Comparing the Prognostic Impacts of Delayed Administration of Appropriate Antimicrobials in Older Patients with Afebrile and Febrile Community-Onset Bacteremia
Abstract
1. Introduction
2. Methods
2.1. Study Design
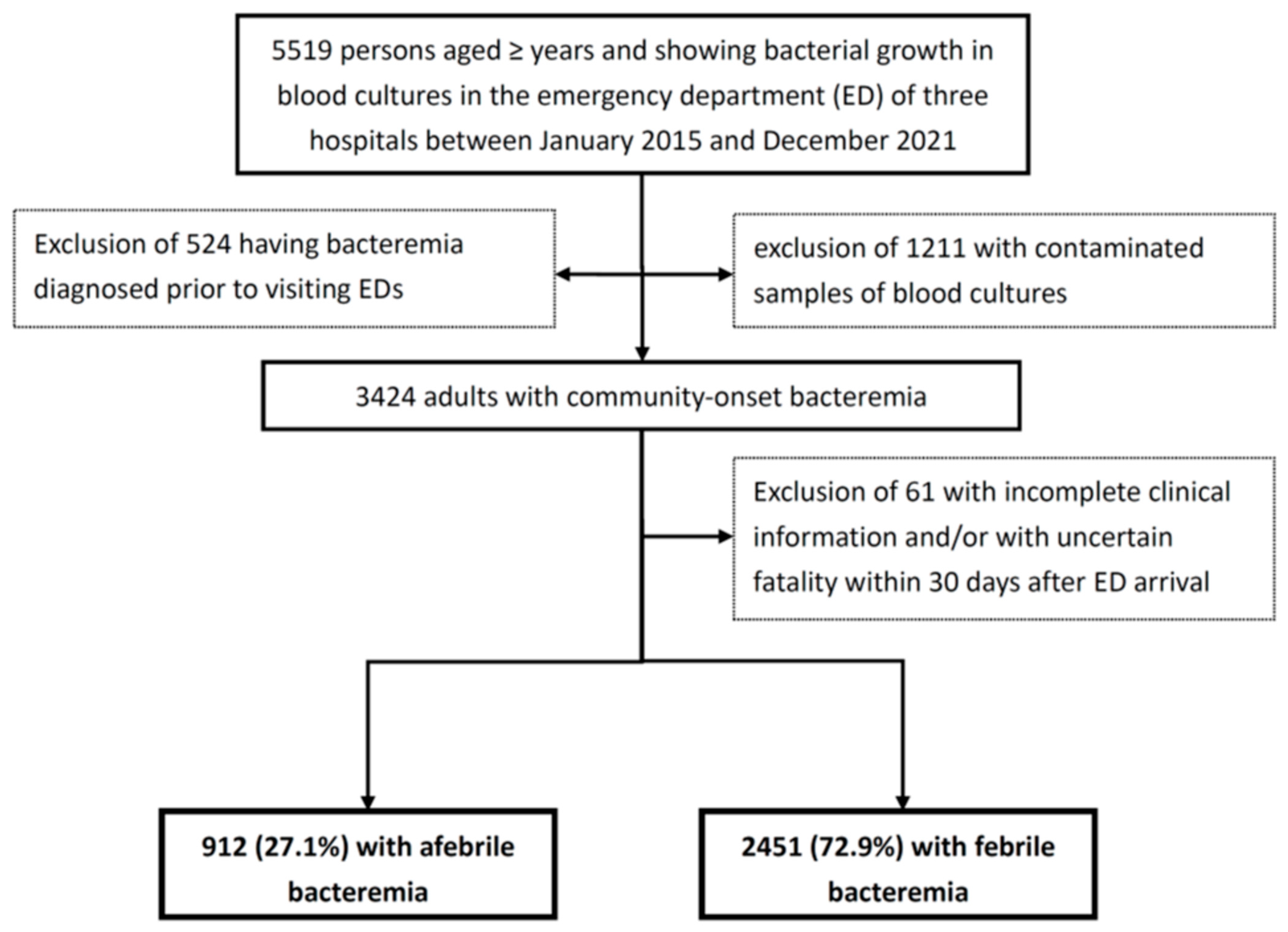
2.2. Patient Population
2.3. Data Collection
2.4. Microbiological Methods
2.5. Definitions
2.6. Statistical Analyses
3. Results
3.1. Clinical Characteristics and Outcomes in Afebrile and Febrile Bacteremia
3.2. Prognostic Effect of Afebrile Bacteremia in Overall Patient Sample
3.3. Prognostic Impacts of Delayed AAT in Patients with Afebrile Bacteremia
| Variables | Patient Number (%) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Fatal, n = 342 | Surviving, n = 570 | OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Delayed AAT, hour | - | - | - | - | 1.003 (1.002–1.003) | <0.001 |
| Patient demographics | ||||||
| Bedridden status | 128 (37.4) | 156 (27.4) | 1.59 (1.19–2.11) | 0.001 | NS | NS |
| Comorbidity | ||||||
| Cardiovascular disease | 206 (60.2) | 392 (68.8) | 0.69 (0.52–0.91) | 0.009 | NS | NS |
| Malignancy | 121 (35.4) | 98 (17.2) | 2.64 (1.93–3.60) | <0.001 | 2.24 (1.49–3.39) | <0.001 |
| Liver cirrhosis | 51 (14.9) | 41 (7.2) | 2.26 (1.46–3.50) | <0.001 | NS | NS |
| Critical illness (MEDS score > 15) at onset | 252 (73.7) | 82 (14.4) | 16.66 (11.91–23.31) | <0.001 | 12.64 (8.84–18.08) | <0.001 |
| Characteristics of bacteremia | ||||||
| Polymicrobial bacteremia | 63 (18.4) | 61 (10.7) | 1.88 (1.29–2.76) | 0.001 | NS | NS |
| Bacteremia source | ||||||
| Lower respiratory tract | 186 (54.4) | 119 (20.9) | 4.52 (3.37–6.06) | <0.001 | NS | NS |
| Urinary tract | 33 (9.6) | 169 (29.6) | 0.25 (0.17–0.38) | <0.001 | 0.30 (0.19–0.49) | <0.001 |
| Skin and soft-tissue infection | 27 (7.9) | 69 (12.1) | 0.62 (0.39–0.99) | 0.045 | NS | NS |
| Biliary tract | 12 (3.5) | 78 (13.7) | 0.23 (0.12–0.43) | <0.001 | 0.23 (0.11–0.49) | <0.001 |
| Primary bacteremia | 5 (1.5) | 22 (3.9) | 0.37 (0.14–0.99) | 0.04 | 0.35 (0.11–1.13) | 0.08 |
| Etiologic pathogen | ||||||
| Escherichia coli | 80 (23.4) | 189 (33.2) | 0.62 (0.45–0.84) | 0.002 | NS | NS |
| Staphylococcus aureus | 67 (19.6) | 82 (14.4) | 1.45 (1.02–2.07) | 0.04 | NS | NS |
| Anaerobes | 35 (10.2) | 31 (5.4) | 1.97 (1.20–3.28) | 0.007 | NS | NS |
| Pseudomonas species | 20 (5.8) | 18 (3.2) | 1.91 (0.99–3.65) | 0.049 | NS | NS |
3.4. Prognostic Impacts of Delayed AAT in Patients with Febrile Bacteremia
| Variables | Patient Number (%) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Fatal, n = 251 | Surviving, n = 2200 | OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Delayed AAT, hour | - | - | - | - | 1.002 (1.001–1.003) | <0.001 |
| Inadequate source control during antimicrobial therapy | 63 (25.1) | 93 (4.2) | 7.59 (5.34–10.81) | <0.001 | 10.70 (6.86–16.68) | <0.001 |
| Patient demographics | ||||||
| Bedridden status | 66 (26.2) | 373 (17.0) | 1.75 (1.29–2.36) | <0.001 | NS | NS |
| Comorbidity | ||||||
| Malignancy | 87 (34.7) | 376 (17.1) | 2.57 (1.94–3.41) | <0.001 | 2.01 (1.40–2.87) | <0.001 |
| Chronic kidney disease | 74 (29.5) | 496 (22.5) | 1.44 (1.08–1.92) | 0.01 | NS | NS |
| Liver cirrhosis | 32 (12.7) | 184 (8.4) | 1.60 (1.07–2.39) | 0.02 | NS | NS |
| Critical illness (MEDS score > 15) at ED | 149 (59.4) | 178 (8.1) | 16.59 (12.36–22.28) | <0.001 | 8.75 (6.14–12.49) | <0.001 |
| Characteristics of bacteremia | ||||||
| Polymicrobial bacteremia | 42 (16.7) | 166 (7.5) | 2.46 (1.71–3.56) | <0.001 | 1.89 (1.17–3.04) | 0.009 |
| Bacteremia source | ||||||
| Low er respiratory tract | 113 (45.0) | 210 (9.5) | 7.76 (5.83–10.33) | <0.001 | 3.27 (2.17–4.91) | <0.001 |
| Urinary tract | 33 (13.1) | 925 (42.0) | 0.21 (0.14–0.30) | <0.001 | 0.38 (0.24–0.61) | <0.001 |
| Biliary tract | 19 (7.6) | 283 (12.9) | 0.56 (0.34–0.90) | 0.02 | 0.56 (0.30–1.04) | 0.07 |
| Infective endocarditis | 10 (4.0) | 33 (1.5) | 2.73 (1.33–5.60) | 0.01 | 3.81 (1.54–9.44) | 0.004 |
| Primary bacteremia | 7 (2.8) | 138 (6.3) | 0.43 (0.20–0.93) | 0.03 | NS | NS |
| Etiologic pathogen | ||||||
| Klebsiella pneumoniae | 74 (29.5) | 461 (21.0) | 1.58 (1.18–2.11) | 0.002 | NS | NS |
| Escherichia coli | 65 (25.9) | 989 (45.0) | 0.43 (0.32–0.58) | <0.001 | NS | NS |
| Staphylococcus aureus | 32 (12.7) | 174 (7.9) | 1.70 (1.14–2.54) | 0.009 | NS | NS |
| Pseudomonas species | 23 (9.2) | 61 (2.8) | 3.54 (2.15–5.82) | <0.001 | 2.71 (1.36–5.42) | 0.005 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goto, M.; Al-Hasan, M. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin. Microbiol. Infect. 2013, 19, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B. Incidence of bloodstream infection: A review of population-based studies. Clin. Microbiol. Infect. 2013, 19, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, M.; Nørgaard, M.; Dethlefsen, C.; Schønheyder, H.C. Temporal changes in the incidence and 30-day mortality associated with bacteremia in hospitalized patients from 1992 through 2006: A population-based cohort study. Clin. Infect. Dis. 2011, 52, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Verway, M.; Brown, K.A.; Marchand-Austin, A.; Diong, C.; Lee, S.; Langford, B.; Schwartz, K.L.; MacFadden, D.R.; Patel, S.N.; Sander, B. Prevalence and mortality associated with bloodstream organisms: A population-wide retrospective cohort study. J. Clin. Microbiol. 2022, 60, e0242921. [Google Scholar] [CrossRef]
- Laupland, K.B.; Church, D.L. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin. Microbiol. Rev. 2014, 27, 647–664. [Google Scholar] [CrossRef]
- Laupland, K.B.; Pasquill, K.; Steele, L.; Parfitt, E.C. Burden of bloodstream infection in older persons: A population-based study. BMC Geriatr. 2021, 21, 31. [Google Scholar] [CrossRef]
- Valles, J.; Rello, J.; Ochagavia, A.; Garnacho, J.; Alcala, M.A. Community-acquired bloodstream infection in critically ill adult patients: Impact of shock and inappropriate antibiotic therapy on survival. Chest 2003, 123, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lee, C.H.; Yang, C.Y.; Hsieh, C.C.; Tang, H.J.; Ko, W.C. Beneficial effects of early empirical administration of appropriate antimicrobials on survival and defervescence in adults with community-onset bacteremia. Crit. Care 2019, 23, 363. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Lin, W.L.; Lin, C.C.; Hsieh, W.H.; Hsieh, C.H.; Wu, M.H.; Wu, J.Y.; Lee, C.C. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 947–953. [Google Scholar] [CrossRef]
- Hernández, C.; Fehér, C.; Soriano, A.; Marco, F.; Almela, M.; Cobos-Trigueros, N.; De La Calle, C.; Morata, L.; Mensa, J.; Martínez, J.A. Clinical characteristics and outcome of elderly patients with community-onset bacteremia. J. Infect. 2015, 70, 135–143. [Google Scholar] [CrossRef]
- Gleckman, R.; Hibert, D. Afebrile bacteremia: A phenomenon in geriatric patients. J. Am. Med. Assoc. 1982, 248, 1478–1481. [Google Scholar] [CrossRef]
- Yo, C.-H.; Lee, M.-T.G.; Hsein, Y.C.; Lee, C.C.; Group, E.R. Risk factors and outcomes of afebrile bacteremia patients in an emergency department. Diag. Microbiol. Infect. Dis. 2016, 86, 455–459. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Internat. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- CLSI Document M100-S33; Performance Standards for Antimicrobial Susceptibility Testing; approved standard. Thirty-second informational supplement. CLSI: Wayne, PA, USA, 2023.
- Lee, C.C.; Lin, W.J.; Shih, H.I.; Wu, C.J.; Chen, P.L.; Lee, H.C.; Lee, N.Y.; Chang, C.M.; Wang, L.R.; Ko, W.C. Clinical significance of potential contaminants in blood cultures among patients in a medical center. J. Microbiol. Immunol. Infect. 2007, 40, 438–444. [Google Scholar]
- David, N.; Gilbert, R.C.M., Jr.; Eliopoulos, G.M.; Chambers, H.F.; Saag, M.S. Selected pharmacologic faetures of antimicrobial agents. In The Sanford Guide to Antimicrobial Therapy; Heji Book Publishing House Headquarters: Taiwan, China, 2023; pp. 143–156. [Google Scholar]
- Leligdowicz, A.; Dodek, P.M.; Norena, M.; Wong, H.; Kumar, A.; Kumar, A.; Anand Kumar the Co-operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between source of infection and hospital mortality in patients who have septic shock. Am. J. Respir. Crit. Care Med. 2014, 189, 1204–1213. [Google Scholar] [CrossRef]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef]
- Schellevis, F.G.; van der Velden, J.; van de Lisdonk, E.; van Eijk, J.T.; van Weel, C. Comorbidity of chronic diseases in general practice. J. Clin. Epidemiol. 1993, 46, 469–473. [Google Scholar] [CrossRef]
- McCabe, W.R. Gram-negative bacteremia. Adv. Intern. Med. 1974, 19, 135–158. [Google Scholar]
- Shapiro, N.I.; Wolfe, R.E.; Moore, R.B.; Smith, E.; Burdick, E.; Bates, D.W. Mortality in Emergency Department Sepsis (MEDS) score: A prospectively derived and validated clinical prediction rule. Crit. Care Med. 2003, 31, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.J., Jr. Temperature and host defense. Microbiol. Rev. 1979, 43, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Hsu, Y.C. Afebrile bacteremia in adult emergency department patients with liver cirrhosis: Clinical characteristics and outcomes. Sci. Rep. 2020, 10, 7617. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. N. Eng. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef]
- Granholm, A.; Møller, M.H.; Krag, M.; Perner, A.; Hjortrup, P.B. Predictive performance of the simplified acute physiology score (SAPS) II and the initial sequential organ failure assessment (SOFA) score in acutely ill intensive care patients: Post-hoc analyses of the SUP-ICU inception cohort study. PLoS ONE 2016, 11, e0168948. [Google Scholar] [CrossRef]
- Sadaka, F.; EthmaneAbouElMaali, C.; Cytron, M.A.; Fowler, K.; Javaux, V.M.; O’Brien, J. Predicting mortality of patients with sepsis: A comparison of APACHE II and APACHE III scoring systems. J. Clin. Med. Resear. 2017, 9, 907. [Google Scholar] [CrossRef]

| Variable | Patient Number (%) | p-Value | |
|---|---|---|---|
| Afebrile n = 912 | Febrile n = 2451 | ||
| Patient demographics | |||
| Age, year, median (IQR) | 79 (72–85) | 77 (71–84) | <0.001 |
| Gender, male | 506 (55.5) | 1178 (48.1) | 0.001 |
| Nursing-home resident | 102 (11.2) | 129 (5.3) | <0.001 |
| Delayed AAT, hour, median (IQR) | 2.7 (1.0–25.2) | 2.0 (1.0–9.0) | <0.001 |
| Critical illness (MEDS score > 15) at ED | 334 (36.6) | 327 (13.3) | <0.001 |
| Major bacteremia source | |||
| Low er respiratory tract | 305 (33.4) | 323 (13.2) | <0.001 |
| Urinary tract | 202 (22.1) | 958 (39.1) | <0.001 |
| Intra-abdominal | 115 (12.6) | 231 (9.4) | 0.07 |
| Skin and soft tissue | 96 (10.5) | 228 (9.3) | 0.29 |
| Biliary tract | 90 (9.9) | 302 (12.3) | 0.049 |
| Bone and joint | 37 (4.1) | 76 (3.1) | 0.17 |
| Primary bacteremia | 27 (3.0) | 145 (5.9) | 0.001 |
| Polymicrobial bacteremia | 124 (13.6) | 208 (8.5) | <0.001 |
| Complicated bacteremia | 251 (27.5) | 640 (26.1) | 0.41 |
| Major etiologic pathogen | |||
| Escherichia coli | 269 (29.5) | 1054 (43.0) | <0.001 |
| Klebsiella pneumoniae | 195 (21.5) | 535 (21.8) | 0.83 |
| Staphylococcus aureus | 149 (16.3) | 206 (8.4) | <0.001 |
| streptococci | 127 (13.9) | 312 (12.7) | 0.36 |
| Anaerobes | 66 (7.2) | 75 (3.1) | <0.001 |
| Enterococci | 46 (5.0) | 86 (3.5) | 0.04 |
| Pseudomonas species | 38 (4.2) | 84 (3.4) | 0.31 |
| Fatal comorbidity (McCabe–Johnson classification) | 279 (30.6) | 528 (21.5) | <0.001 |
| Major comorbidity | |||
| Cardiovascular disease | 598 (65.6) | 1689 (88.9) | 0.07 |
| Diabetes mellitus | 390 (42.8) | 1084 (44.2) | 0.45 |
| Neurological disease | 372 (40.8) | 761 (31.0) | <0.001 |
| Chronic kidney disease | 246 (27.0) | 570 (23.3) | 0.03 |
| Malignancy | 219 (24.0) | 463 (18.9) | <0.001 |
| Liver cirrhosis | 92 (10.1) | 216 (8.8) | 0.25 |
| Crude mortality rates | |||
| 3-day | 186 (20.4) | 97 (4.0) | <0.001 |
| 15-day | 286 (31.4) | 193 (7.9) | <0.001 |
| 30-day | 342 (37.5) | 251 (10.2) | <0.001 |
| Variables | Patient Number (%) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Fatal, n = 593 | Surviving, n = 2770 | OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Temperature, each degree decrease from 38.0 °C | - | - | - | - | 1.91 (1.72–2.12) | <0.001 |
| Treatment for bacteremia | ||||||
| Inappropriate empirical antimicrobial therapy ** | 159 (26.8) | 547 (19.7) | 1.49 (1.21–1.83) | <0.001 | 1.88 (1.41–2.51) | <0.001 |
| Inadequate source control during antimicrobial therapy | 163 (10.6) | 94 (3.4) | 3.38 (2.43–4.72) | <0.001 | 9.08 (5.93–13.92) | <0.001 |
| Patient demographics | ||||||
| Gender, male | 319 (53.8) | 1365 (49.3) | 1.20 (1.00–1.43) | 0.046 | 1.26 (0.99–1.61) | 0.06 |
| Bedridden status | 194 (32.7) | 529 (19.1) | 2.06 (1.69–2.51) | <0.001 | NS | NS |
| Comorbidity | ||||||
| Cardiovascular disease | 366 (61.7) | 1921 (69.4) | 0.71 (0.59–0.86) | <0.001 | NS | NS |
| Malignancy | 208 (35.1) | 474 (17.1) | 2.62 (2.15–3.18) | <0.001 | 3.43 (2.43–4.83) | <0.001 |
| Neurological disease | 231 (39.0) | 902 (32.6) | 1.32 (1.10–1.59) | 0.003 | NS | NS |
| Chronic kidney disease | 183 (27.5) | 653 (23.6) | 1.23 (1.01–1.50) | 0.04 | NS | NS |
| Liver cirrhosis | 83 (14.0) | 225 (8.1) | 1.84 (1.41–2.41) | <0.001 | NS | NS |
| Critical illness (MEDS score > 15) at onset | 401 (67.6) | 260 (9.4) | 20.16 (16.27–24.98) | 0.001 | 9.89 (7.59–12.88) | <0.001 |
| Characteristics of bacteremia | ||||||
| Polymicrobial bacteremia | 105 (17.7) | 227 (8.2) | 2.42 (1.88–3.10) | <0.001 | 1.51 (1.06–2.16) | 0.02 |
| Bacteremia source | ||||||
| Low er respiratory tract | 299 (50.4) | 329 (11.9) | 7.55 (6.19–9.20) | <0.001 | 1.76 (1.30–2.38) | <0.001 |
| Urinary tract | 66 (11.1) | 1094 (39.5) | 0.19 (0.15–0.25) | <0.001 | 0.34 (0.21–0.56) | <0.001 |
| Biliary tract | 31 (5.2) | 361 (13.0) | 0.37 (0.25–0.54) | 0.001 | NS | NS |
| Infective endocarditis | 20 (3.4) | 41 (1.5) | 2.32 (1.35–4.00) | 0.002 | 2.84 (1.42–5.69) | 0.003 |
| Primary bacteremia | 12 (2.0) | 160 (5.8) | 0.35 (0.19–0.61) | <0.001 | 0.34 (0.17–0.70) | 0.003 |
| Liver abscess | 8 (1.3) | 105 (3.8) | 0.35 (0.17–0.72) | 0.003 | 0.22 (0.09–0.52) | 0.001 |
| Etiologic pathogen | ||||||
| Klebsiella pneumoniae | 150 (25.3) | 581 (21.0) | 1.28 (1.04–1.57) | 0.02 | 1.33 (0.98–1.80) | 0.06 |
| Escherichia coli | 145 (24.5) | 1178 (42.5) | 0.44 0.36–0.54) | <0.001 | NS | NS |
| Staphylococcus aureus | 99 (16.7) | 256 (9.2) | 1.97 (1.53–2.53) | <0.001 | NS | NS |
| Anaerobes | 47 (7.9) | 94 (3.4) | 2.45 (1.71–3.52) | <0.001 | NS | NS |
| Pseudomonas species | 43 (7.3) | 79 (2.9) | 2.66 (1.82–3.90) | <0.001 | 2.02 (1.16–3.50) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, S.-C.; Chen, P.-L.; Ho, C.-Y.; Hong, M.-Y.; Lee, C.-C.; Ko, W.-C. Comparing the Prognostic Impacts of Delayed Administration of Appropriate Antimicrobials in Older Patients with Afebrile and Febrile Community-Onset Bacteremia. Antibiotics 2024, 13, 465. https://doi.org/10.3390/antibiotics13050465
Hsueh S-C, Chen P-L, Ho C-Y, Hong M-Y, Lee C-C, Ko W-C. Comparing the Prognostic Impacts of Delayed Administration of Appropriate Antimicrobials in Older Patients with Afebrile and Febrile Community-Onset Bacteremia. Antibiotics. 2024; 13(5):465. https://doi.org/10.3390/antibiotics13050465
Chicago/Turabian StyleHsueh, Shu-Chun, Po-Lin Chen, Ching-Yu Ho, Ming-Yuan Hong, Ching-Chi Lee, and Wen-Chien Ko. 2024. "Comparing the Prognostic Impacts of Delayed Administration of Appropriate Antimicrobials in Older Patients with Afebrile and Febrile Community-Onset Bacteremia" Antibiotics 13, no. 5: 465. https://doi.org/10.3390/antibiotics13050465
APA StyleHsueh, S.-C., Chen, P.-L., Ho, C.-Y., Hong, M.-Y., Lee, C.-C., & Ko, W.-C. (2024). Comparing the Prognostic Impacts of Delayed Administration of Appropriate Antimicrobials in Older Patients with Afebrile and Febrile Community-Onset Bacteremia. Antibiotics, 13(5), 465. https://doi.org/10.3390/antibiotics13050465







