Treating Infected Non-Healing Venous Leg Ulcers with Medical-Grade Honey: A Prospective Case Series
Abstract
1. Introduction
2. Results
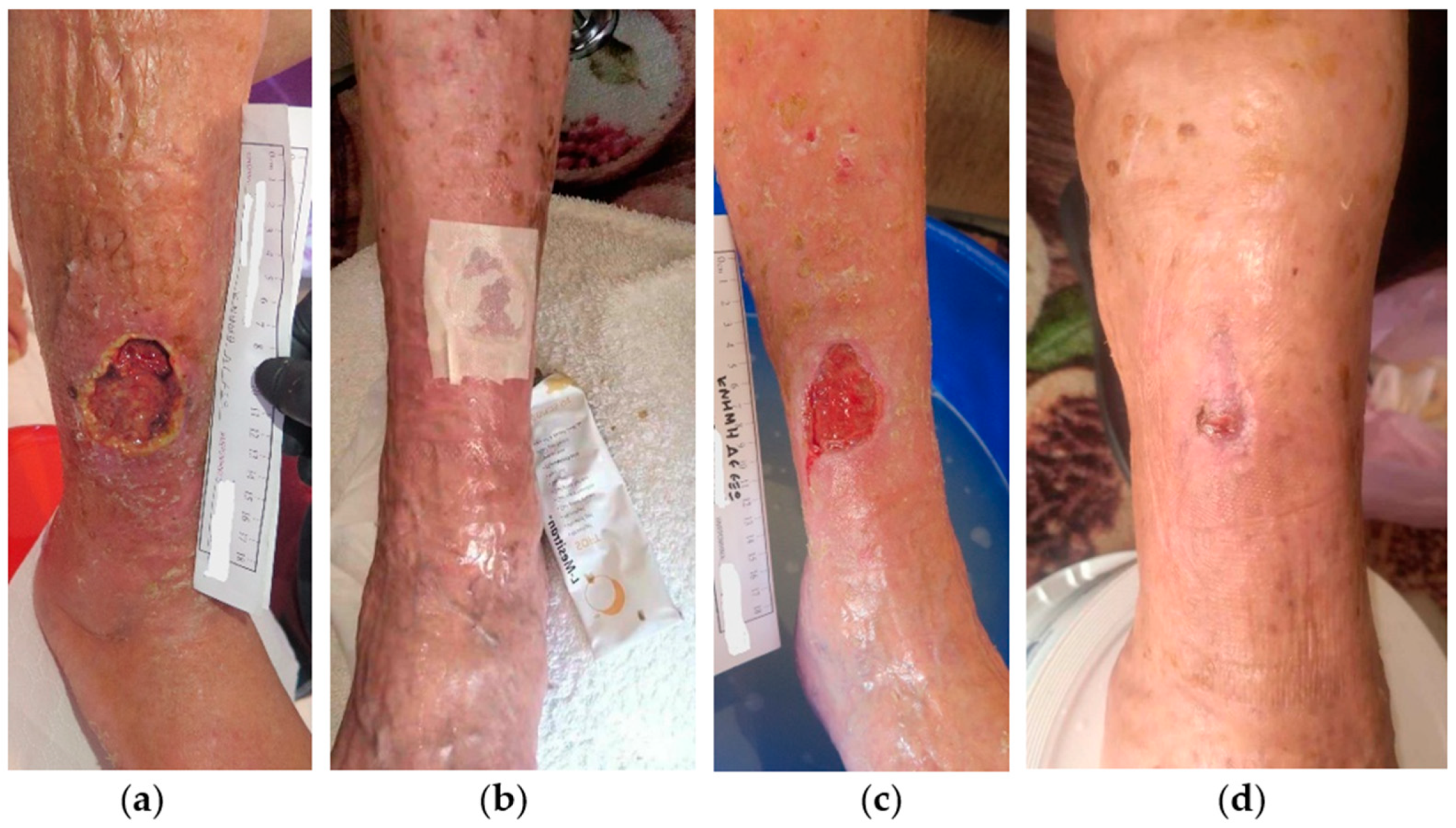
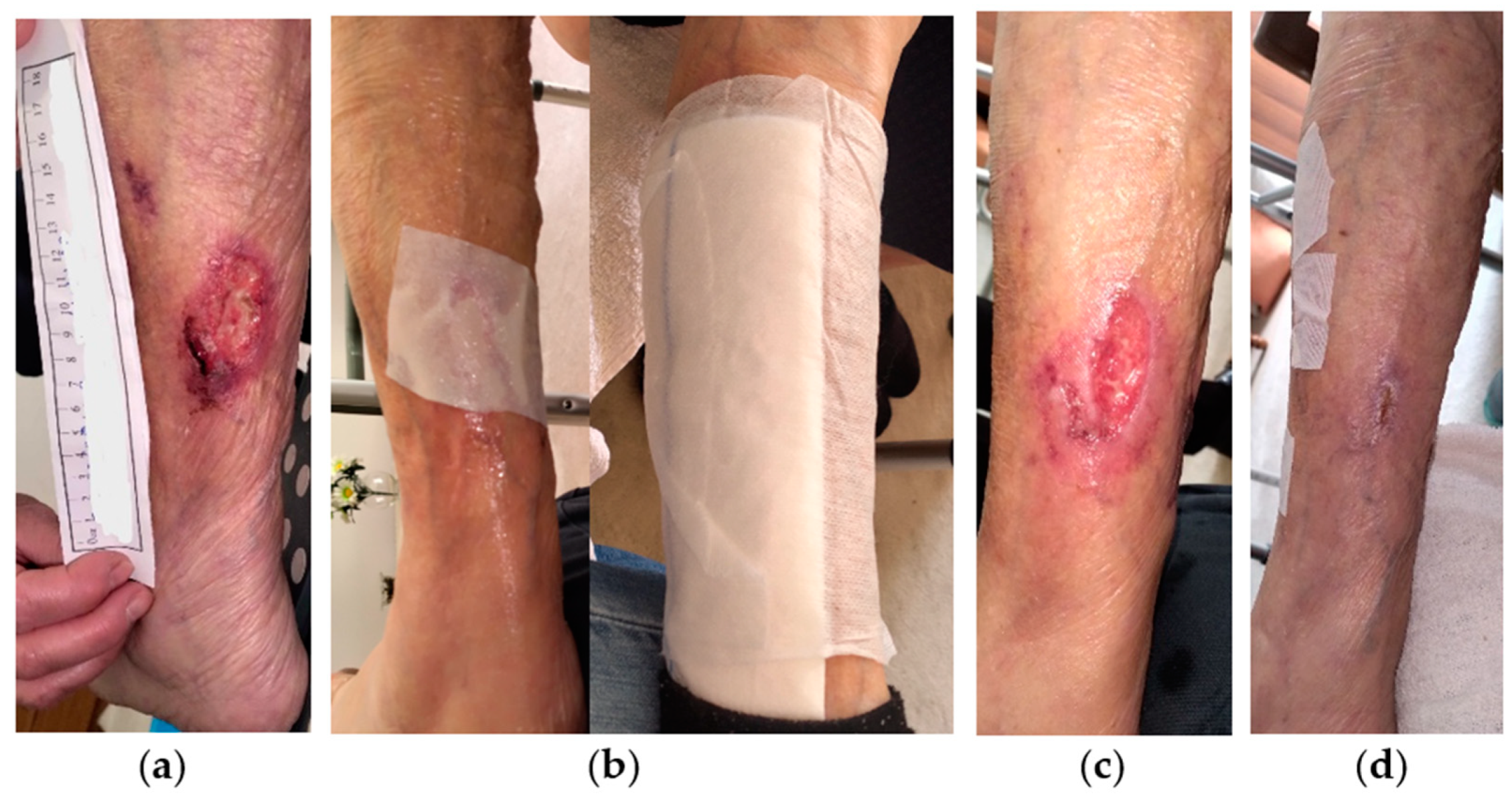
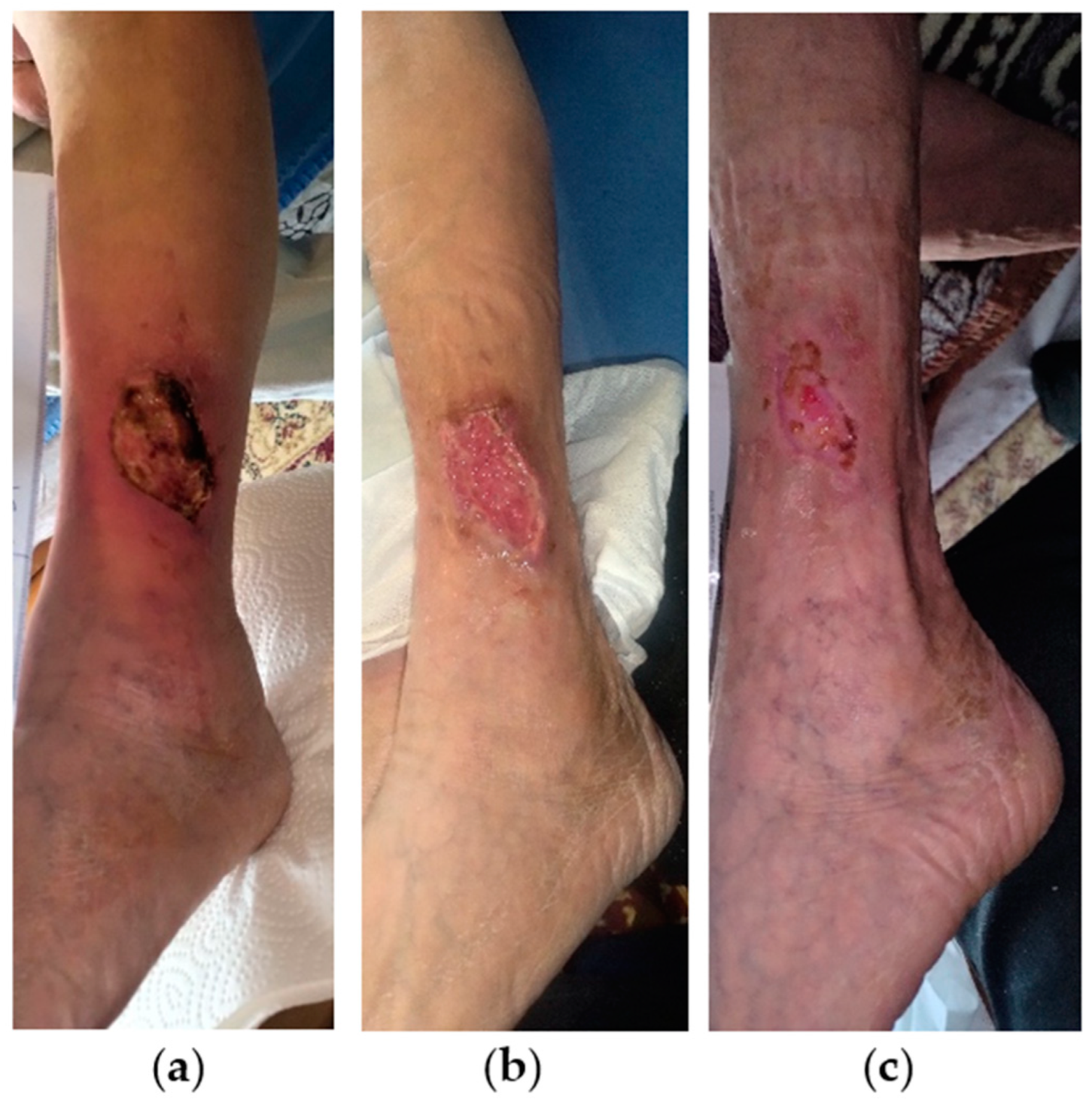
2.1. Case 1
2.2. Case 2
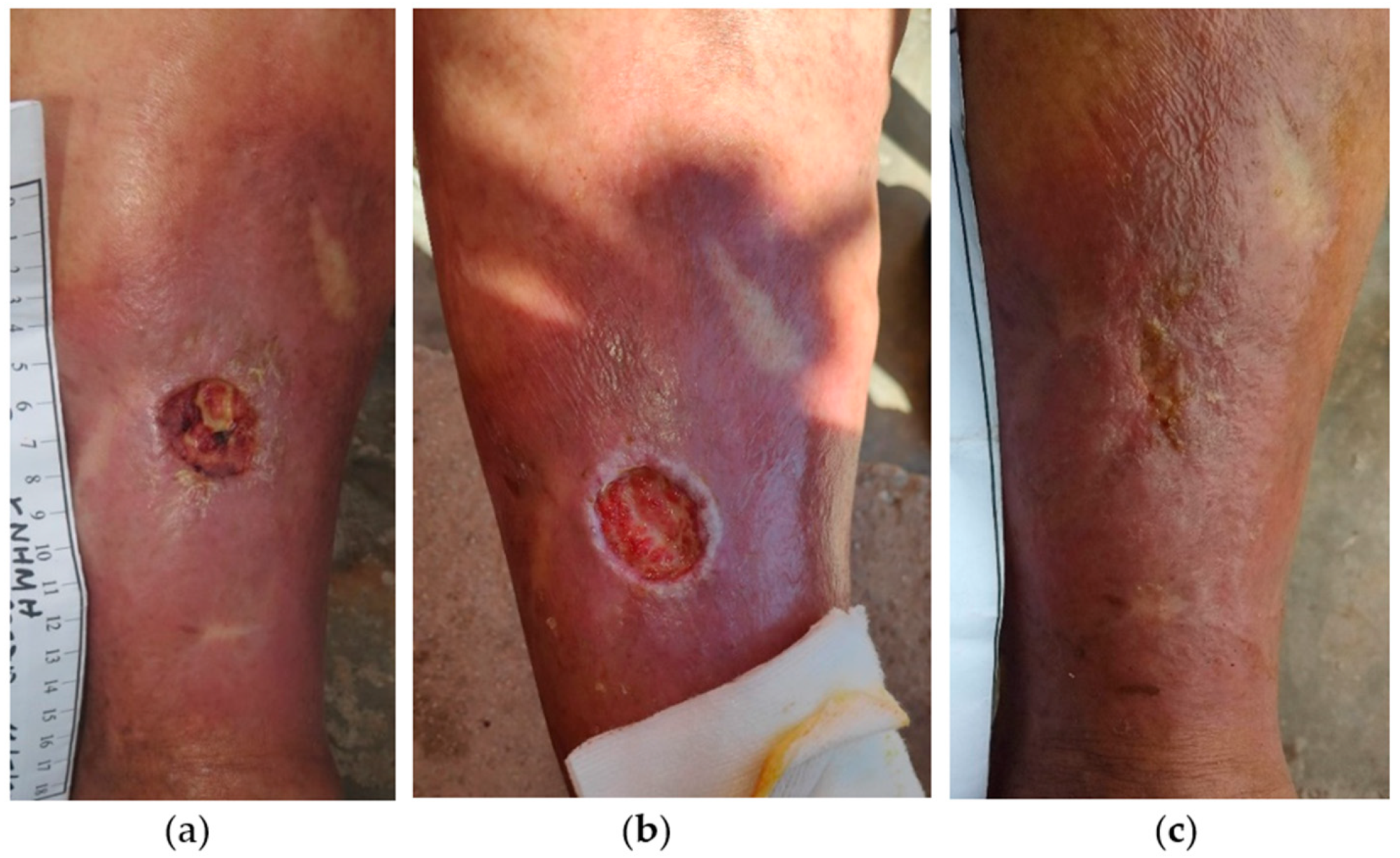
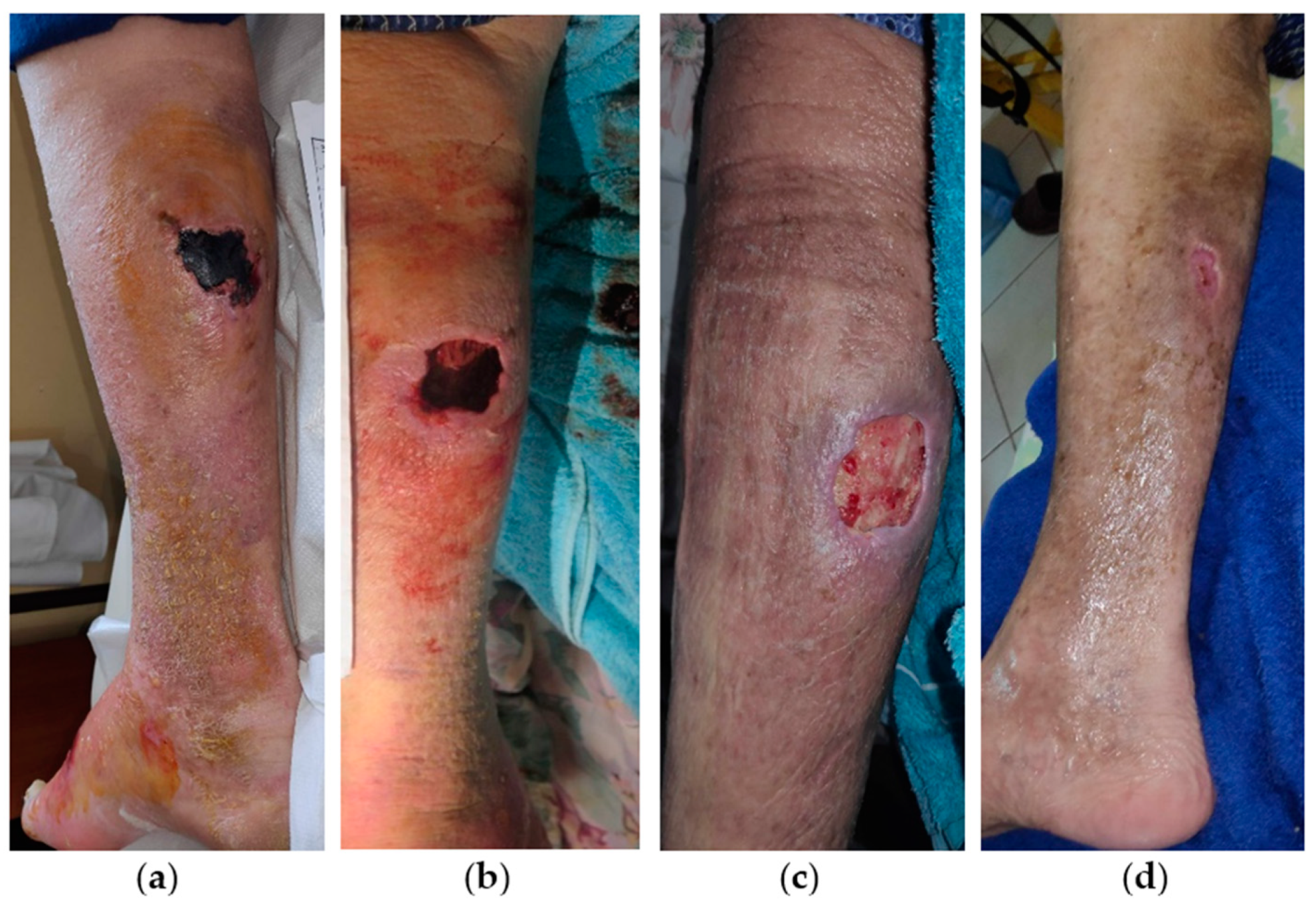
2.3. Case 3
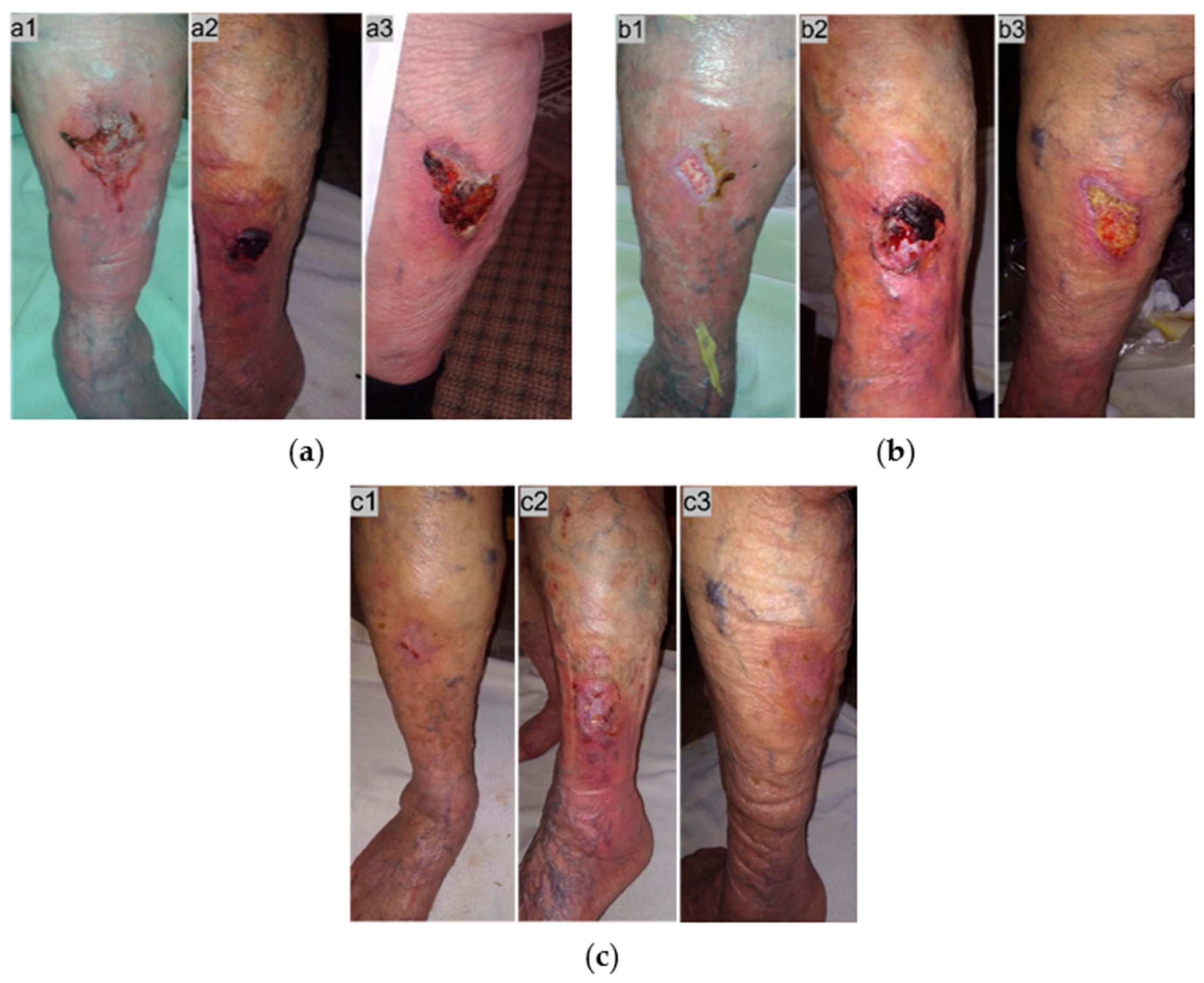
2.4. Case 4
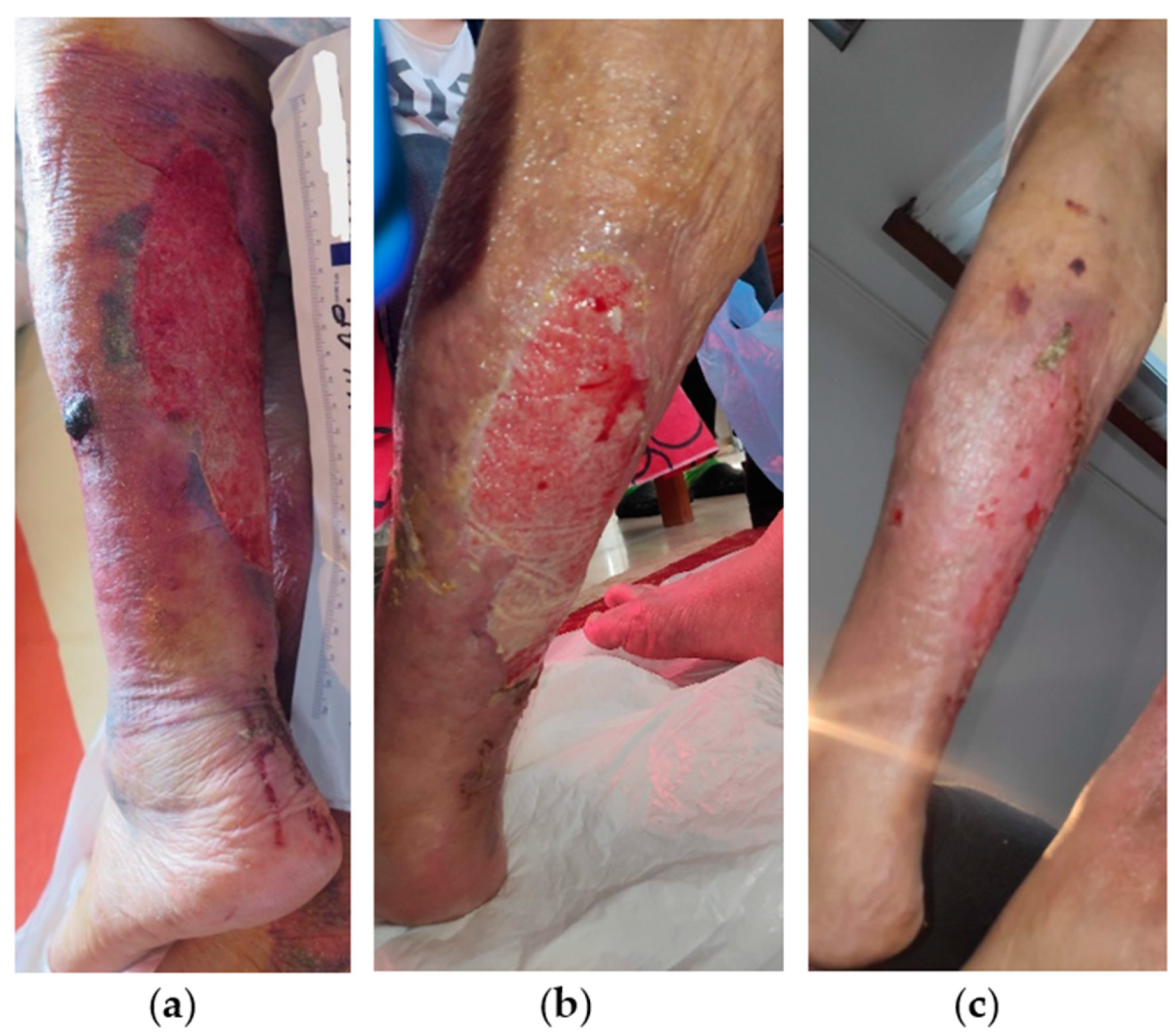
2.5. Case 5
2.6. Case 6
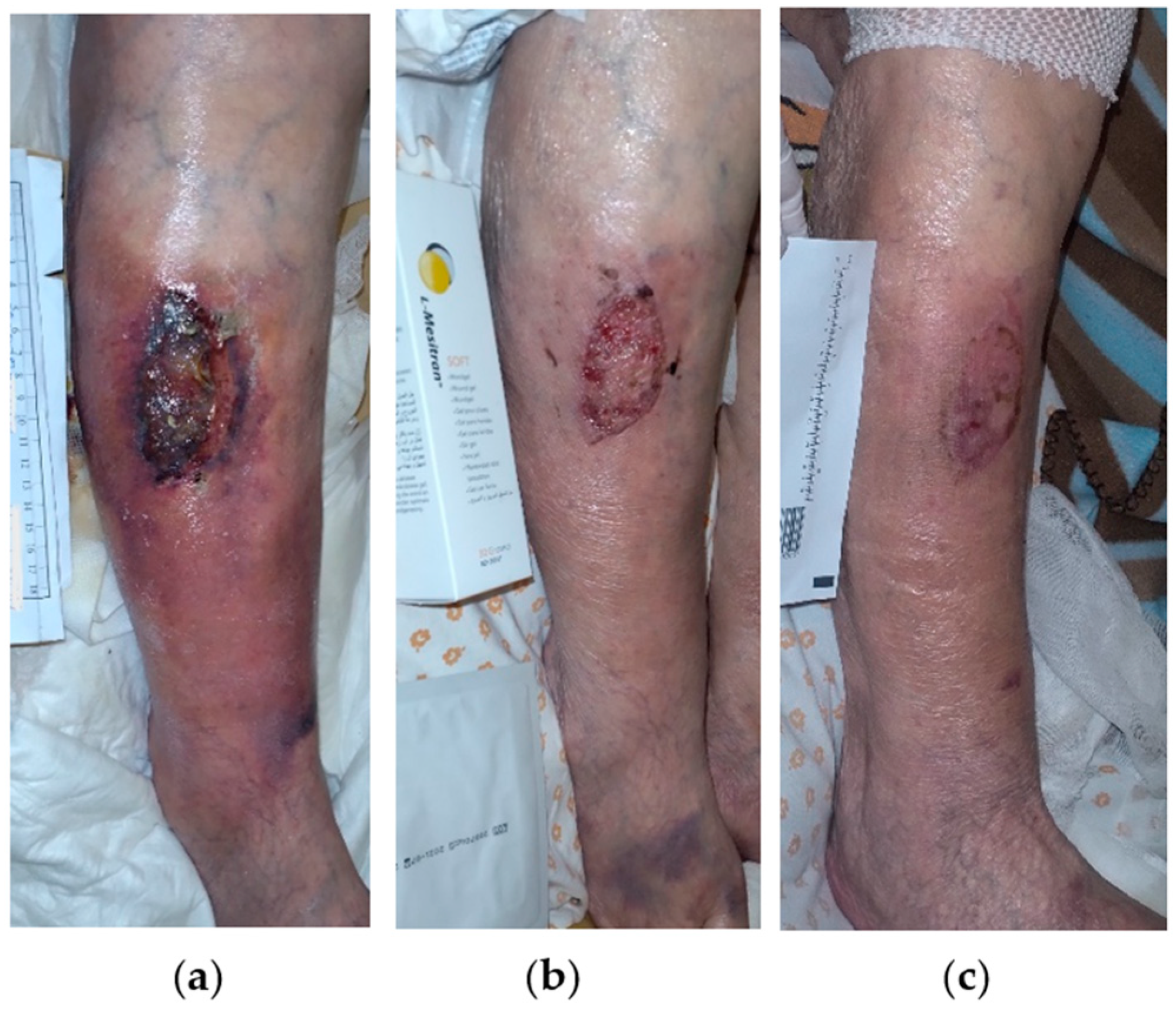
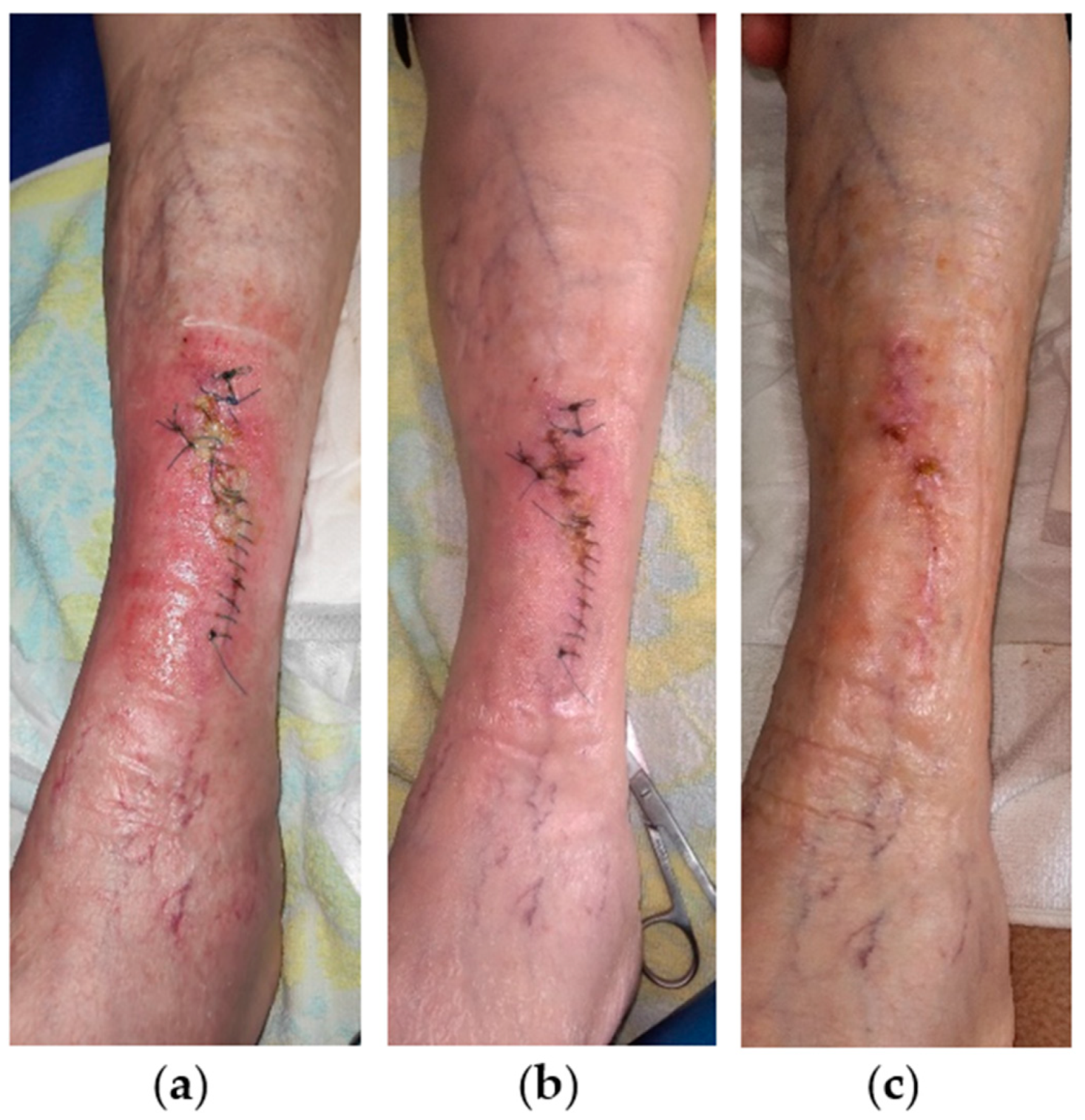
2.7. Case 7
2.8. Case 8
2.9. Case 9
2.10. General Population Data
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. L-Mesitran Wound Care Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bohn, G.A. Key concepts in healing venous leg ulcers. Wounds 2023, 35, S1–S6. [Google Scholar]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef]
- Probst, S.; Weller, C.D.; Bobbink, P.; Saini, C.; Pugliese, M.; Skinner, M.B.; Gethin, G. Prevalence and incidence of venous leg ulcers-a protocol for a systematic review. Syst. Rev. 2021, 10, 148. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Abbade, L.P.; Lastória, S. Venous ulcer: Epidemiology, physiopathology, diagnosis and treatment. Int. J. Dermatol. 2005, 44, 449–456. [Google Scholar] [CrossRef]
- Alam, W.; Hasson, J.; Reed, M. Clinical approach to chronic wound management in older adults. J. Am. Geriatr. Soc. 2021, 69, 2327–2334. [Google Scholar] [CrossRef]
- O’Donnell, T.F., Jr.; Passman, M.A.; Marston, W.A.; Ennis, W.J.; Dalsing, M.; Kistner, R.L.; Lurie, F.; Henke, P.K.; Gloviczki, M.L.; Eklöf, B.G.; et al. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J. Vasc. Surg. 2014, 60, 3S–59S. [Google Scholar] [CrossRef]
- Ma, H.; O’Donnell, T.F., Jr.; Rosen, N.A.; Iafrati, M.D. The real cost of treating venous ulcers in a contemporary vascular practice. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 355–361. [Google Scholar] [CrossRef]
- Folguera-Álvarez, C.; Garrido-Elustondo, S.; Rico-Blázquez, M.; Verdú-Soriano, J. Factors Associated with the Quality of Life of Patients With Venous Leg Ulcers in Primary Care: Cross-Sectional Study. Int. J. Low Extrem. Wounds 2022, 21, 521–528. [Google Scholar] [CrossRef]
- Kumar, P.; Khan, I.A.; Das, A.; Shah, H. Chronic venous disease. Part 1: Pathophysiology and clinical features. Clin. Exp. Dermatol. 2022, 47, 1228–1239. [Google Scholar] [CrossRef]
- Langemo, D.K.; Hanson, D.; Anderson, J.; Thompson, P.; Hunter, S. Use of honey for wound healing. Adv. Skin Wound Care 2009, 22, 113–118. [Google Scholar] [CrossRef]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.; Cremers, N.A. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef]
- De Groot, T.; Janssen, T.; Faro, D.; Cremers, N.A.J.; Chowdhary, A.; Meis, J.F. Antifungal Activity of a Medical-Grade Honey Formulation against Candida auris. J. Fungi 2021, 7, 50. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Saikaly, S.K.; Khachemoune, A. Honey and Wound Healing: An Update. Am. J. Clin. Dermatol. 2017, 18, 237–251. [Google Scholar] [CrossRef]
- Naik, P.P.; Chrysostomou, D.; Cinteza, M.; Pokorná, A.; Cremers, N.A. When time does not heal all wounds-the use of medical grade honey in wound healing: A case series. J. Wound Care 2022, 31, 548–558. [Google Scholar] [CrossRef]
- Vivas, A.; Lev-Tov, H.; Kirsner, R.S. Venous Leg Ulcers. Ann. Intern. Med. 2016, 165, ITC17–ITC32. [Google Scholar] [CrossRef]
- Schnack, L.L.; Andersen, C.; Wu, S. Complicated scenarios associated with venous leg ulcers. Wounds 2023, 35, S7–S16. [Google Scholar]
- Alavi, A.; Sibbald, R.G.; Phillips, T.J.; Miller, O.F.; Margolis, D.J.; Marston, W.; Woo, K.; Romanelli, M.; Kirsner, R.S. What’s new: Management of venous leg ulcers: Approach to venous leg ulcers. J. Am. Acad. Dermatol. 2016, 74, 627–640, quiz 641–642. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, B. Venous leg ulcers: Pathophysiology and Classification. Indian Dermatol. Online J. 2014, 5, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Raetz, J.; Wilson, M.; Collins, K. Varicose Veins: Diagnosis and Treatment. Am. Fam. Physician 2019, 99, 682–688. [Google Scholar]
- Gethin, G.; Vellinga, A.; Tawfick, W.; O’Loughlin, A.; McIntosh, C.; Mac Gilchrist, C.; Murphy, L.; Ejiugwo, M.; O’Regan, M.; Cameron, A.; et al. The profile of patients with venous leg ulcers: A systematic review and global perspective. J. Tissue Viability 2021, 30, 78–88. [Google Scholar] [CrossRef]
- Bérard, A.; Abenhaim, L.; Platt, R.; Kahn, S.R.; Steinmetz, O. Risk factors for the first-time development of venous ulcers of the lower limbs: The influence of heredity and physical activity. Angiology 2002, 53, 647–657. [Google Scholar] [CrossRef]
- Vlajinac, H.; Marinkovic, J.; Maksimovic, M.; Radak, D. Factors related to venous ulceration: A cross-sectional study. Angiology 2014, 65, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Melikian, R.; O’Donnell, T.F., Jr.; Suarez, L.; Iafrati, M.D. Risk factors associated with the venous leg ulcer that fails to heal after 1 year of treatment. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 98–105. [Google Scholar] [CrossRef]
- Bui, U.T.; Edwards, H.; Finlayson, K. Identifying risk factors associated with infection in patients with chronic leg ulcers. Int. Wound J. 2018, 15, 283–290. [Google Scholar] [CrossRef]
- Niculet, E.; Bobeica, C.; Tatu, A.L. Glucocorticoid-Induced Skin Atrophy: The Old and the New. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1041–1050. [Google Scholar] [CrossRef]
- Cwajda-Białasik, J.; Mościcka, P.; Jawień, A.; Szewczyk, M.T. Microbiological Status of Venous Leg Ulcers and Its Predictors: A Single-Center Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 12965. [Google Scholar] [CrossRef]
- Holubová, A.; Chlupáčová, L.; Cetlová, L.; Cremers, N.A.J.; Pokorná, A. Medical-Grade Honey as an Alternative Treatment for Antibiotics in Non-Healing Wounds-A Prospective Case Series. Antibiotics 2021, 10, 918. [Google Scholar] [CrossRef]
- Papanikolaou, G.E.; Gousios, G.; Cremers, N.A.J. Use of Medical-Grade Honey to Treat Clinically Infected Heel Pressure Ulcers in High-Risk Patients: A Prospective Case Series. Antibiotics 2023, 12, 605. [Google Scholar] [CrossRef]
- Mayer, A.; Slezak, V.; Takac, P.; Olejnik, J.; Majtan, J. Treatment of non-healing leg ulcers with honeydew honey. J. Tissue Viability 2014, 23, 94–97. [Google Scholar] [CrossRef]
- Dunford, C.E.; Hanano, R. Acceptability to patients of a honey dressing for non-healing venous leg ulcers. J. Wound Care 2004, 13, 193–197. [Google Scholar] [CrossRef]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.D.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair. Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Gethin, G.; Cowman, S. Bacteriological changes in sloughy venous leg ulcers treated with manuka honey or hydrogel: An RCT. J. Wound Care 2008, 17, 241–244, 246–247. [Google Scholar] [CrossRef]
- Nair, H.K.R.; Tatavilis, N.; Pospíšilová, I.; Kučerová, J.; Cremers, N.A.J. Medical-Grade Honey Kills Antibiotic-Resistant Bacteria and Prevents Amputation in Diabetics with Infected Ulcers: A Prospective Case Series. Antibiotics 2020, 9, 529. [Google Scholar] [CrossRef]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Majtan, J.; Bohova, J.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Madakova, Z.; Majtan, T.; Majtan, V.; Klaudiny, J. Fir honeydew honey flavonoids inhibit TNF-α-induced MMP-9 expression in human keratinocytes: A new action of honey in wound healing. Arch. Dermatol. Res. 2013, 305, 619–627. [Google Scholar] [CrossRef]
- Gethin, G.; Cowman, S. Manuka honey vs. hydrogel—A prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. J. Clin. Nurs. 2009, 18, 466–474. [Google Scholar] [CrossRef]
- Jull, A.; Walker, N.; Parag, V.; Molan, P.; Rodgers, A. Honey as Adjuvant Leg Ulcer Therapy trial collaborators. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br. J. Surg. 2008, 95, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, D.; Pokorná, A.; Cremers, N.A.J.; Peters, L.J.F. Medical-Grade Honey Is a Versatile Wound Care Product for the Elderly. JAR Life 2024, 13, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Smaropoulos, E.; Cremers, N.A.J. Medical-Grade Honey for the Treatment of Extravasation-Induced Injuries in Preterm Neonates: A Case Series. Adv. Neonatal Care 2021, 21, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Holubová, A.; Chlupáčová, L.; Krocová, J.; Cetlová, L.; Peters, L.J.F.; Cremers, N.A.J.; Pokorná, A. The Use of Medical Grade Honey on Infected Chronic Diabetic Foot Ulcers—A Prospective Case-Control Study. Antibiotics 2023, 12, 1364. [Google Scholar] [CrossRef]
- Gillespie, D.L.; Writing Group III of the Pacific Vascular Symposium 6; Kistner B, Glass C, Bailey B, Chopra A, Ennis B, Marston B, Masuda E, Moneta G, Nelzen O, Raffetto J, Raju S, Vedantham S, Wright D, Falanga, V. Venous ulcer diagnosis, treatment, and prevention of recurrences. J. Vasc. Surg. 2010, 52, 8S–14S. [Google Scholar] [CrossRef]
| Case | Gender/Age (Years) | VLU Location/Dimensions (cm) | Wound Age (Weeks)/ Previous Treatment | Relevant Comorbidities | Local Signs of Infection | Time for Infection Resolution (Weeks) | Time for Wound Healing (Weeks) |
|---|---|---|---|---|---|---|---|
| 1 | Female 79 | Right 6 × 6 | 4 povidone-iodine solution and mupirocin calcium cream | CVI, AHT, COPD, heart failure, atrial fibrillation, obesity, limited mobility | slough, high amount of exudate malodor, pain, erythema, delayed healing | 4 | 11 |
| 2 | Female 80 | Right 3 × 3 | 20 povidone-iodine solution | CVI, AHT, obesity | slough, moderate amount of exudate, edema, delayed healing | 3 | 18 |
| 3 | Female 91 | Right: 5 × 4 Left lateral: 3 × 3 Left posterior: 6 × 5 | 2 povidone-iodine solution | CVI, DVT, heart failure, atrial fibrillation, limited mobility | necrotic tissue, moderate amount of exudate, erythema, edema, pain | Right: 3 Left posterior: 2 Left lateral: 1 | Right: 5 Left posterior: 6 Left lateral: 3 |
| 4 | Female 84 | Right 9 × 6 | 4 not treated | CVI, COPD, CVD, heart failure, atrial fibrillation, obesity, permanent immobility | necrotic tissue, malodor, edema, pain moderate amount of exudate, erythema, hyperthermia, delayed healing | 2 | 4 |
| 5 | Female 87 | Left 5 × 4 | 1 not treated | CVI, AHT, PAD, limited mobility | slough, low amount of exudate, rolled and macerated edges | 2 | 6 |
| 6 | Male 83 | Right 5 × 5 | 2 not treated | CVI, AHT, T2DM, heart failure, atrial fibrillation, limited mobility | necrotic tissue, edema, erythema, pain | 2 | 11 |
| 7 | Female 88 | Left 17 × 10 | >16 antiseptic solutions & antibiotic creams | CVI, AHT, heart failure, atrial fibrillation, obesity, permanent immobility | slough, moderate amount of exudate, edema, pain, delayed healing | 2 | 3 |
| 8 | Female 84 | Left 12 × 1 | 2 povidone-iodine solution | CVI, multiple myeloma, limited mobility | slough, erythema, moderate amount of exudate | 1 | 3 |
| 9 | Female 75 | Left 6 × 5 | 4 silver sulfadiazine cream | CVI, AHT, T2DM, limited mobility | necrotic eschar, slough, moderate amount of exudate, erythema, edema, pain, delayed healing | 2 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papanikolaou, G.E.; Gousios, G.; Cremers, N.A.J.; Peters, L.J.F. Treating Infected Non-Healing Venous Leg Ulcers with Medical-Grade Honey: A Prospective Case Series. Antibiotics 2024, 13, 614. https://doi.org/10.3390/antibiotics13070614
Papanikolaou GE, Gousios G, Cremers NAJ, Peters LJF. Treating Infected Non-Healing Venous Leg Ulcers with Medical-Grade Honey: A Prospective Case Series. Antibiotics. 2024; 13(7):614. https://doi.org/10.3390/antibiotics13070614
Chicago/Turabian StylePapanikolaou, Georgios E., Georgios Gousios, Niels A. J. Cremers, and Linsey J. F. Peters. 2024. "Treating Infected Non-Healing Venous Leg Ulcers with Medical-Grade Honey: A Prospective Case Series" Antibiotics 13, no. 7: 614. https://doi.org/10.3390/antibiotics13070614
APA StylePapanikolaou, G. E., Gousios, G., Cremers, N. A. J., & Peters, L. J. F. (2024). Treating Infected Non-Healing Venous Leg Ulcers with Medical-Grade Honey: A Prospective Case Series. Antibiotics, 13(7), 614. https://doi.org/10.3390/antibiotics13070614








