Antimicrobial Resistance of Listeria monocytogenes Strains Isolated in Food and Food-Processing Environments in Italy
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Antibiotic Susceptibility Testing
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luque-Sastre, L.; Arroyo, C.; Fox, E.M.; McMahon, B.J.; Bai, L.; Li, F.; Fanning, S. Antimicrobial Resistance in Listeria Species. Microbiol. Spectr. 2018, 6, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.; Niedermeyer, J.; Gould, N.; Brown, P.; Strules, J.; Parsons, A.W.; Bernardo Mesa-Cruz, J.; Kelly, M.J.; Hooker, M.J.; Chamberlain, M.J.; et al. Listeria monocytogenes at the Human–Wildlife Interface: Black Bears (Ursus americanus) as Potential Vehicles for Listeria. Microb. Biotechnol. 2020, 13, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Radoshevich, L.; Cossart, P. Listeria Monocytogenes: Towards a Complete Picture of Its Physiology and Pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Karthik, K.; Tiwari, R.; Shabbir, M.Z.; Barbuddhe, S.; Malik, S.V.S.; Singh, R.K. Listeriosis in Animals, Its Public Health Significance (Food-Borne Zoonosis) and Advances in Diagnosis and Control: A Comprehensive Review. Vet. Q. 2015, 35, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Pirone-Davies, C.; Chen, Y.; Pightling, A.; Ryan, G.; Wang, Y.; Yao, K.; Hoffmann, M.; Allard, M.W. Genes Significantly Associated with Lineage II Food Isolates of Listeria monocytogenes. BMC Genom. 2018, 19, 708. [Google Scholar] [CrossRef]
- Lee, S.; Chen, Y.; Gorski, L.; Ward, T.J.; Osborne, J.; Kathariou, S. Listeria monocytogenes Source Distribution Analysis Indicates Regional Heterogeneity and Ecological Niche Preference among Serotype 4b Clones. mBio 2018, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.E.; Abd El-Hamid, M.I.; El-Gedawy, A.; Bendary, M.M.; ELTarabili, R.M.; Alhomrani, M.; Alamri, A.S.; Alghamdi, S.A.; Arnout, M.; Binjawhar, D.N.; et al. New Insights into Listeria monocytogenes Antimicrobial Resistance, Virulence Attributes and Their Prospective Correlation. Antibiotics 2022, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A Review of Listeria monocytogenes: An Update on Outbreaks, Virulence, Dose-Response, Ecology, and Risk Assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Goulet, V.; King, L.A.; Vaillant, V.; de Valk, H. What Is the Incubation Period for Listeriosis? BMC Infect. Dis. 2013, 13, 11. [Google Scholar] [CrossRef]
- Koopmans, M.M.; Brouwer, M.C.; Vázquez-Boland, J.A.; van de Beek, D. Human Listeriosis. Clin. Microbiol. Rev. 2023, 36, e00060-19. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar]
- Pagliano, P.; Arslan, F.; Ascione, T. Epidemiology and treatment of the commonest form of listeriosis: Meningitis and bacteraemia. Infez. Med. 2017, 3, 210–216. [Google Scholar]
- Baquero, F.; Lanza, F.V.; Duval, M.; Coque, T.M. Ecogenetics of Antibiotic Resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef] [PubMed]
- Duma, M.N.; Ciupescu, L.M.; Dan, S.D.; Crisan-Reget, O.L.; Tabaran, A. Virulence and Antimicrobial Resistance of Listeria monocytogenes Isolated from Ready-to-Eat Food Products in Romania. Microorganisms 2024, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Peruzy, M.F.; Capuano, F.; Proroga, Y.T.R.; Cristiano, D.; Carullo, M.R.; Murru, N. Antimicrobial Susceptibility Testing for Salmonella serovars Isolated from Food Samples: Five-Year Monitoring (2015–2019). Antibiotics 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Bao, H.; Yang, Z.; He, T.; Tian, Y.; Zhou, Y.; Pang, M.; Wang, R.; Zhang, H. Antimicrobial Susceptibility, Multilocus Sequence Typing, and Virulence of Listeria Isolated from a Slaughterhouse in Jiangsu, China. BMC Microbiol. 2021, 21, 327. [Google Scholar] [CrossRef] [PubMed]
- Montalti, M.; Soldà, G.; Capodici, A.; Di Valerio, Z.; Gribaudo, G.; La Fauci, G.; Salussolia, A.; Scognamiglio, F.; Zannoner, A.; Gori, D. Antimicrobial Resistance (AMR) in Italy over the Past Five Years: A Systematic Review. Biologics 2022, 2, 151–164. [Google Scholar] [CrossRef]
- Mpundu, P.; Mbewe, A.R.; Muma, J.B.; Mwasinga, W.; Mukumbuta, N.; Munyeme, M. A Global Perspective of Antibiotic-Resistant Listeria monocytogenes Prevalence in Assorted Ready to Eat Foods: A Systematic Review. Vet. World 2021, 14, 2219–2229. [Google Scholar] [CrossRef]
- Morobe, I.C.; Nyila, M.A.; Gashe, B.A.; Matsheka, M.I. Prevalence, Antimicrobial Resistance Profiles of Listeria monocytognes from Various Foods in Gaborone, Botswana. Afr. J. Biotechnol. 2009, 8, 6383–6387. [Google Scholar] [CrossRef]
- Alonso-Hernando, A.; Capita, R.; Prieto, M.; Alonso-Calleja, C. Comparison of Antibiotic Resistance Patterns in Listeria monocytogenes and Salmonella enterica Strains Pre-Exposed and Exposed to Poultry Decontaminants. Food Control 2009, 20, 1108–1111. [Google Scholar] [CrossRef]
- Kayode, A.J.; Semerjian, L.; Osaili, T.; Olapade, O.; Okoh, A.I. Occurrence of Multidrug-Resistant Listeria monocytogenes in Environmental Waters: A Menace of Environmental and Public Health Concern. Front. Environ. Sci. 2021, 9, 737435. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Okoh, A.I.; Lues, R. Occurrence and Multidrug Resistance in Strains of Listeria monocytogenes Recovered from the Anaerobic Co-Digestion Sludge Contained in a Single Stage Steel Biodigester: Implications for Antimicrobial Stewardship. Microorganisms 2023, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of Antibiotic Resistance in Listeria monocytogenes Isolated from Food Products: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Harakeh, S.; Saleh, I.; Zouhairi, O.; Baydoun, E.; Barbour, E.; Alwan, N. Antimicrobial Resistance of Listeria monocytogenes Isolated from Dairy-Based Food Products. Sci. Total Environ. 2009, 407, 4022–4027. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Ha, J.H.; Lee, M.K.; Cho, Y.S. Antimicrobial Susceptibility and Serotyping of Listeria monocytogenes Isolated from Ready-to-Eat Seafood and Food Processing Environments in Korea. Food Sci. Biotechnol. 2017, 26, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.Z.; Paixão, R.; Gobbi, D.D.S.; Raimundo, D.C.; Ferreira, T.P.; Moreno, A.M.; Hofer, E.; Reis, C.M.F.; Matté, G.R.; Matté, M.H. Characterization of Antibiotic Resistance in Listeria Spp. Isolated from Slaughterhouse Environments, Pork and Human Infections. J. Infect. Dev. Ctries. 2014, 8, 416–423. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, X.; Aspridou, Z.; Misiou, O.; Dong, P.; Zhang, Y. The Prevalence and Antibiotic-Resistant of Listeria monocytogenes in Livestock and Poultry Meat in China and the EU from 2001 to 2022: A Systematic Review and Meta-Analysis. Foods 2023, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, M.; Kooti, S.; Mohammadi, B.; Salimi, Y.; Akya, A. A Systematic Review and Meta-Analysis of Listeria monocytogenes Isolated from Human and Non-Human Sources: The Antibiotic Susceptibility Aspect. Diagn. Microbiol. Infect. Dis. 2022, 102, 115634. [Google Scholar] [CrossRef]
- Caruso, M.; Fraccalvieri, R.; Pasquali, F.; Santagada, G.; Latorre, L.M.; Difato, L.M.; Miccolupo, A.; Normanno, G.; Parisi, A. Antimicrobial Susceptibility and Multilocus Sequence Typing of Listeria monocytogenes Isolated over 11 Years from Food, Humans, and the Environment in Italy. Foodborne Pathog. Dis. 2020, 17, 284–294. [Google Scholar] [CrossRef]
- Tamburro, M.; Sammarco, M.L.; Fanelli, I.; Ripabelli, G. Characterization of Listeria Monocytogenes Serovar 1/2a, 1/2b, 1/2c and 4b by High Resolution Melting Analysis for Epidemiological Investigations. Int. J. Food Microbiol. 2019, 310, 108289. [Google Scholar] [CrossRef] [PubMed]
- Yücel, N.; Çitak, S.; Önder, M. Prevalence and Antibiotic Resistance of Listeria Species in Meat Products in Ankara, Turkey. Food Microbiol. 2005, 22, 241–245. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Osaili, T.M.; Awad, A.A.; Olaimat, A.N.; Shaker, R.R.; Holley, R.A. Occurrence and Antibiotic Susceptibility of Listeria monocytogenes Isolated from Raw and Processed Meat Products in Amman, Jordan. CYTA—J. Food 2015, 13, 346–352. [Google Scholar] [CrossRef]
- Rahimi, E.; Ameri, M.; Momtaz, H. Prevalence and Antimicrobial Resistance of Listeria Species Isolated from Milk and Dairy Products in Iran. Food Control 2010, 21, 1448–1452. [Google Scholar] [CrossRef]
- Kevenk, T.O.; Terzi Gulel, G. Prevalence, Antimicrobial Resistance and Serotype Distribution of Listeria monocytogenes Isolated from Raw Milk and Dairy Products. J. Food Saf. 2016, 36, 11–18. [Google Scholar] [CrossRef]
- Patterson, J. Prevalence of Antibiotic Resistant Listeria monocytogenes in Sea Foods of Tuticorin Coast, Southeastern India. Eur. J. Appl. Sci. 2016, 8, 356–364. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Prevalence, Genetic Diversity and Antimicrobial Resistance of Listeria monocytogenes Isolated from Fresh and Smoked Fish in Poland. Food Microbiol. 2017, 64, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Peruzy, M.F.; Murru, N.; Perugini, A.G.; Capuano, F.; Delibato, E.; Mercogliano, R.; Korkeala, H.; Proroga, Y.T.R. Evaluation of Virulence Genes in Yersinia enterocolitica Strains Using SYBR Green Real-Time PCR. Food Microbiol. 2017, 65, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.; Bland, R.; Waite-Cusic, J.; Kovacevic, J. Diversity and Antimicrobial Resistance of Listeria Spp. and L. monocytogenes Clones from Produce Handling and Processing Facilities in the Pacific Northwest. Food Control 2021, 123, 107665. [Google Scholar] [CrossRef]
- Wilson, A.; Gray, J.; Scott Chandry, P.; Fox, E.M. Phenotypic and Genotypic Analysis of Antimicrobial Resistance among Listeria monocytogenes Isolated from Australian Food Production Chains. Genes 2018, 9, 80. [Google Scholar] [CrossRef]
- de Vasconcelos Byrne, V.; Hofer, E.; Vallim, D.C.; de Castro Almeida, R.C. Occurrence and Antimicrobial Resistance Patterns of Listeria monocytogenes Isolated from Vegetables. Braz. J. Microbiol. 2016, 47, 438–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acciari, V.A.; Torresi, M.; Migliorati, G.; Di Giannatale, E.; Semprini, P.; Prencipe, V. Caratterizzazione Di Ceppi Listeria monocytogenes Isolati Da Formaggi a Pasta Molle e Semi-Molle Prelevati Nella Regione Abruzzo. Vet. Ital. 2011, 47, 5–13. [Google Scholar]
- CLSI. CLSI Document M100-S24—Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Proroga, Y.T.R.; Mancusi, A.; Peruzy, M.F.; Carullo, M.R.; Montone, A.M.I.; Fulgione, A.; Capuano, F. Characterization of Salmonella typhimurium and Its Monophasic Variant 1,4,[5],12:I:- Isolated from Different Sources. Folia Microbiol. 2019, 64, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Wolfensberger, A.; Kuster, S.P.; Marchesi, M.; Zbinden, R.; Hombach, M. The Effect of Varying Multidrug-Resistence (MDR) Definitions on Rates of MDR Gram-Negative Rods. Antimicrob. Resist. Infect. Control. 2019, 8, 193. [Google Scholar] [CrossRef]
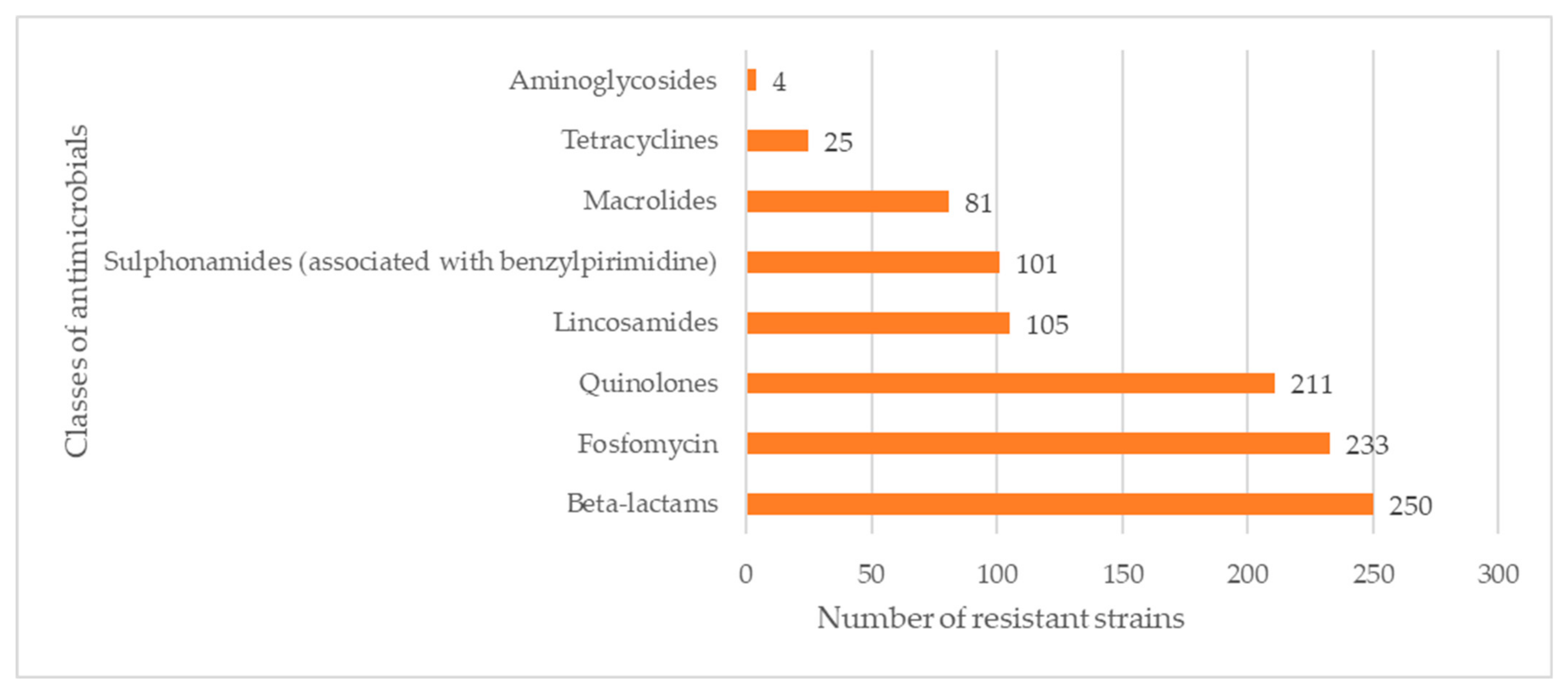
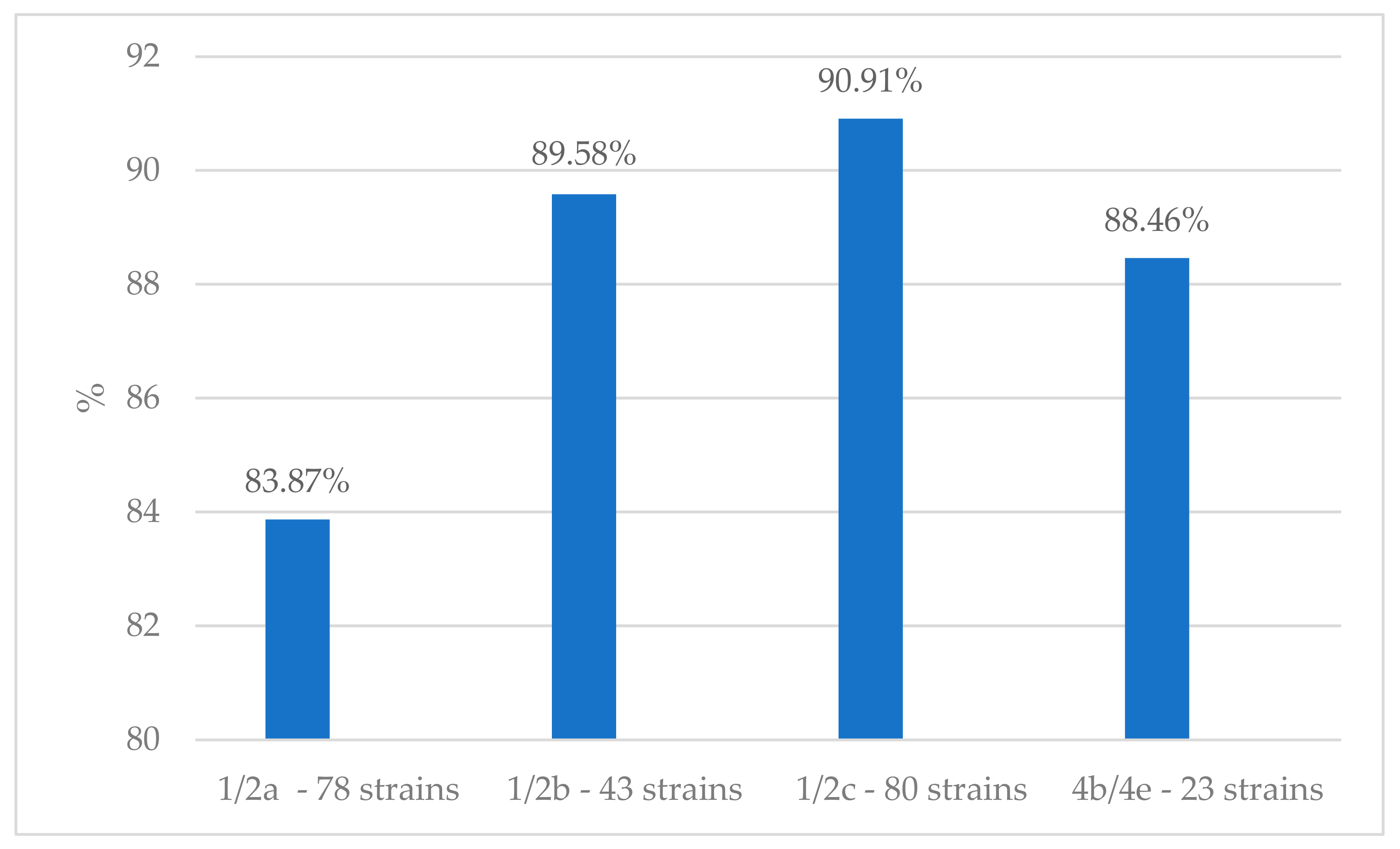
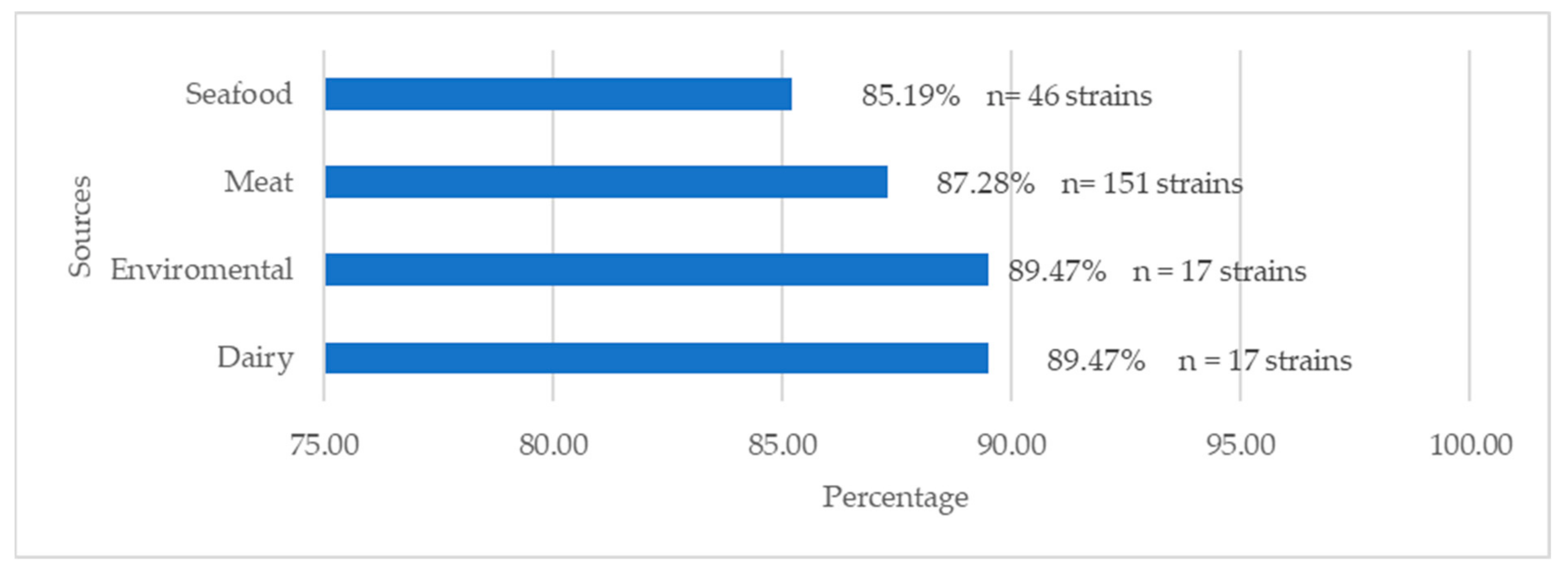
| 1/2a (93 Strains) | 1/2b (48 Strains) | 1/2c (88 Strains) | 1/a (2 Strains) | 3a (3 Strains) | 3b (1 Strain) | 4b/4e (26 Strains) | 4d/4e (1 Strain) | 4e (2 Strains) | Unidentified (5 Strains) | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | 33.33% | 54.17% | 47.73% | 0.00% | 66.67% | 0.00% | 57.69% | 0.00% | 100.00% | 60.00% | 44.98% |
| (n: 31) | (n: 26) | (n: 42) | (n: 2) | (n: 15) | (n: 2) | (n: 3) | (n = 121) | ||||
| Lincomycin | 25.81% | 45.83% | 48.86% | 0.00% | 33.33% | 0.00% | 38.46% | 0.00% | 50.00% | 80.00% | 39.03% |
| (n: 24) | (n: 22) | (n: 43) | (n: 1) | (n: 10) | (n: 1) | (n: 4) | (n = 105) | ||||
| Oxacillin | 84.95% | 93.75% | 86.36% | 100.00% | 66.67% | 100.00% | 100.00% | 100.00% | 100.00% | 80.00% | 88.48% |
| (n: 79) | (n: 45) | (n: 76) | (n: 2) | (n: 2) | (n: 1) | (n: 26) | (n: 1) | (n: 2) | (n: 4) | (n = 238) | |
| Flumenique | 80.65% | 75.00% | 79.55% | 50.00% | 66.67% | 100.00% | 80.77% | 0.00% | 100.00% | 60.00% | 78.44% |
| (n: 75) | (n: 36) | (n: 70) | (n: 1) | (n: 2) | (n: 1) | (n: 21) | (n: 2) | (n: 3) | (n = 211) | ||
| Fosfomycin | 78.49% | 95.83% | 88.64% | 100.00% | 100.00% | 0.00% | 80.77% | 100.00% | 100.00% | 100.00% | 85.87% |
| (n: 73) | (n: 46) | (n: 78) | (n: 2) | (n: 3) | (n: 21) | (n: 1) | (n: 2) | (n: 5) | (n = 231) | ||
| Gentamycin | 3.23% | 0.00% | 1.14% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 1.49% |
| (n: 3) | (n: 1) | (n = 4) | |||||||||
| Meropenem | 11.83% | 10.42% | 15.91% | 0.00% | 0.00% | 0.00% | 11.54% | 0.00% | 50.00% | 20.00% | 13.01% |
| (n: 11) | (n: 5) | (n: 14) | (n: 3) | (n: 1) | (n: 1) | (n = 35) | |||||
| Erithromycin | 34.41% | 14.58% | 37.50% | 0.00% | 0.00% | 0.00% | 11.54% | 100.00% | 100.00% | 60.00% | 30.11% |
| (n: 32) | (n: 7) | (n: 33) | (n: 3) | (n: 1) | (n: 2) | (n: 3) | (n = 81) | ||||
| Sulfametoxazole–thrymethoprim | 45.16% | 22.92% | 36.36% | 0.00% | 33.33% | 0.00% | 38.46% | 0.00% | 100.00% | 60.00% | 37.54% |
| (n: 42) | (n: 11) | (n: 32) | (n: 1) | (n: 10) | (n: 2) | (n: 3) | (n = 101) | ||||
| Tetracycline | 8.60% | 8.33% | 11.36% | 0.00% | 0.00% | 0.00% | 3.85% | 0.00% | 50.00% | 20.00% | 9.29% |
| (n: 8) | (n: 4) | (n: 10) | (n: 1) | (n: 1) | (n: 1) | (n = 25) |
| Dairy Products (19 Strains) | Meat Products (173 Strains) | Seafood (54 Strains) | Confectionary Products (1 Strain) | Enviromental (19 Strains) | Sauces (2 Strains) | Ready-to-Eat Rice Dishes (1 Strain) | Total | |
|---|---|---|---|---|---|---|---|---|
| Ampicillin | 63.16 (n: 12) | 43.93 (n: 76) | 35.19 (n: 19) | 100 (n: 1) | 63.16 (n: 12) | 50 (n: 1) | 0.00 | 44.98 (n = 121) |
| Lincomycin | 42.11 (n: 8) | 42.19 (n: 73) | 29.63 (n: 16) | 100 (n: 1) | 31.58 (n: 6) | 50 (n: 1) | 0.00 | 39.03 (n = 105) |
| Oxacillin | 94.74 (n: 18) | 85.55 (n: 148) | 90.74 (n: 49) | 100 (n: 1) | 100 (n: 19) | 100 (n: 2) | 100 (n: 1) | 88.47 (n = 238) |
| Flumenique | 73.68 (n: 14) | 79.19 (n: 137) | 75.92 (n: 41) | 100 (n: 1) | 78.95 (n: 15) | 100 (n: 2) | 100 (n 1) | 79.18 (n = 213) |
| Fosfomycin | 84.21 (n: 16) | 83.81 (n: 145) | 87.04 (n: 47) | 100 (n: 1) | 100 (n: 19) | 100 (n: 2) | 100 (n: 1) | 85.87 (n = 231) |
| Gentamicin | 0.00 | 1.73 (n: 3) | 1.85 (n: 1) | 0.00 | 0.00 | 0.00 | 0.00 | 1.48 (n = 4) |
| Meropenem | 15.79 (n: 3) | 15.61 (n: 27) | 5.56 (n: 3) | 0.00 | 10.53 (n: 2) | 0.00 | 0.00 | 13.01 (n = 35) |
| Erithomycin | 21.05 (n: 4) | 33.53 (n: 58) | 27.78 (n: 15) | 0.00 | 21.05 (n: 4) | 0.00 | 0.00 | 30.11 (n = 81) |
| Sulfametoxazole–thrymethoprim | 21.05 (n: 4) | 39.31 (n: 68) | 33.33 (n: 18) | 100 (n: 1) | 47.37 (n: 9) | 50 (n: 1) | 0.00 | 37.54 (n = 101) |
| Tetracycline | 10.52 (n: 2) | 10.98 (n: 19) | 5.56 (n: 3) | 0.00 | 5.26 (n: 1) | 0.00 | 0.00 | 9.29 (n = 25) |
| Species | Serovar | Meat Products | Seafood | Dairy Products | Environment | Sauces | Rice Dishes | Confectionary Products | Total |
|---|---|---|---|---|---|---|---|---|---|
| Listeria monocytogenes | 1/2a | 47 | 27 | 7 | 9 | 2 | 1 | 0 | 93 |
| 1/2b | 19 | 21 | 6 | 2 | 0 | 0 | 0 | 48 | |
| 1/2c | 85 | 0 | 1 | 2 | 0 | 0 | 0 | 88 | |
| 1/a | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |
| 3a | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | |
| 3b | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| 4b/4e | 17 | 1 | 3 | 4 | 0 | 0 | 1 | 26 | |
| 4d/4e | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 4e | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Unidentified | 2 | 0 | 1 | 2 | 0 | 0 | 0 | 5 |
| Molecule | Diameter of the Halo of Inhibition (mm) | References | ||
|---|---|---|---|---|
| Sensible | Intermediate | Resistant | ||
| Ampicillin | ≥16 | <16 | [38] | |
| Meropenem | ≥26 | <26 | [38] | |
| Erytromycin | ≥25 | <25 | [38] | |
| Trimetoprim–Sulfametoxazole | ≥29 | <29 | [38] | |
| Tetracycline | ≥19 | 15–18 | ≤14 | [39] |
| Gentamicin | >18 | <18 | The breakpoints of Staphylococcus aureus (from EUCAST 2023 [38]) resistance were considered [40] | |
| Oxacillin | ≥18 | ≤17 | [41] | |
| Fosfomycin | ≥24 | ≤24 | The breakpoints of E. coli (from EUCAST 2023 [38]) resistance were considered [39] | |
| Lincomycin | ≤9 | [42] | ||
| Flumequine | ≥20 | 13–19 | <12.5 | The breakpoints of Enterobacteriales (from CLSI 2014 [43]) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rippa, A.; Bilei, S.; Peruzy, M.F.; Marrocco, M.G.; Leggeri, P.; Bossù, T.; Murru, N. Antimicrobial Resistance of Listeria monocytogenes Strains Isolated in Food and Food-Processing Environments in Italy. Antibiotics 2024, 13, 525. https://doi.org/10.3390/antibiotics13060525
Rippa A, Bilei S, Peruzy MF, Marrocco MG, Leggeri P, Bossù T, Murru N. Antimicrobial Resistance of Listeria monocytogenes Strains Isolated in Food and Food-Processing Environments in Italy. Antibiotics. 2024; 13(6):525. https://doi.org/10.3390/antibiotics13060525
Chicago/Turabian StyleRippa, Antonio, Stefano Bilei, Maria Francesca Peruzy, Maria Grazia Marrocco, Patrizia Leggeri, Teresa Bossù, and Nicoletta Murru. 2024. "Antimicrobial Resistance of Listeria monocytogenes Strains Isolated in Food and Food-Processing Environments in Italy" Antibiotics 13, no. 6: 525. https://doi.org/10.3390/antibiotics13060525
APA StyleRippa, A., Bilei, S., Peruzy, M. F., Marrocco, M. G., Leggeri, P., Bossù, T., & Murru, N. (2024). Antimicrobial Resistance of Listeria monocytogenes Strains Isolated in Food and Food-Processing Environments in Italy. Antibiotics, 13(6), 525. https://doi.org/10.3390/antibiotics13060525







