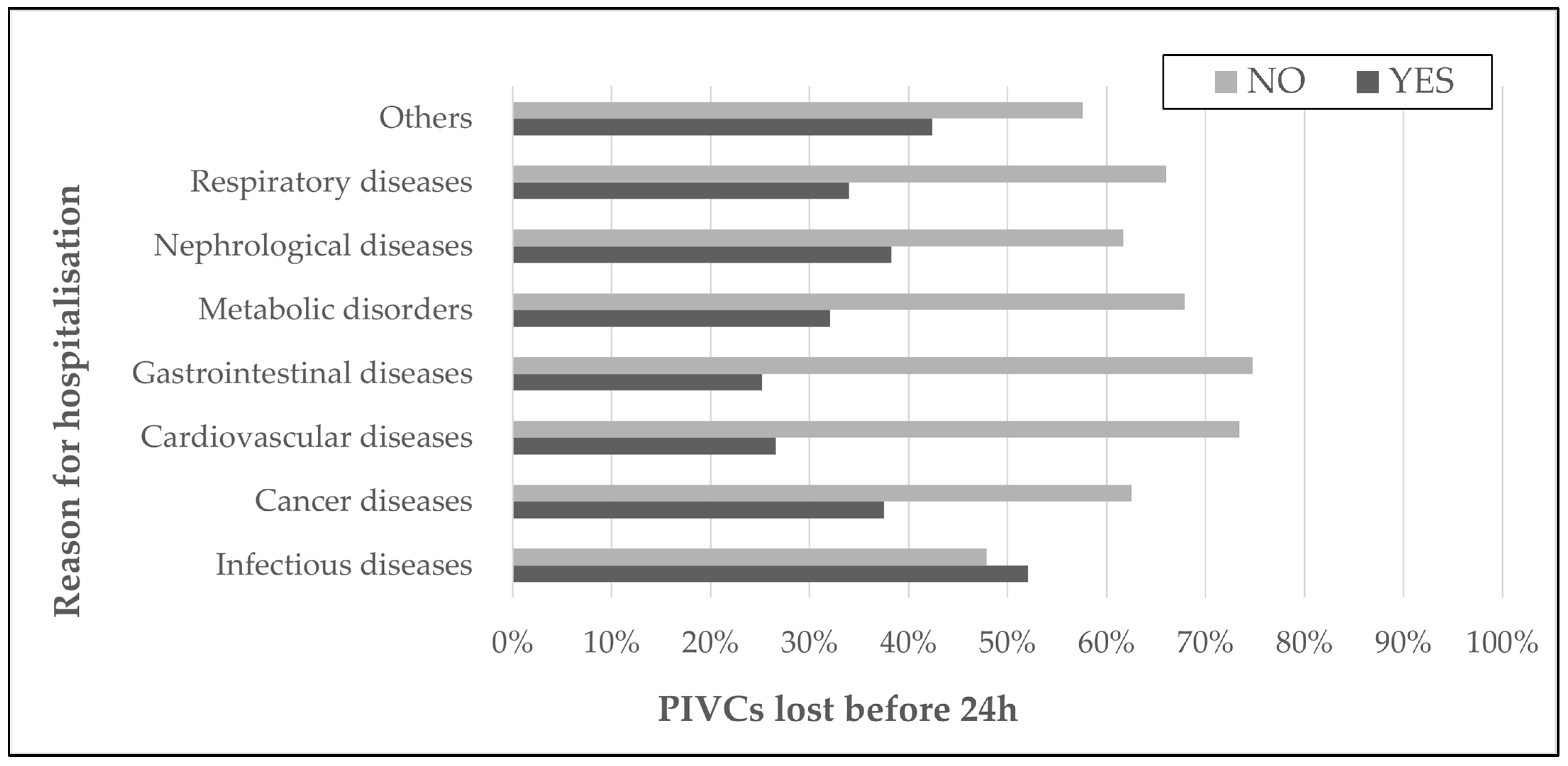
| Main reason for hospitalization (n, %) | Infectious diseases | 261 | 22.0% |
| Chronic diseases | 915 | 78.0% | |
| Chronic diseases (n, %) | Cancer diseases | 32 | 3.8% |
| Cardiovascular diseases | 290 | 31.7% | |
| Gastrointestinal tract, liver, biliary tract and pancreas diseases | 155 | 16.9% | |
| Metabolic disorders | 131 | 14.3% | |
| Nephrological diseases | 79 | 8.6% | |
| Respiratory diseases | 94 | 10.3% | |
| Others | 132 | 14.4% | |
| Admissions due to COVID-19 (n, %) | 159 | 13.5% | |
| Previous hospitalizations up to 3-months back (n, %) | 237 | 20.1% | |
| In-hospital deaths (n, %) | 130 | 11.0% | |
| Fatality case rate (%) | All hospitalized | 11.1% | |
| Patients with PIVC- | 11.2% | ||
| Length of hospital stay (days) (Median (IQR)) | 9 (6–12) | ||
| Length of intravenous therapy (days) (Median (IQR)) | 7 (5–11) | ||
| Study Patient Population with Peripheral Intravenous Catheters | ||||
|---|---|---|---|---|
| Total number of inserted PIVC (n) | 3377 | |||
| Number of used PIVC per patient (Me, IQR) | 2 (1–4) | |||
| PIVC patient days | 9678 | |||
| Location of PIVC (n, %) | Optimal | Forearm | 955 | 28.3% |
| Suboptimal | Hand | 787 | 23.3% | |
| Antecubital fossa | 598 | 17.7% | ||
| Wrist | 507 | 15.0% | ||
| Foot | 118 | 3.5% | ||
| Other | 409 | 12.1% | ||
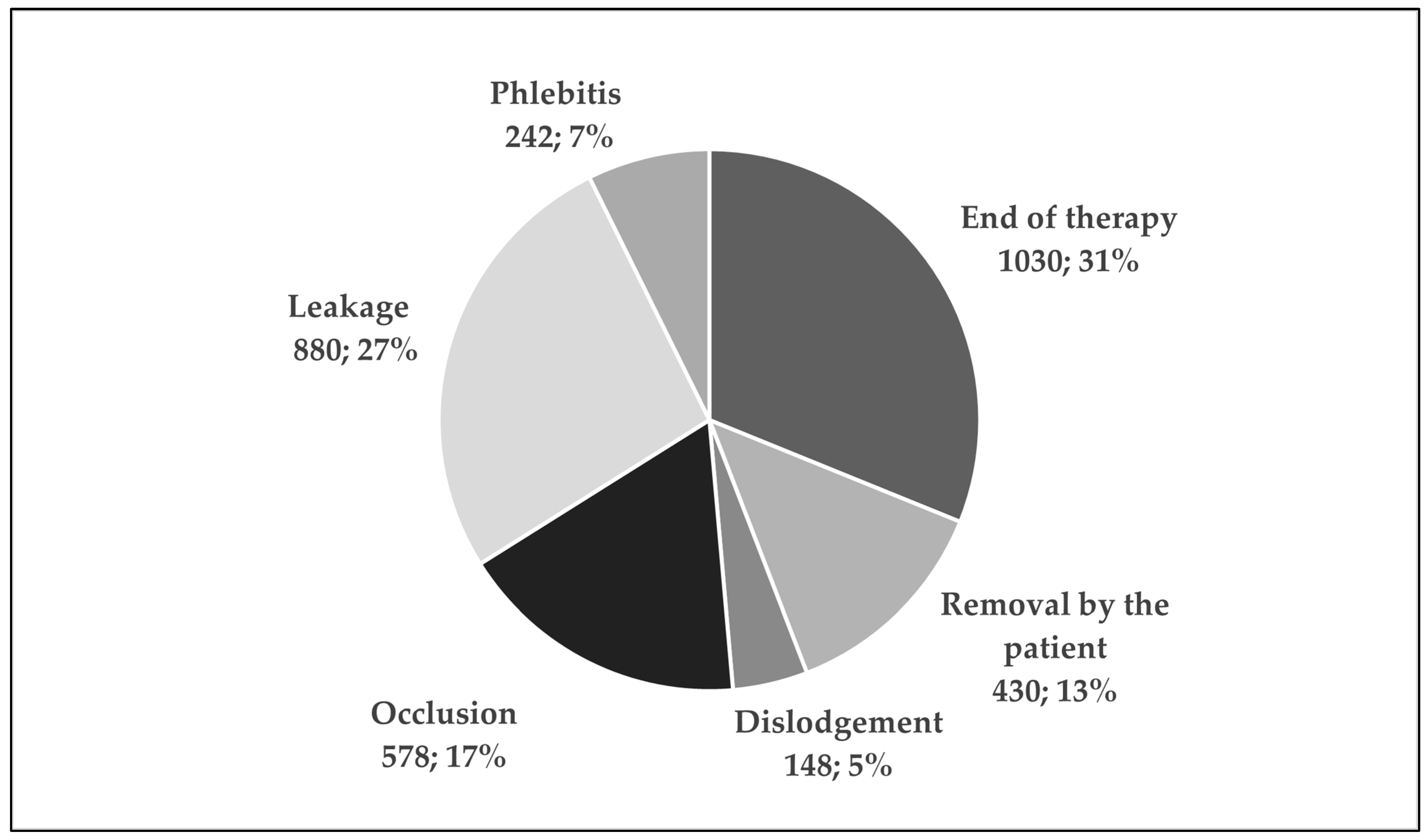
| PIVC that stopped functioning within 24 h (n, %) | 764 | 22.6% | ||
| Patients with at least one PIVC that stopped functioning within 24 h (n, %) | 425 | 36.1% | ||
| Differences in patients’ characteristics | PIVC < 24 h (n); p < 0.001 | |||
| Yes | No | |||
| Age [years, Median, (IQR)] | 76 (68–84) | 73 (64–81) | ||
| Length of stay [days, Median, IQR] | 11 (7–15) | 8 (6–11) | ||
| Length of intravenous therapy [days, Median, (IQR)] | 9 (6–14) | 6 (4–9) | ||
| Phlebitis Scale, venous inflammation signs (phlebitis) when removing the PIVC (n, %) * | 0—no sign | 2617 | 89.0% | |
| 1—possible 1st signs | 309 | 10.5% | ||
| 2—early stage | 13 | 0.4% | ||
| 3—medium stage | 1 | 0.1% | ||
| ATC Classification System, Only IV Solutions | Consumptions of Drugs * | ||
|---|---|---|---|
| DDD | mL | ||
| Anti-infective For Systemic Use | Antibacterials for systemic use (J01) | 5009.9 | N/A |
| Antimycotics for systemic use (J02) | 100.0 | N/A | |
| Antivirals for systemic use (J05) | 69 | N/A | |
| Systemic Hormonal Preparations **, (H) | Corticosteroids for systemic use (H02) | 3746.7 | N/A |
| Cardiovascular System (C) | Loop diuretics (C03) | 2298.3 | N/A |
| Others (C01A, C01B, C01CA, C01D *, C02) | 728.3 | 100.0 | |
| Alimentary Tract and Metabolism (A) | 2195.7 | 1200.0 | |
| Blood And Blood Forming Organs (B) | Electrolyte solutions (B05B *) | N/A | 5,705,600.0 |
| Others (B02, B03A *, B05A *, B05B *) | 116.3 | 620,680.0 | |
| Musculoskeletal System (M): M01 | 133.3 | N/A | |
| Nervous System (N): N01B, N02, N05, N05C | 1109.2 | N/A | |
| Respiratory System (R): R03, R06 | 9.7 | N/A | |
| TOTAL | 15,516.4 | 6,327,580.0 | |
| TOTAL per PIVC patient days | 1.6 | 653.8 | |
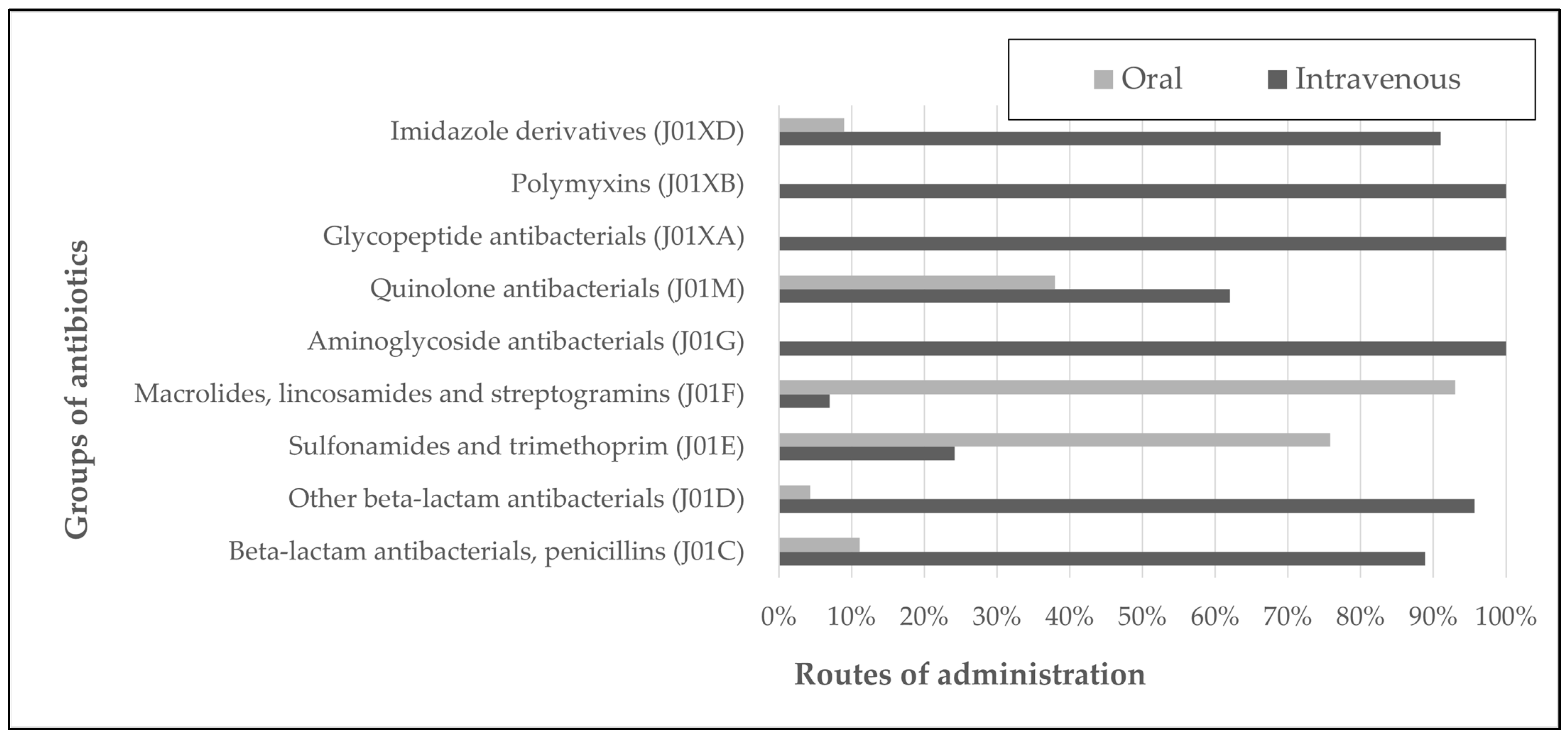
| Antibacterials for Systemic Use (J01) | Route of Administration (DDD) | ||||
|---|---|---|---|---|---|
| 2021 | 2022 | Total Amount | |||
| IV | Oral | IV | Oral | ||
| Beta-lactam antibacterials, penicillins (J01C) | 600.0 | 62.3 | 915.8 | 127.0 | 1705.1 |
| Other beta-lactam antibacterials (J01D) | 1139.6 | 90.0 | 1340.2 | 21.0 | 2590.8 |
| Sulfonamides and trimethoprim (J01E) | 72.0 | 160.0 | 65.6 | 272.0 | 569.6 |
| Macrolides, lincosamides and streptogramins (J01F) | 13.7 | 261.0 | 27.7 | 294.0 | 596.4 |
| Aminoglycoside antibacterials (J01G) | 36.5 | 0.0 | 84.8 | 0.0 | 121.3 |
| Quinolone antibacterials (J01M) | 83.8 | 45.0 | 60.0 | 44.5 | 233.3 |
| Glycopeptide antibacterials (J01XA) | 165.5 | 0.0 | 160.0 | 0.0 | 325.5 |
| Polymyxins (J01XB) | 12.0 | 0.0 | 6.0 | 0.0 | 18.0 |
| Imidazole derivatives (J01XD) | 159.7 | 13.3 | 67.0 | 10.0 | 250.0 |
| SUM | 2282.8 | 631.6 | 2727.1 | 768.5 | 6410.0 |
| TOTAL antibiotic (oral and intravenous) consumption per patient days | 0.6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekiełko, P.; Mucha, A.; Stawowczyk, E.; Wójkowska-Mach, J. Peripheral Intravenous Therapy in Internal Medicine Department—Antibiotics and Other Drugs’ Consumption and Characteristics of Vascular Access Devices in 2-Year Observation Study. Antibiotics 2024, 13, 664. https://doi.org/10.3390/antibiotics13070664
Piekiełko P, Mucha A, Stawowczyk E, Wójkowska-Mach J. Peripheral Intravenous Therapy in Internal Medicine Department—Antibiotics and Other Drugs’ Consumption and Characteristics of Vascular Access Devices in 2-Year Observation Study. Antibiotics. 2024; 13(7):664. https://doi.org/10.3390/antibiotics13070664
Chicago/Turabian StylePiekiełko, Piotr, Anna Mucha, Ewa Stawowczyk, and Jadwiga Wójkowska-Mach. 2024. "Peripheral Intravenous Therapy in Internal Medicine Department—Antibiotics and Other Drugs’ Consumption and Characteristics of Vascular Access Devices in 2-Year Observation Study" Antibiotics 13, no. 7: 664. https://doi.org/10.3390/antibiotics13070664
APA StylePiekiełko, P., Mucha, A., Stawowczyk, E., & Wójkowska-Mach, J. (2024). Peripheral Intravenous Therapy in Internal Medicine Department—Antibiotics and Other Drugs’ Consumption and Characteristics of Vascular Access Devices in 2-Year Observation Study. Antibiotics, 13(7), 664. https://doi.org/10.3390/antibiotics13070664








