Guanethidine Restores Tetracycline Sensitivity in Multidrug-Resistant Escherichia coli Carrying tetA Gene
Abstract
1. Introduction
2. Results
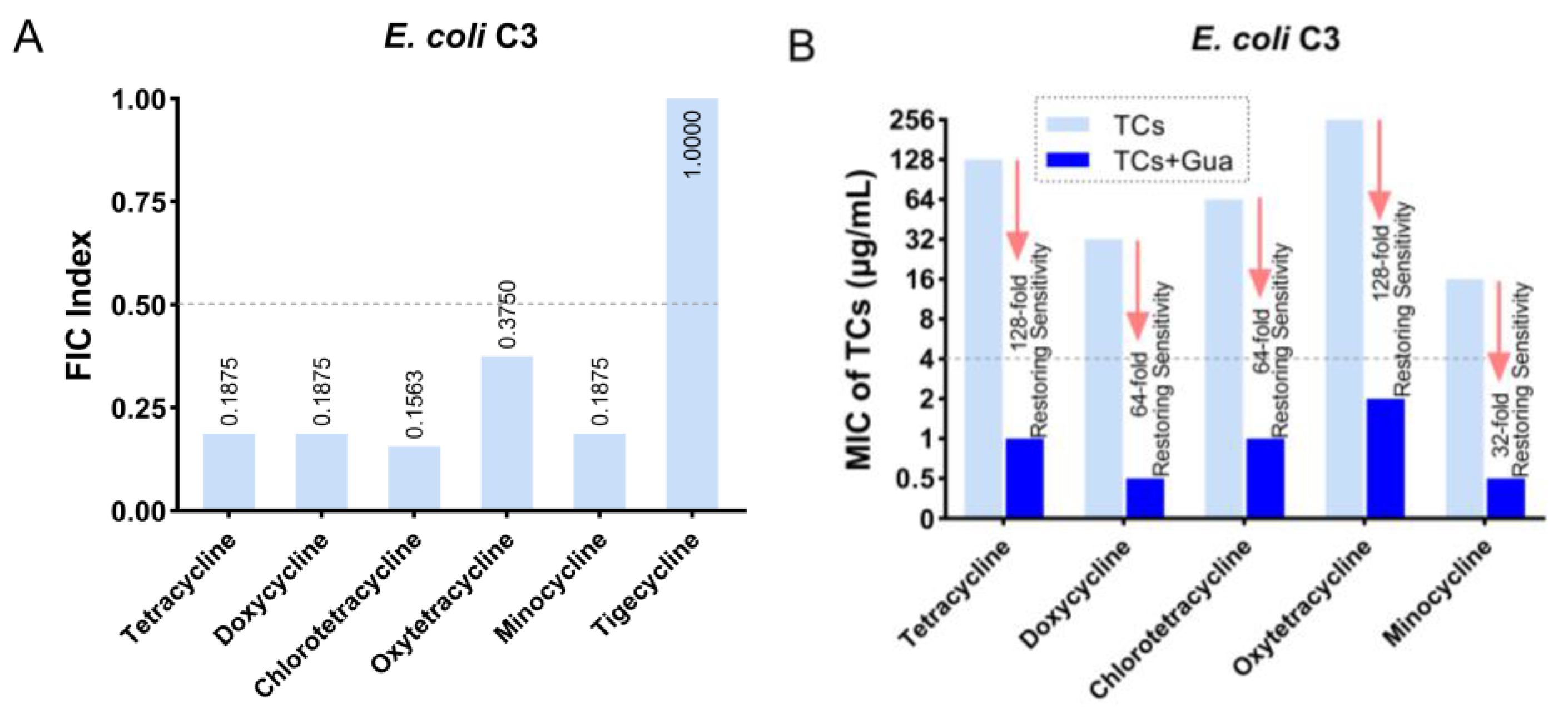
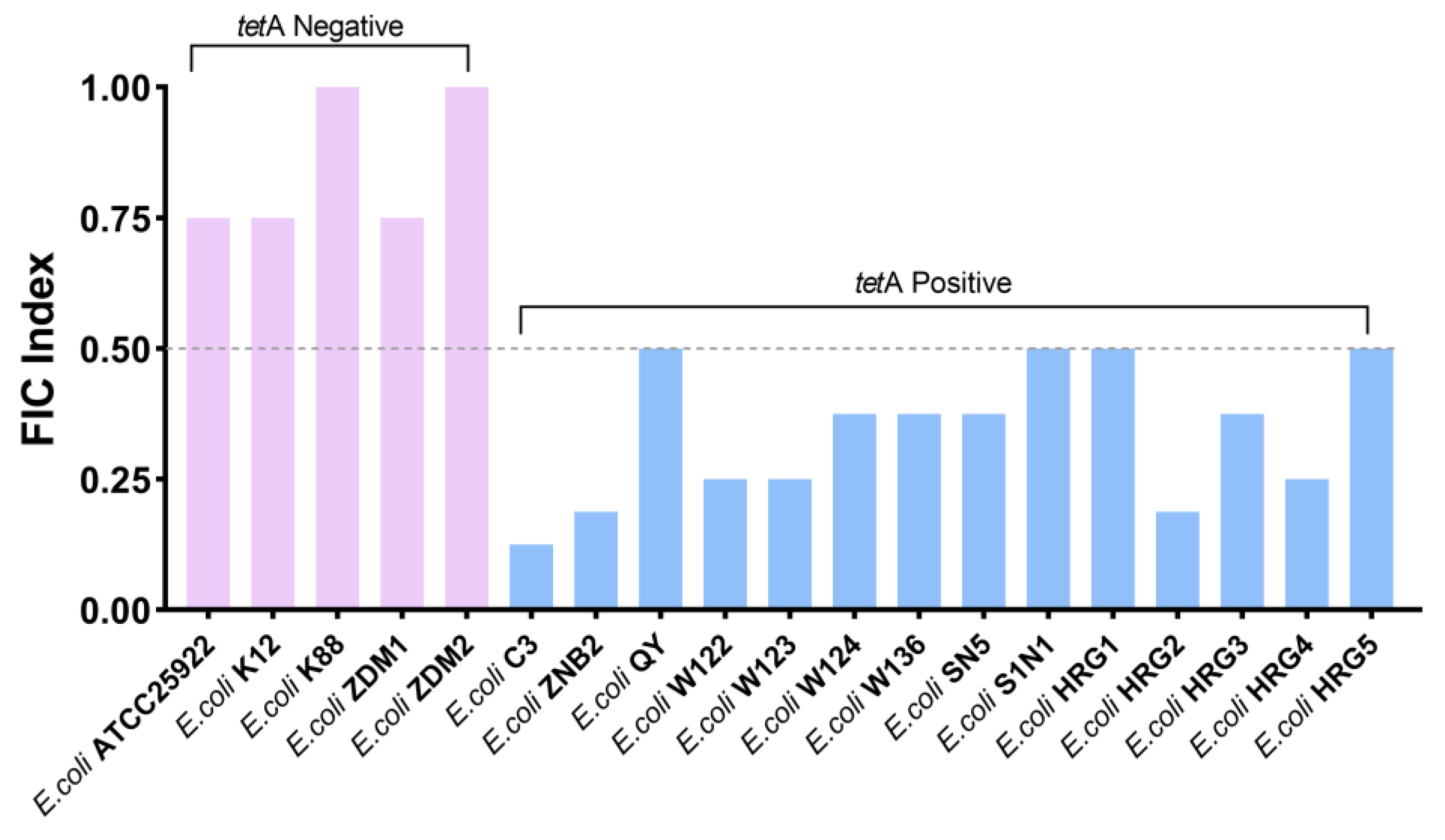
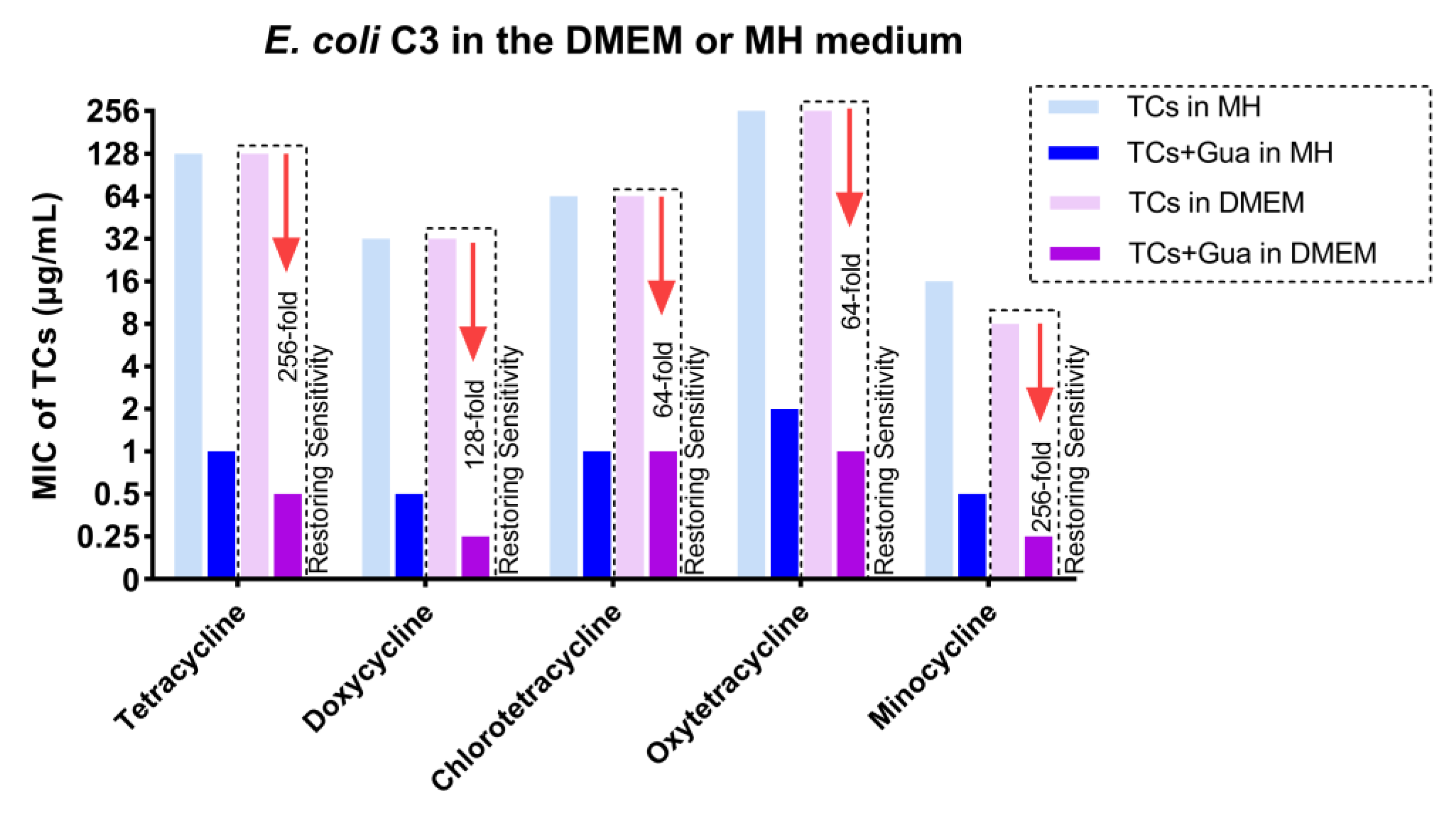
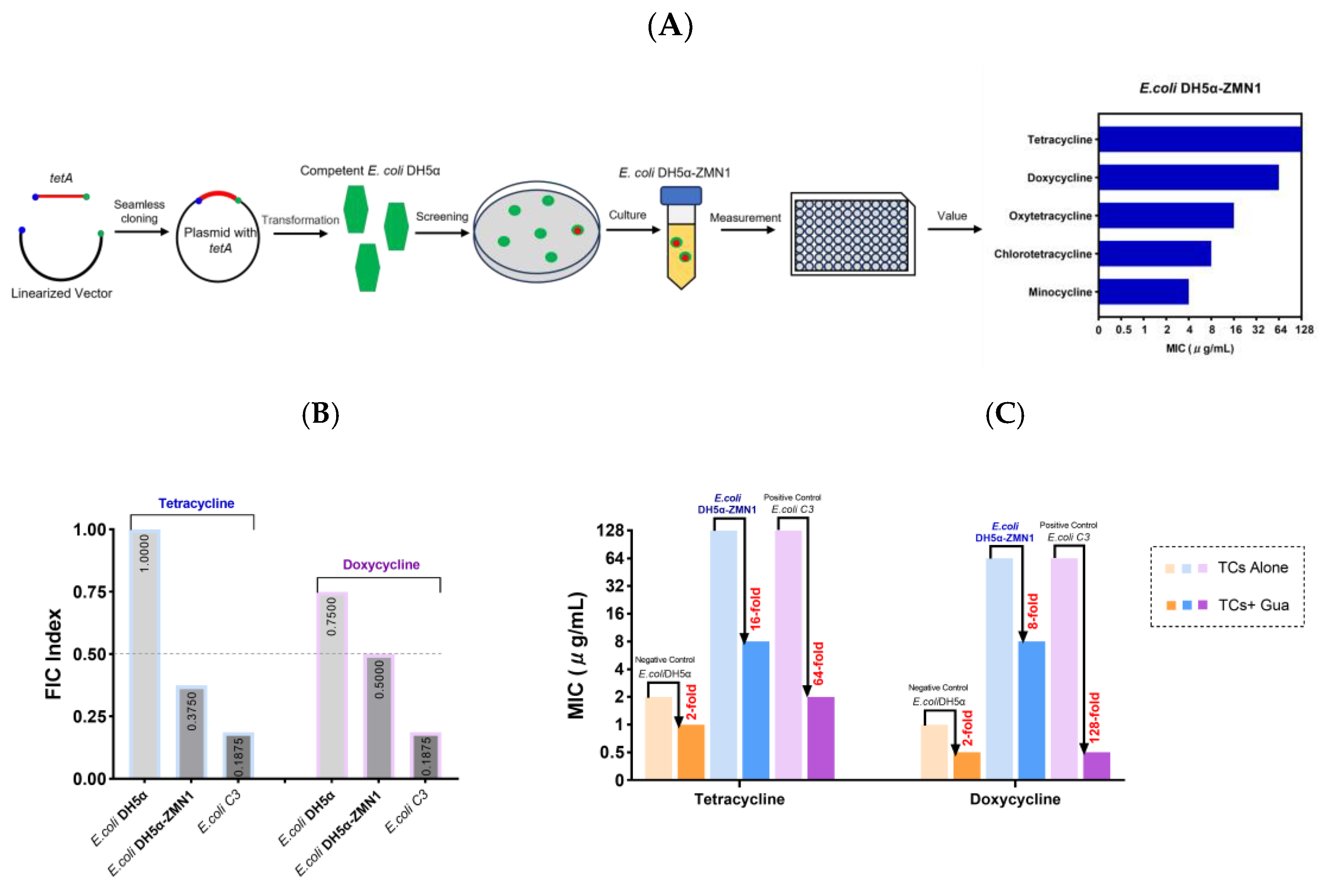
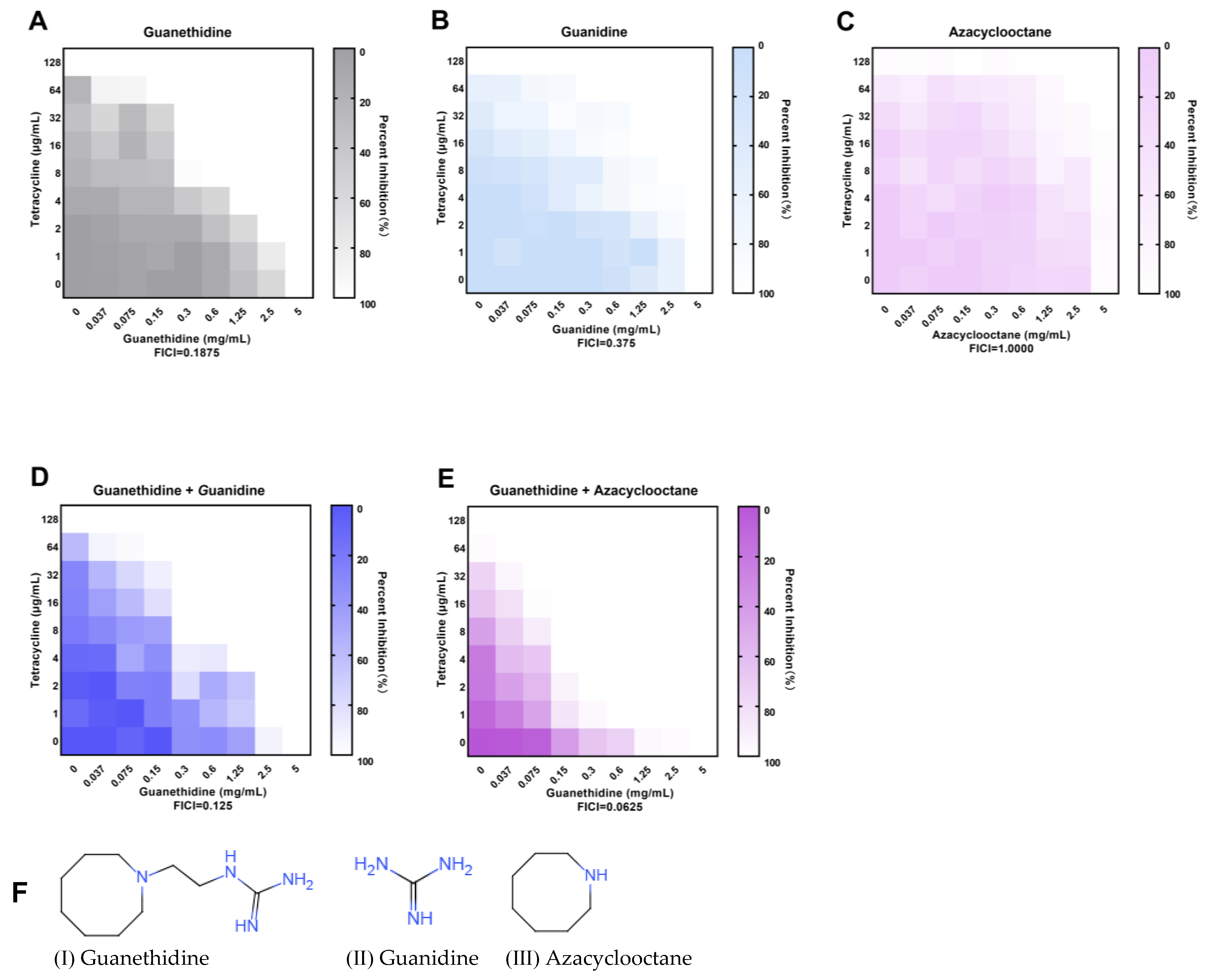
2.1. Guanethidine Potentiates Tetracyclines against Multidrug-Resistant E. coli
2.2. Guanethidine Retards the Development of Resistance in E. coli
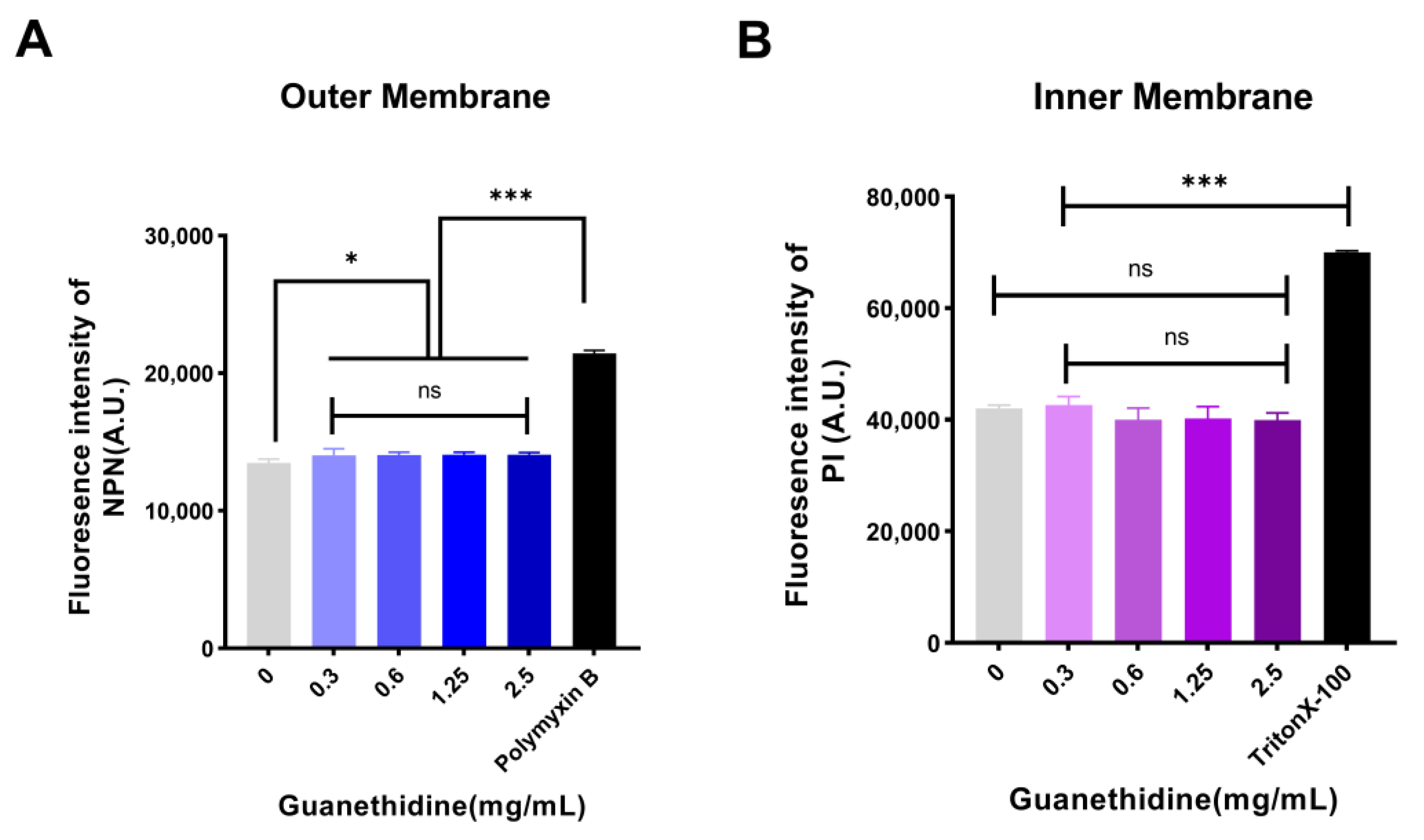
2.3. Guanethidine Affects the Integrity of the Outer Membrane
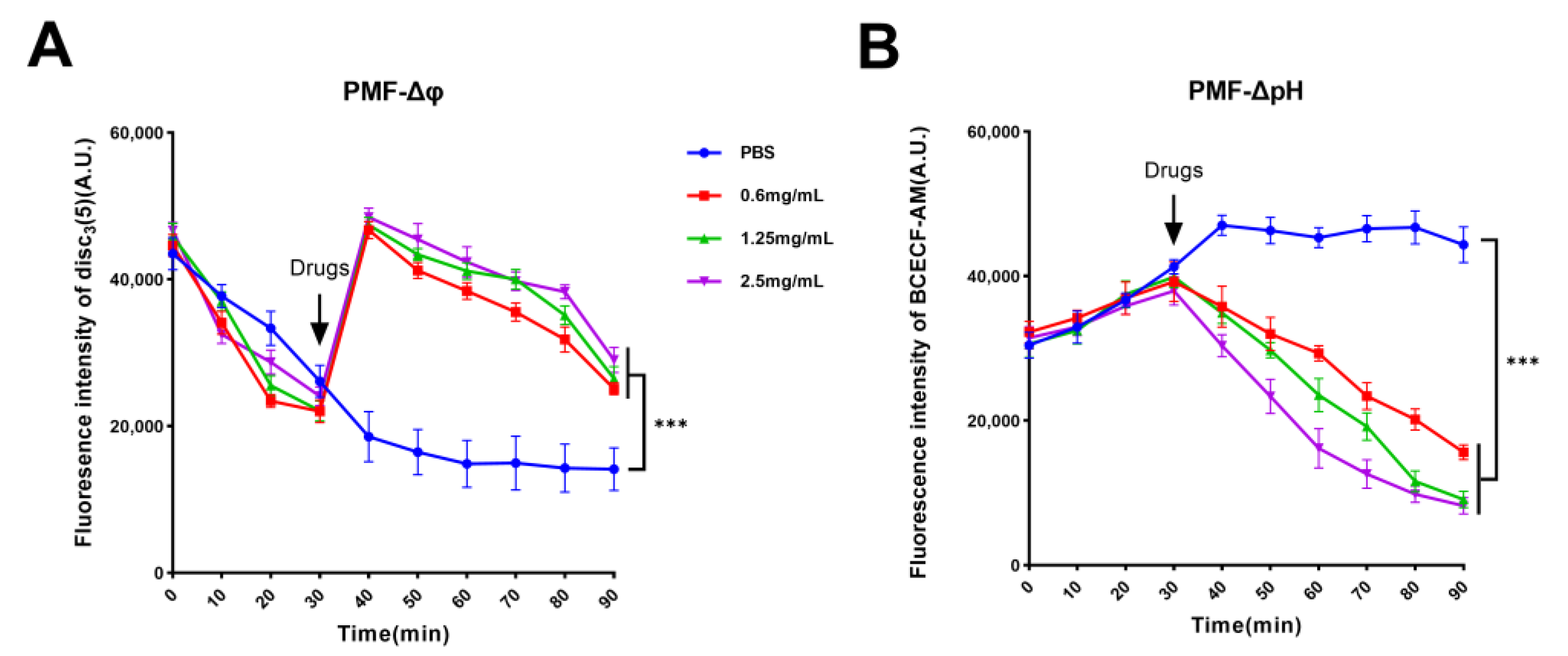
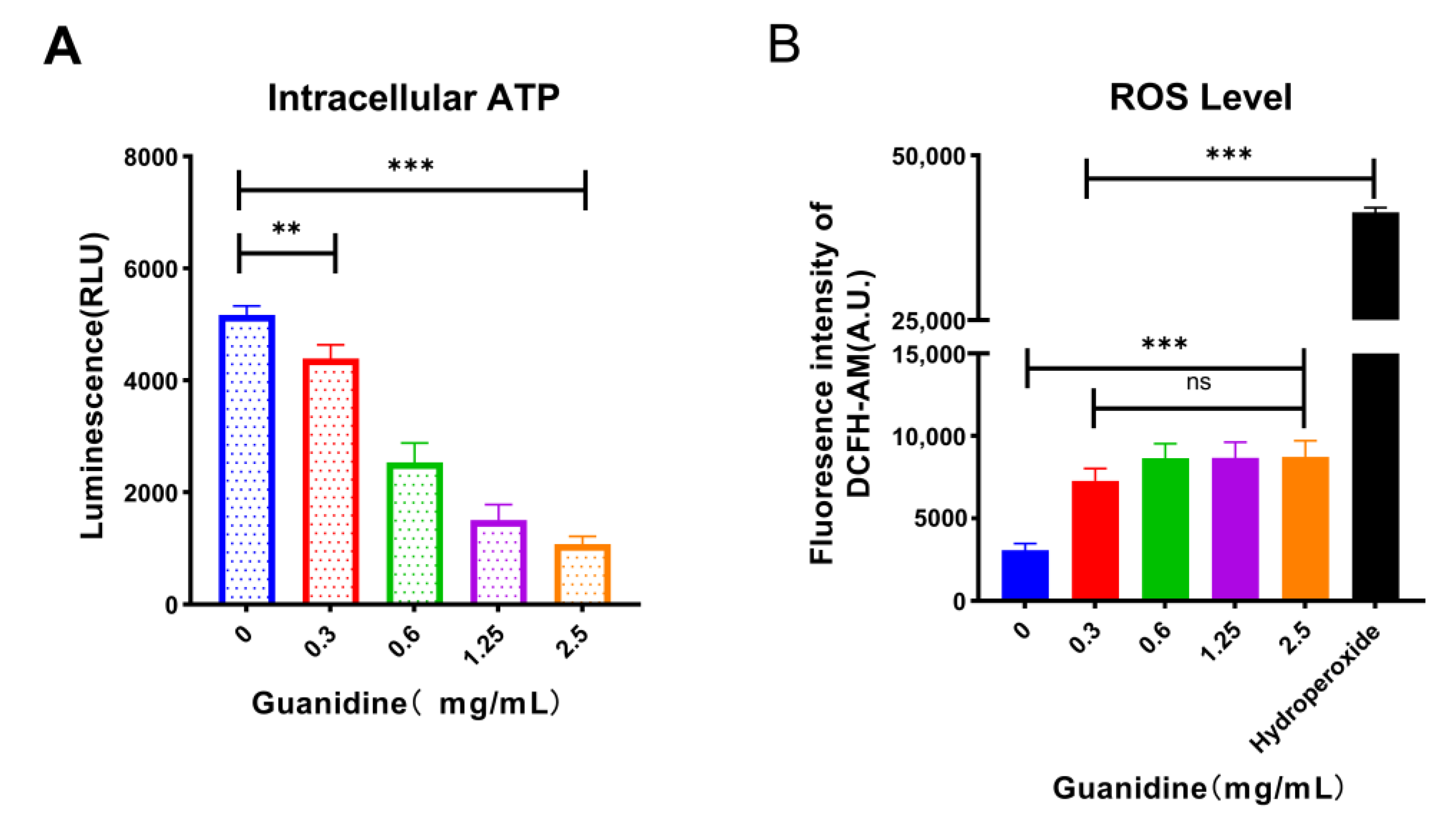
2.4. Guanethidine Affects Bacterial PMF
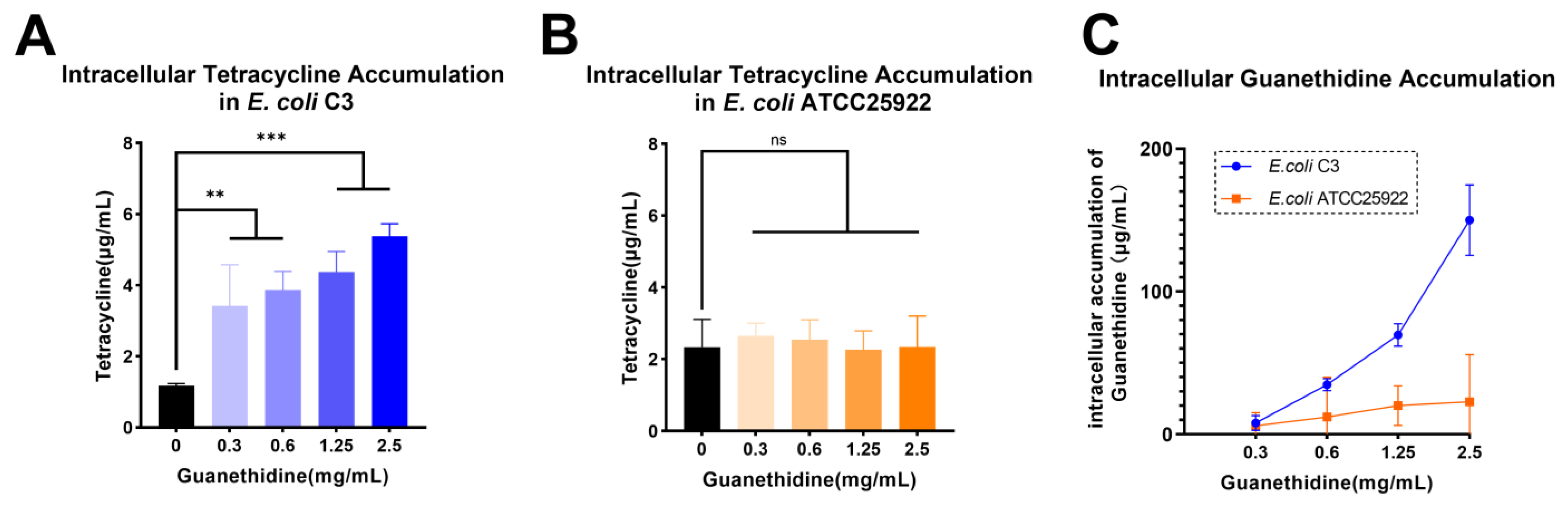
2.5. Guanethidine Addition Is Correlated with an Enhanced Intracellular Concentration of Tetracycline in E. coli
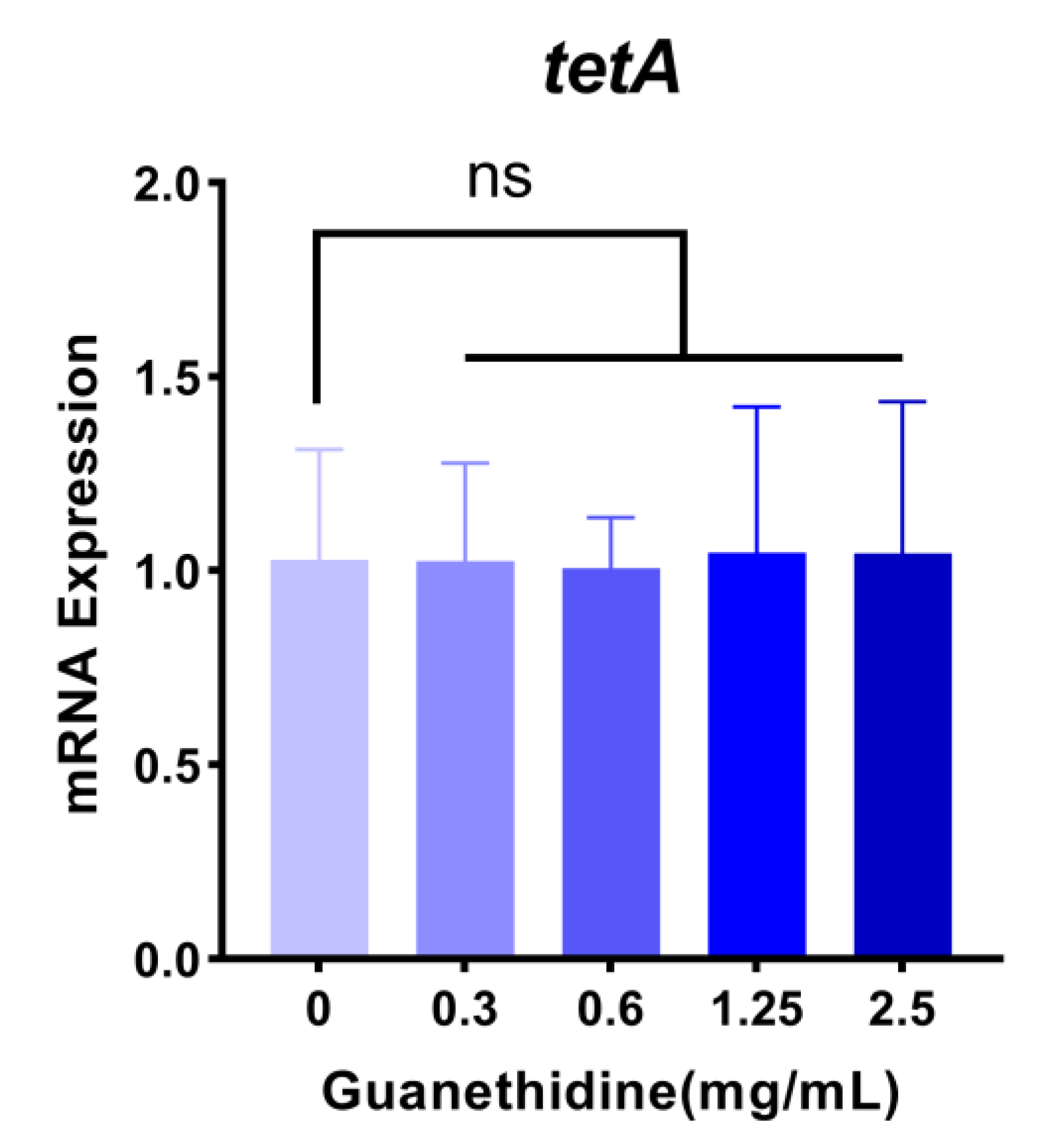
2.6. Guanethidine Did Not Significantly Affect the Expression Level nor the Integrity of the TetA Protein
2.7. Guanethidine Inhibits the Activity of Efflux Pump Protein TetA
2.7.1. Guanethidine Enhances the Effectiveness of Tetracycline against Genetically Engineered E. coli Expressing the tetA Gene
2.7.2. The Molecular Docking Analysis Demonstrates the Stable Binding of Guanethidine to the TetA Pocket
2.8. The Guanidine Group Is the Crucial Group of the Synergistic Effect
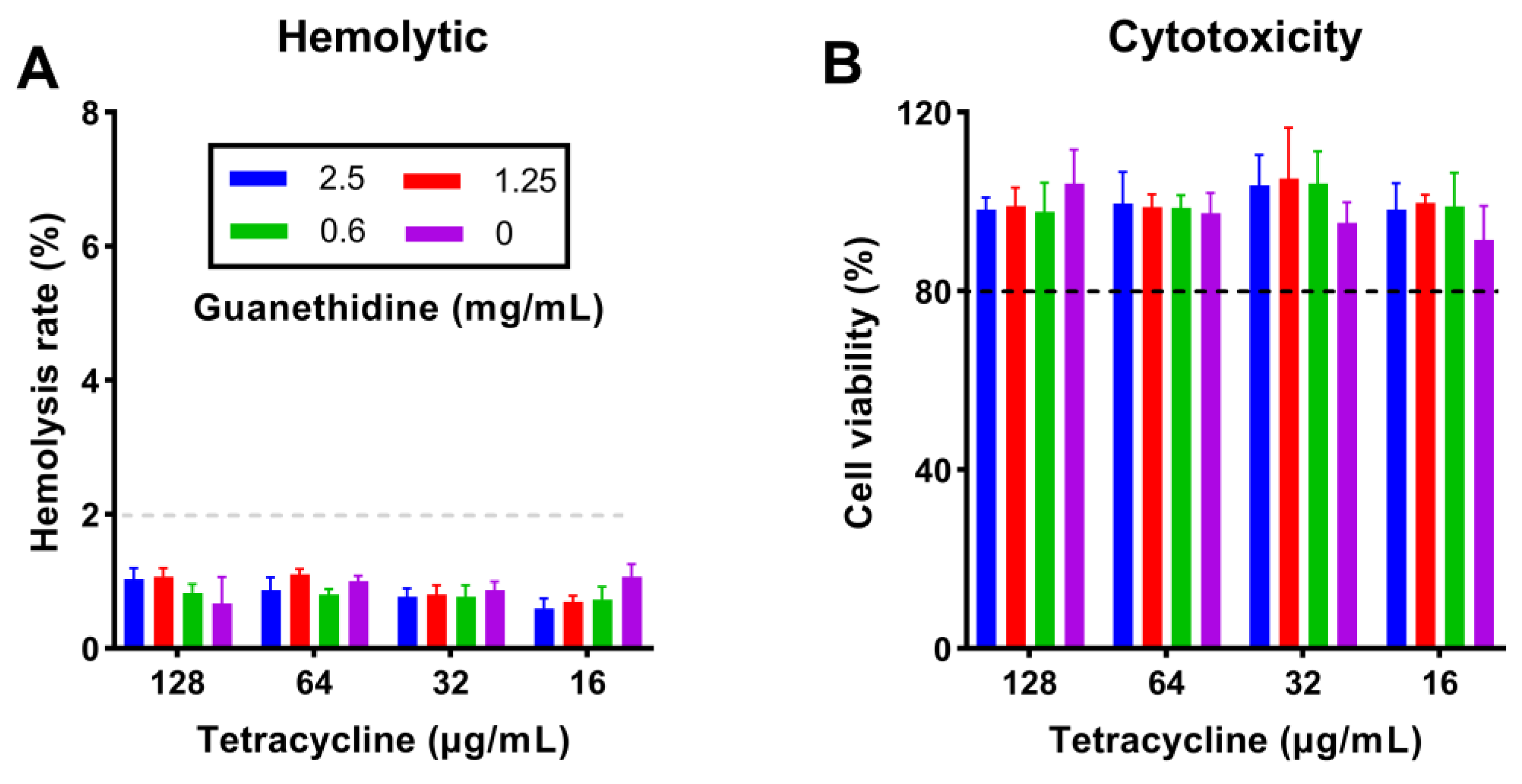
2.9. The Combination Did Not Increase the Toxicity of In Vitro Therapy
2.10. The Combination Did Not Alter Blood Routine or Biochemical Parameters in Healthy Mouse Models
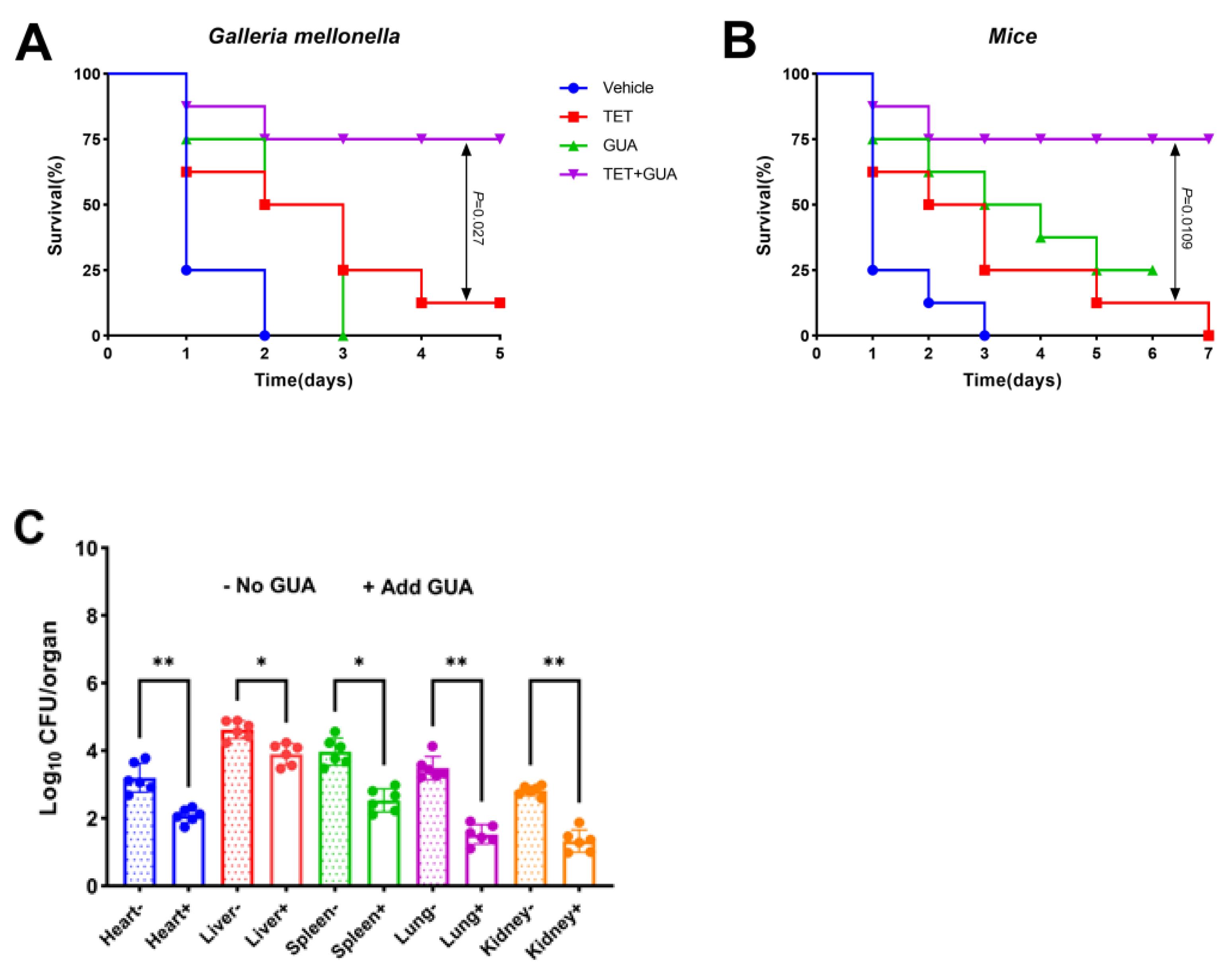
2.11. The Combination Enhanced the Survival Rate of Animals Infected with Multidrug-Resistant E. coli C3
3. Discussion
4. Materials and Methods
4.1. Bacteria and Reagents
4.2. MIC Measurements
4.3. FIC Index Determination
4.4. Determination of Growth Curve and Time–Kill Curve
4.5. Resistance Development Studies
4.6. Fluorescence Assay
4.6.1. Outer Membrane Permeability Assay
4.6.2. Inner Membrane Permeability Assay
4.6.3. Membrane Potential Gradient Assay
4.6.4. Proton Motive Force Assay
4.6.5. Total ROS Measurement
4.7. ATP Determination
4.8. Construct and Transform the Plasmid Containing the tetA Gene
4.8.1. The tetA Gene Was Amplified
4.8.2. The Amplification of the pBAD Plasmid
4.8.3. Construction and Transformation of Plasmids
4.9. Molecular Docking
4.10. RT-PCR Analysis
4.11. Gene tetA Integrity Analysis
4.12. Antibiotics Accumulation Analysis
4.13. Measurement of Hemolysis Activity
4.14. Cytotoxicity Assays
4.15. In Vivo Toxicity Test
4.16. Galleria Mellonella Infection Mode
4.17. Mouse Intraperitoneal Infection Model
4.18. Determination of Bacterial Load in Organs
4.19. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.I. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Bronstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Nikaido, H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob. Agents Chemother. 1989, 33, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, G.; Acharya, Y.; Haldar, J. Antibiotic Adjuvants: A Versatile Approach to Combat Antibiotic Resistance. ACS Omega 2023, 8, 10757–10783. [Google Scholar] [CrossRef]
- Douafer, H.; Andrieu, V.; Phanstiel, O.; Brunel, J.M. Antibiotic Adjuvants: Make Antibiotics Great Again! J. Med. Chem. 2019, 62, 8665–8681. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Antibiotic adjuvants: An alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit. Rev. Microbiol. 2019, 45, 301–314. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Y.; Yang, K.; Li, R.; Xiao, X.; Zhu, K.; Wang, Z. Metformin Restores Tetracyclines Susceptibility against Multidrug Resistant Bacteria. Adv. Sci. 2020, 7, 1902227. [Google Scholar] [CrossRef] [PubMed]
- Whalen, K.E.; Poulson-Ellestad, K.L.; Deering, R.W.; Rowley, D.C.; Mincer, T.J. Enhancement of antibiotic activity against multidrug-resistant bacteria by the efflux pump inhibitor 3,4-dibromopyrrole-2,5-dione isolated from a Pseudoalteromonas sp. J. Nat. Prod. 2015, 78, 402–412. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Huang, X.; Ding, S.; Wang, Y.; Shen, J.; Zhu, K. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 2020, 5, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Fuente-Nunez, C.d.l.; Reffuveille, F.; Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS. Pathog. 2014, 10, 1004152. [Google Scholar] [CrossRef]
- Messele, Y.E.; Werid, G.M.; Petrovski, K. Meta-Analysis on the Global Prevalence of Tetracycline Resistance in Escherichia coli Isolated from Beef Cattle. Vet. Sci. 2023, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.F.; Berg, J.L. Efficacy of a feed-additive antibacterial combination for improving feedlot cattle performance and health. Can. Vet. J. 1995, 36, 223. [Google Scholar] [PubMed]
- Mesa, N.G.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Chopra, I. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Speer, B.S.; Shoemaker, N.B.; Salyers, A.A. Bacterial resistance to tetracycline: Mechanisms, transfer, and clinical significance. Clin. Microbiol. Rev. 1992, 5, 387–399. [Google Scholar] [CrossRef]
- Roberts, M. Tetracycline resistance determinants: Mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol. Rev. 1996, 19, 1–24. [Google Scholar] [CrossRef]
- Stavropoulos, T.A.; Strathdee, C.A. Expression of the tetA(C) tetracycline efflux pump in Escherichia coli confers osmotic sensitivity. FEMS Microbiol. Lett. 2000, 190, 147–150. [Google Scholar] [CrossRef]
- Moller, T.S.; Overgaard, M.; Nielsen, S.S.; Bortolaia, V.; Sommer, M.O.; Guardabassi, L.; Olsen, J.E. Relation between tetR and tetA expression in tetracycline resistant Escherichia coli. BMC Microbiol. 2016, 16, 39. [Google Scholar] [CrossRef]
- Sophie, N.; Julien, C.; Annick, D.; Adeline, P.; Frederic, D.; Christian, L. Role of AcrAB-TolC multidrug efflux pump in drug-resistance acquisition by plasmid transfer. Science 2019, 364, 778–782. [Google Scholar] [CrossRef]
- Seong-Heun, K.; Dorothy, S.; Daniele, C. Antimicrobial drugs bearing guanidine moieties: A review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef]
- Hannon, C.L.; Anslyn, E.V. The Guanidinium Group: Its Biological Role and Synthetic Analogs; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- David, C.N.; Malachy Chigozie, U.; Clement, O.A.; Mushtak, T.S.A.; Joseph, C.I.; Uchenna, V.C.; Morteza, S. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, 24655. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, M.; Muhammad, I.; Cui, Q.; Zhang, H.; Jia, Y.; Xu, Q.; Kong, L.; Ma, H. An Antibacterial Peptide with High Resistance to Trypsin Obtained by Substituting d-Amino Acids for Trypsin Cleavage Sites. Antibiotics 2021, 10, 1465. [Google Scholar] [CrossRef]
- Yang, B.; Tong, Z.; Shi, J.; Wang, Z.; Liu, Y. Bacterial proton motive force as an unprecedented target to control antimicrobial resistance. Med. Res. Rev. 2023, 43, 1068–1090. [Google Scholar] [CrossRef]
- Wright, D.J.; Tate, C.G. Isolation and characterisation of transport-defective substrate-binding mutants of the tetracycline antiporter TetA(B). Biochim. Biophys. Acta 2015, 1848, 2261–2270. [Google Scholar] [CrossRef]
- Shih, Y.E.; Chen, C.T. Synthesis of Guanethidine Sulfate. Bull. Inst. Chem. Acad. Sin. 1980, 27, 19–22. [Google Scholar]
- Tosin, T. The staggering death toll of drug-resistant bacteria. Nature 2022. [Google Scholar] [CrossRef]
- Namita, S.; Anil, K.C.; Sweety, D.; Pooja, C.; Aruna, P.; Prity, G. Antibiotic Adjuvants: A Promising Approach to Combat Multidrug Resistant Bacteria. Curr. Drug. Targets 2021, 22, 1334–1345. [Google Scholar] [CrossRef]
- Ejim, L.; Farha, M.A.; Falconer, S.B.; Wildenhain, J.; Coombes, B.K.; Tyers, M.; Brown, E.D.; Wright, G.D. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat. Chem. Biol. 2011, 7, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Suss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell 2017, 171, 1354–1367. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Q.; Wang, R.; Wang, H.; Wong, Y.T.; Wang, M.; Hao, Q.; Yan, A.; Kao, R.Y.; Ho, P.L.; et al. Resensitizing carbapenem- and colistin-resistant bacteria to antibiotics using auranofin. Nat. Commun. 2020, 11, 5263. [Google Scholar] [CrossRef] [PubMed]
- Lulu, H.; Junyan, L.; Jingdong, C.; Qinhui, R.; Ze, L.; Haifeng, H.; Zhiyong, L.; Lei, Z.; Wei, L.; Wei, G.; et al. Structural insight into substrate preference for TET-mediated oxidation. Nature 2015, 527, 118–122. [Google Scholar] [CrossRef]
- Ghai, I. A Barrier to Entry: Examining the Bacterial Outer Membrane and Antibiotic Resistance. Appl. Sci. 2023, 13, 4238. [Google Scholar] [CrossRef]
- Ding, X.; Yang, C.; Moreira, W.; Yuan, P.; Periaswamy, B.; de Sessions, P.F.; Zhao, H.; Tan, J.; Lee, A.; Ong, K.X.; et al. A Macromolecule Reversing Antibiotic Resistance Phenotype and Repurposing Drugs as Potent Antibiotics. Adv. Sci. 2020, 7, 2001374. [Google Scholar] [CrossRef]
- Ana, R.G.; Carla, L.V.; Ana, S.P.; Elisiário, J.T.; Fernanda, M.F.R. Synthetic and natural guanidine derivatives as antitumor and antimicrobial agents: A review. Bioorg. Chem. 2023, 138, 106600. [Google Scholar] [CrossRef]
- Orner, B.P.; Hamilton, A.D. The Guanidinium Group in Molecular Recognition: Design and Synthetic Approaches. J. Incl. Phenom. Macrocycl. Chem. 2001, 41, 141–147. [Google Scholar] [CrossRef]
- CLSI. Performance standards for antimicrobial susceptibility testing. Clinical. Lab. Stand. Inst. 2020, 23, 49. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Y.; Yang, K.; Li, R.; Xiao, X.; Wang, Z. Anti-HIV agent azidothymidine decreases Tet(X)-mediated bacterial resistance to tigecycline in Escherichia coli. Commun. Biol. 2020, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids Against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, 2100749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X.; Cui, Q.; Zhu, D.; Wisal, M.A.; Yu, H.; Kong, L.; Ma, H. Famotidine Enhances Rifampicin Activity against Acinetobacter baumannii by Affecting OmpA. J. Bacteriol. 2023, 205, e00187-23. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.M.; Ramirez, D.; Arthur, G.; Zhanel, G.; Schweizer, F. Guanidinylated Polymyxins as Outer Membrane Permeabilizers Capable of Potentiating Rifampicin, Erythromycin, Ceftazidime and Aztreonam against Gram-Negative Bacteria. Antibiotics 2022, 11, 1277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Z.; Shi, J.; Jia, Y.; Deng, T.; Wang, Z. Reversion of antibiotic resistance in multidrug-resistant pathogens using non-antibiotic pharmaceutical benzydamine. Commun. Biol. 2021, 4, 1328. [Google Scholar] [CrossRef]
- Iu, H.T.; Fong, P.M.; Yam, H.C.; Gao, P.; Yan, B.; Lai, P.M.; Tang, V.Y.; Li, K.H.; Ma, C.W.; Ng, K.H.; et al. Identification of a Small Molecule Compound Active against Antibiotic-Tolerant Staphylococcus aureus by Boosting ATP Synthesis. Int. J. Mol. Sci. 2023, 24, 6242. [Google Scholar] [CrossRef]
- Li, X.T.; Thomason, L.C.; Sawitzke, J.A.; Costantino, N.; Court, D.L. Positive and negative selection using the tetA-sacB cassette: Recombineering and P1 transduction in Escherichia coli. Nucleic Acids Res. 2013, 41, 204. [Google Scholar] [CrossRef]
- Shilling, P.J.; Khananisho, D.; Cumming, A.J.; Soderstrom, B.; Daley, D.O. Signal amplification of araC pBAD using a standardized translation initiation region. Synth. Biol. 2022, 7, 9. [Google Scholar] [CrossRef]
- Motohashi, K. A simple and efficient seamless DNA cloning method using SLiCE from Escherichia coli laboratory strains and its application to SLiP site-directed mutagenesis. BMC Biotechnol. 2015, 15, 47. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput.-Aid. Drug. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Wang, X.; Song, K.; Li, L.; Chen, L. Structure-Based Drug Design Strategies and Challenges. Curr. Top. Med. Chem. 2018, 18, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Dos, S.R.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed]
- Stone, L.K.; Baym, M.; Lieberman, T.D.; Chait, R.; Clardy, J.; Kishony, R. Compounds that select against the tetracycline-resistance efflux pump. Nat. Chem. Biol. 2016, 12, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Wang, Y.; Zhang, Y.; Wang, Z.; Wang, X.; Muhammad, I.; Kong, L.; Pei, Z.; Ma, H.; Jiang, X. High Cell Selectivity and Bactericidal Mechanism of Symmetric Peptides Centered on d-Pro–Gly Pairs. Int. J. Mol. Sci. 2020, 21, 1140. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Y.; Yang, K.; Tong, Z.; Shi, J.; Li, R.; Xiao, X.; Ren, W.; Hardeland, R.; Reiter, R.J.; et al. Melatonin overcomes MCR-mediated colistin resistance in Gram-negative pathogens. Theranostics 2020, 10, 10697–10711. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, M.; Zhang, Z.; Wang, L.; Wang, Y.; Liu, L.; Wang, D.; Zhang, X.; Zhao, L.; Zhao, Y.; et al. Guanethidine Restores Tetracycline Sensitivity in Multidrug-Resistant Escherichia coli Carrying tetA Gene. Antibiotics 2024, 13, 973. https://doi.org/10.3390/antibiotics13100973
Zhao X, Zhang M, Zhang Z, Wang L, Wang Y, Liu L, Wang D, Zhang X, Zhao L, Zhao Y, et al. Guanethidine Restores Tetracycline Sensitivity in Multidrug-Resistant Escherichia coli Carrying tetA Gene. Antibiotics. 2024; 13(10):973. https://doi.org/10.3390/antibiotics13100973
Chicago/Turabian StyleZhao, Xiaoou, Mengna Zhang, Zhendu Zhang, Lei Wang, Yu Wang, Lizai Liu, Duojia Wang, Xin Zhang, Luobing Zhao, Yunhui Zhao, and et al. 2024. "Guanethidine Restores Tetracycline Sensitivity in Multidrug-Resistant Escherichia coli Carrying tetA Gene" Antibiotics 13, no. 10: 973. https://doi.org/10.3390/antibiotics13100973
APA StyleZhao, X., Zhang, M., Zhang, Z., Wang, L., Wang, Y., Liu, L., Wang, D., Zhang, X., Zhao, L., Zhao, Y., Jin, X., Liu, X., & Ma, H. (2024). Guanethidine Restores Tetracycline Sensitivity in Multidrug-Resistant Escherichia coli Carrying tetA Gene. Antibiotics, 13(10), 973. https://doi.org/10.3390/antibiotics13100973




