Impact of Nutrient Starvation on Biofilm Formation in Pseudomonas aeruginosa: An Analysis of Growth, Adhesion, and Spatial Distribution
Abstract
1. Introduction
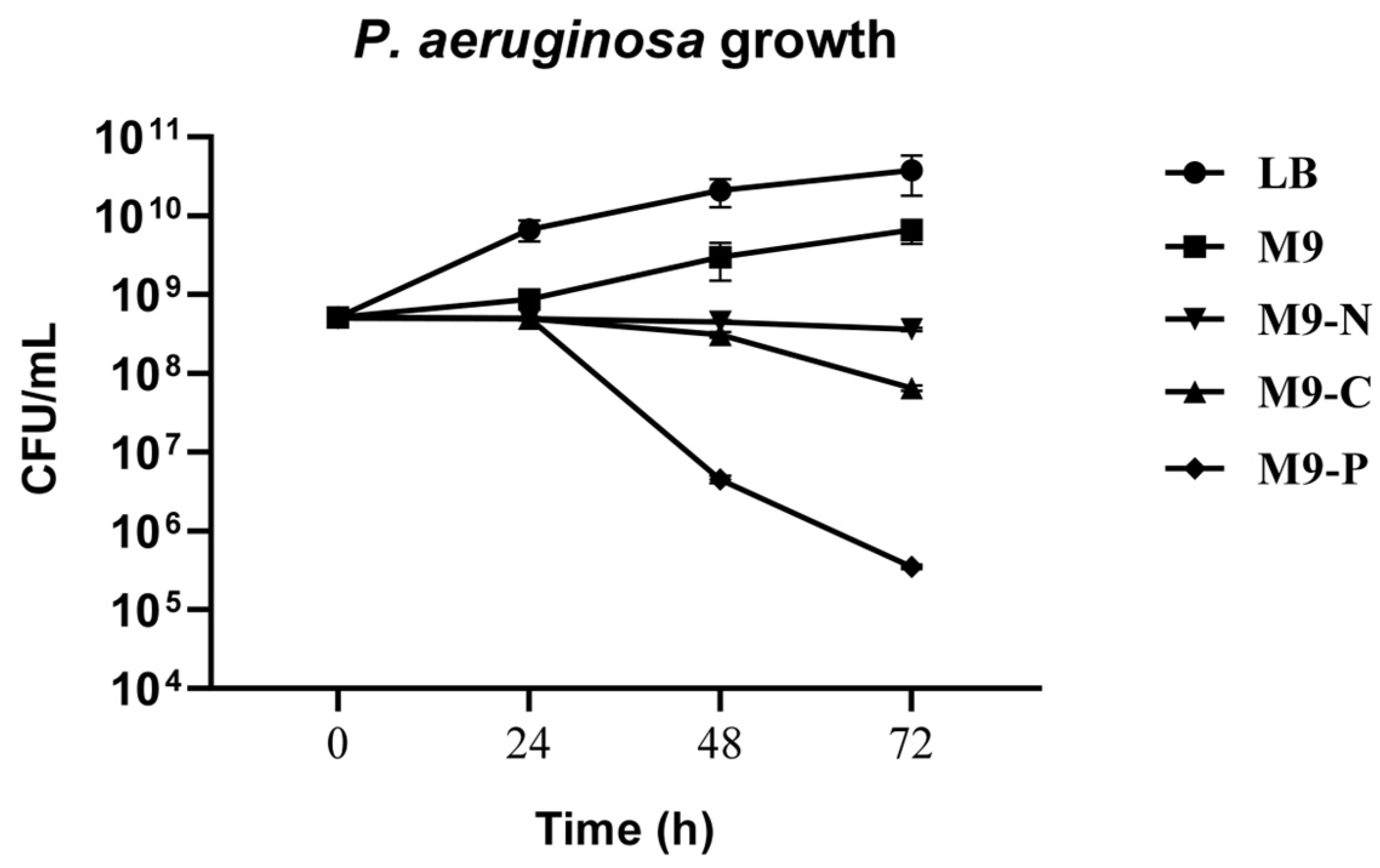
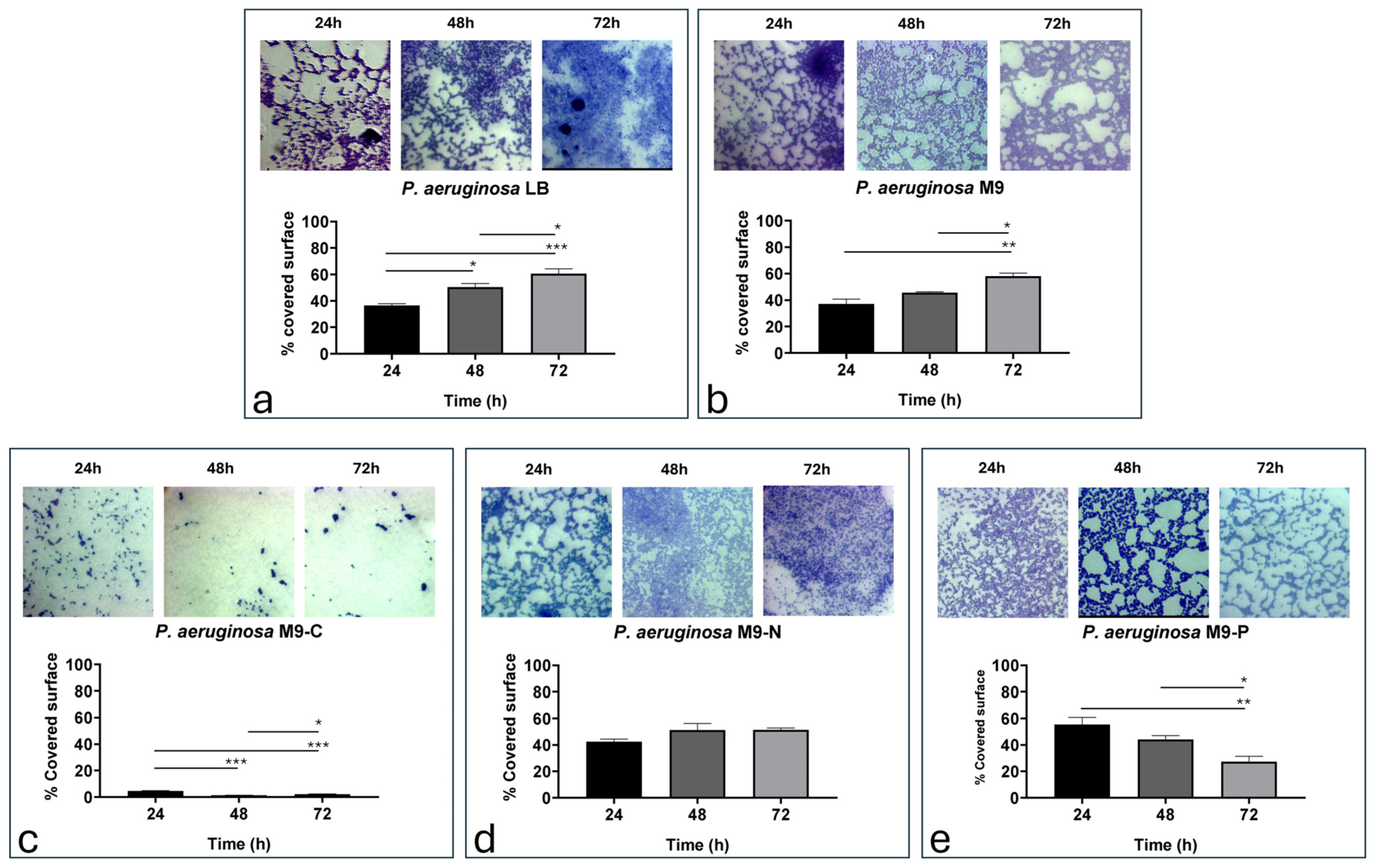
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maji, K.; Lavanya, M. Microbiologically Influenced Corrosion in Stainless Steel by Pseudomonas aeruginosa: An Overview. J. Bio Tribo Corros. 2024, 10, 16. [Google Scholar] [CrossRef]
- Scott, B.; Wilkinson, B. P05 Identifying Novel Drug Targets of the ESKAPE Pathogen Pseudomonas aeruginosa. JAC Antimicrob. Resist. 2024, 6, dlad143.009. [Google Scholar] [CrossRef]
- Venkateswaran, P.; Vasudevan, S.; David, H.; Shaktivel, A.; Shanmugam, K.; Neelakantan, P.; Solomon, A.P. Revisiting ESKAPE Pathogens: Virulence, Resistance, and Combating Strategies Focusing on Quorum Sensing. Front. Cell Infect. Microbiol. 2023, 13, 1159798. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, S.; Kumar, P.; Tripathi, V.N. Quorum Sensing in Gram-Negative Pathogens, a Fresh Look. Microbe 2024, 4, 100108. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, J. Quorum Sensing in Pseudomonas aeruginosa and Its Relationship to Biofilm Development. In Introduction to Biofilm Engineering; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2019; Volume 1323, pp. 1–16. ISBN 978-0-8412-3473-4. [Google Scholar]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Song, Y.; Tang, H.; Bao, R. Comparative Analysis of Five Type II TA Systems Identified in Pseudomonas aeruginosa Reveals Their Contributions to Persistence and Intracellular Survival. Front. Cell. Infect. Microbiol. 2023, 13, 1127786. [Google Scholar] [CrossRef]
- Veetilvalappil, V.V.; Manuel, A.; Aranjani, J.M.; Tawale, R.; Koteshwara, A. Pathogenic Arsenal of Pseudomonas aeruginosa: An Update on Virulence Factors. Future Microbiol. 2022, 17, 465–481. [Google Scholar] [CrossRef]
- Song, Y.; Wu, X.; Li, Z.; Ma, Q.q.; Bao, R. Molecular Mechanism of Siderophore Regulation by the Pseudomonas aeruginosa BfmRS Two-Component System in Response to Osmotic Stress. Commun. Biol. 2024, 7, 295. [Google Scholar] [CrossRef]
- Yang, H.; Jin, L.; Zhao, D.; Lian, Z.; Appu, M.; Huang, J.; Zhang, Z. Antibacterial and Antibiofilm Formation Activities of Pyridinium-Based Cationic Pillar[5]Arene Against Pseudomonas aeruginosa. J. Agric. Food Chem. 2021, 69, 4276–4283. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Zhu, J.; Huang, J.; Zhang, H. Inhibition of Bacterial Adhesion and Biofilm Formation of Sulfonated Chitosan against Pseudomonas aeruginosa. Carbohydr. Polym. 2019, 206, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the Structure and Contribution towards Bacterial Resistance in Antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Gondil, V.S.; Subhadra, B. Biofilms and Their Role on Diseases. BMC Microbiol. 2023, 23, 203. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Petralia, S.; Calabrese, G.; Conoci, S. An Integrated Biosensor Platform for Extraction and Detection of Nucleic Acids. Biotechnol. Bioeng. 2020, 117, 1554–1561. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Hall-Stoodley, L.; Stoodley, P. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I. Mimicking Biofilm Formation and Development: Recent Progress in in Vitro and in Vivo Biofilm Models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three Faces of Biofilms: A Microbial Lifestyle, a Nascent Multicellular Organism, and an Incubator for Diversity. NPJ Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Preda, V.G.; Săndulescu, O. Communication Is the Key: Biofilms, Quorum Sensing, Formation and Prevention. Discoveries 2019, 7, e10. [Google Scholar] [CrossRef]
- Iaconis, A.; De Plano, L.M.; Caccamo, A.; Franco, D.; Conoci, S. Anti-Biofilm Strategies: A Focused Review on Innovative Approaches. Microorganisms 2024, 12, 639. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J.D.P.; Straub, H.; Pietsch, F.; Lemare, M.; Ahrens, C.H.; Schreiber, F.; Webb, J.S.; van der Mei, H.C.; Ren, Q. Role of the Flagellar Hook in the Structural Development and Antibiotic Tolerance of Pseudomonas aeruginosa Biofilms. ISME J. 2022, 16, 1176–1186. [Google Scholar] [CrossRef]
- Zhai, Y.; Tian, W.; Chen, K.; Lan, L.; Kan, J.; Shi, H. Flagella-Mediated Adhesion of Escherichia coli O157:H7 to Surface of Stainless Steel, Glass and Fresh Produces during Sublethal Injury and Recovery. Food Microbiol. 2024, 117, 104383. [Google Scholar] [CrossRef]
- Ruhal, R.; Kataria, R. Biofilm Patterns in Gram-Positive and Gram-Negative Bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef]
- Schniederberend, M.; Williams, J.F.; Shine, E.; Shen, C.; Jain, R.; Emonet, T.; Kazmierczak, B.I. Modulation of Flagellar Rotation in Surface-Attached Bacteria: A Pathway for Rapid Surface-Sensing after Flagellar Attachment. PLoS Pathog. 2019, 15, e1008149. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The Influence of Surface Chemistry on the Kinetics and Thermodynamics of Bacterial Adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef]
- Abdel-Rhman, S.H. Role of Pseudomonas aeruginosa Lipopolysaccharides in Modulation of Biofilm and Virulence Factors of Enterobacteriaceae. Ann. Microbiol. 2019, 69, 299–305. [Google Scholar] [CrossRef]
- Huszczynski, S.M.; Lam, J.S.; Khursigara, C.M. The Role of Pseudomonas aeruginosa Lipopolysaccharide in Bacterial Pathogenesis and Physiology. Pathogens 2019, 9, 6. [Google Scholar] [CrossRef]
- Harmsen, M.; Yang, L.; Pamp, S.J.; Tolker-Nielsen, T. An Update on Pseudomonas aeruginosa Biofilm Formation, Tolerance, and Dispersal. FEMS Immunol. Med. Microbiol. 2010, 59, 253–268. [Google Scholar] [CrossRef]
- Nair, H.A.S.; Subramoni, S.; Poh, W.H.; Hasnuddin, N.T.B.; Tay, M.; Givskov, M.; Tolker-Nielsen, T.; Kjelleberg, S.; McDougald, D.; Rice, S.A. Carbon Starvation of Pseudomonas aeruginosa Biofilms Selects for Dispersal Insensitive Mutants. BMC Microbiol. 2021, 21, 255. [Google Scholar] [CrossRef] [PubMed]
- Dubern, J.-F.; Halliday, N.; Cámara, M.; Winzer, K.; Barrett, D.A.; Hardie, K.R.; Williams, P. Growth Rate and Nutrient Limitation as Key Drivers of Extracellular Quorum Sensing Signal Molecule Accumulation in Pseudomonas aeruginosa. Microbiology 2023, 169, 001316. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Alexandre, K.; Etienne, M. Tolerance and Persistence of Pseudomonas aeruginosa in Biofilms Exposed to Antibiotics: Molecular Mechanisms, Antibiotic Strategies and Therapeutic Perspectives. Front. Microbiol. 2020, 11, 2057. [Google Scholar] [CrossRef]
- Akiyama, T.; Williamson, K.S.; Schaefer, R.; Pratt, S.; Chang, C.B.; Franklin, M.J. Resuscitation of Pseudomonas aeruginosa from Dormancy Requires Hibernation Promoting Factor (PA4463) for Ribosome Preservation. Proc. Natl. Acad. Sci. USA 2017, 114, 3204–3209. [Google Scholar] [CrossRef]
- Díaz-Salazar, C.; Calero, P.; Espinosa-Portero, R.; Jiménez-Fernández, A.; Wirebrand, L.; Velasco-Domínguez, M.G.; López-Sánchez, A.; Shingler, V.; Govantes, F. The Stringent Response Promotes Biofilm Dispersal in Pseudomonas Putida. Sci. Rep. 2017, 7, 18055. [Google Scholar] [CrossRef]
- Scheffler, R.J.; Sugimoto, Y.; Bratton, B.P.; Ellison, C.K.; Koch, M.D.; Donia, M.S.; Gitai, Z. Pseudomonas aeruginosa Detachment from Surfaces via a Self-Made Small Molecule. J. Biol. Chem. 2021, 296, 100279. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y.; Lin, S.; Zhang, W.; Shu, G.; Lin, J.; Li, H.; Xu, F.; Tang, H.; Peng, G.; et al. Strategies for Interfering with Bacterial Early Stage Biofilms. Front. Microbiol. 2021, 12, 675843. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial Adhesion and Biofilms on Surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Duanis-Assaf, T.; Reches, M. Factors Influencing Initial Bacterial Adhesion to Antifouling Surfaces Studied by Single-Cell Force Spectroscopy. iScience 2024, 27, 108803. [Google Scholar] [CrossRef]
- Rosales, A.B.; Causserand, C.; Coetsier, C.; Formosa-Dague, C. Probing the Reduction of Adhesion Forces between Biofilms and Anti-Biofouling Filtration Membrane Surfaces Using FluidFM Technology. Colloids Surf. B Biointerfaces 2024, 234, 113701. [Google Scholar] [CrossRef]
- Balducci, E.; Papi, F.; Capialbi, D.E.; Del Bino, L. Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens. Int. J. Mol. Sci. 2023, 24, 4030. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Trimble, M.J.; Cheng, J.T.; Vallé, Q.; Hancock, R.E.W. Critical Assessment of Methods to Quantify Biofilm Growth and Evaluate Antibiofilm Activity of Host Defence Peptides. Biomolecules 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, W.; Xia, A.; Zhang, R.; Huang, Y.; Yang, S.; Ni, L.; Jin, F. Carbon Starvation Induces the Expression of PprB-Regulated Genes in Pesudomonas Aeruginosa. Appl. Environ. Microbiol. 2019, 85, e01705-19. [Google Scholar] [CrossRef]
- Bains, M.; Fernández, L.; Hancock, R.E.W. Phosphate Starvation Promotes Swarming Motility and Cytotoxicity of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2012, 78, 6762–6768. [Google Scholar] [CrossRef] [PubMed]
- Poulin, M.B.; Kuperman, L.L. Regulation of Biofilm Exopolysaccharide Production by Cyclic Di-Guanosine Monophosphate. Front. Microbiol. 2021, 12, 730980. [Google Scholar] [CrossRef]
- Neznansky, A.; Blus-Kadosh, I.; Yerushalmi, G.; Banin, E.; Opatowsky, Y. The Pseudomonas aeruginosa Phosphate Transport Protein PstS Plays a Phosphate-Independent Role in Biofilm Formation. FASEB J. 2014, 28, 5223–5233. [Google Scholar] [CrossRef]
- Tan, X.; Cheng, X.; Xiao, J.; Liu, Q.; Du, D.; Li, M.; Sun, Y.; Zhou, J.; Zhu, G. Alkaline Phosphatase LapA Regulates Quorum Sensing–Mediated Virulence and Biofilm Formation in Pseudomonas aeruginosa PAO1 under Phosphate Depletion Stress. Microbiol. Spectr. 2023, 11, e02060-23. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Liu, S.-Y.; Guo, J.-S.; Fang, F.; Chen, Y.-P.; Yan, P. Mechanisms of Survival Mediated by the Stringent Response in Pseudomonas aeruginosa under Environmental Stress in Drinking Water Systems: Nitrogen Deficiency and Bacterial Competition. J. Hazard. Mater. 2023, 448, 130941. [Google Scholar] [CrossRef]
- Hall, C.W.; Farkas, E.; Zhang, L.; Mah, T.-F. Potentiation of Aminoglycoside Lethality by C4-Dicarboxylates Requires RpoN in Antibiotic-Tolerant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e01313-19. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, X.; Zhang, Y.; Zhu, M.; Yang, P.; Yuan, J.; Xie, Y.; Zhou, T.; Wang, W.; Chen, S.; et al. RpoN-Dependent Direct Regulation of Quorum Sensing and the Type VI Secretion System in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2018, 200, e00205-18. [Google Scholar] [CrossRef]
- Lloyd, M.G.; Vossler, J.L.; Nomura, C.T.; Moffat, J.F. Blocking RpoN Reduces Virulence of Pseudomonas aeruginosa Isolated from Cystic Fibrosis Patients and Increases Antibiotic Sensitivity in a Laboratory Strain. Sci. Rep. 2019, 9, 6677. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.G.; Lundgren, B.R.; Hall, C.W.; Gagnon, L.B.-P.; Mah, T.-F.; Moffat, J.F.; Nomura, C.T. Targeting the Alternative Sigma Factor RpoN to Combat Virulence in Pseudomonas aeruginosa. Sci. Rep. 2017, 7, 12615. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Plano, L.M.; Caratozzolo, M.; Conoci, S.; Guglielmino, S.P.P.; Franco, D. Impact of Nutrient Starvation on Biofilm Formation in Pseudomonas aeruginosa: An Analysis of Growth, Adhesion, and Spatial Distribution. Antibiotics 2024, 13, 987. https://doi.org/10.3390/antibiotics13100987
De Plano LM, Caratozzolo M, Conoci S, Guglielmino SPP, Franco D. Impact of Nutrient Starvation on Biofilm Formation in Pseudomonas aeruginosa: An Analysis of Growth, Adhesion, and Spatial Distribution. Antibiotics. 2024; 13(10):987. https://doi.org/10.3390/antibiotics13100987
Chicago/Turabian StyleDe Plano, Laura Maria, Manuela Caratozzolo, Sabrina Conoci, Salvatore P. P. Guglielmino, and Domenico Franco. 2024. "Impact of Nutrient Starvation on Biofilm Formation in Pseudomonas aeruginosa: An Analysis of Growth, Adhesion, and Spatial Distribution" Antibiotics 13, no. 10: 987. https://doi.org/10.3390/antibiotics13100987
APA StyleDe Plano, L. M., Caratozzolo, M., Conoci, S., Guglielmino, S. P. P., & Franco, D. (2024). Impact of Nutrient Starvation on Biofilm Formation in Pseudomonas aeruginosa: An Analysis of Growth, Adhesion, and Spatial Distribution. Antibiotics, 13(10), 987. https://doi.org/10.3390/antibiotics13100987









