Surveillance of Multidrug-Resistant Pathogens in Neonatal Intensive Care Units of Palermo, Italy, during SARS-CoV-2 Pandemic
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Aim of the Study
4.2. Collection of Samples and Microbiological Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development. Stemming the Superbug Tide: Just a Few Dollars More: OECD. 2019. Available online: https://www.oecd.org (accessed on 1 September 2023).
- Okeke, I.N.; Laxminarayan, R.; Bhutta, Z.A.; Duse, A.G.; Jenkins, P.; O’Brien, T.F.; Pablos-Mendez, A.; Klugman, K.P. Antimicrobial resistance in developing countries. Part I: Recent trends and current status. Lancet Infect. Dis. 2005, 5, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Day-Stirk, F.; McConville, F.; Campbell, J.; Laski, L.; Guerra-Arias, M.; Hoope-Bender, P.T.; Michel-Schuldt, M.; de Bernis, L. Delivering the evidence to improve the health of women and newborns: State of the World’s Midwifery, report 2014. Reprod. Health 2014, 17, 1189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef]
- Tewabe, T.; Mohammed, S.; Tilahun, Y.; Melaku, B.; Fenta, M.; Dagnaw, T.; Belachew, A.; Molla, A.; Belete, H. Clinical outcome and risk factors of neonatal sepsis among neonates in Felege Hiwot Referral Hospital, Bahir Dar, Amhara Regional State, North West Ethiopia 2016: A retrospective chart review. BMC Res. Notes 2017, 10, 265. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022–2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modeling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-20 (accessed on 3 August 2023).
- OECD. Antimicrobial Resistance: Tackling the Burden in the European Union, Briefing Note for EU/EEA Countries. 2019. Available online: https://www.oecd.org/health/health-systems/AMR-Tackling-the-Burden-in-the-EU-OECD-ECDC-Briefing-Note-2019.pdf (accessed on 3 August 2023).
- Piano Nazionale di Contrasto dell’Antimicrobico-Resistenza (PNCAR) 2017–2020. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2660 (accessed on 3 August 2023).
- Pascale, R.; Bussini, L.; Gaibani, P.; Bovo, F.; Fornaro, G.; Lombardo, D.; Giannella, M. Carbapenem-resistant bacteria in an intensive care unit during the coronavirus disease 2019 (COVID-19) pandemic: A multicenter before-and-after cross-sectional study. Infect. Control Hosp. Epidemiol. 2022, 43, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Sands, K.E.; Huang, S.S.; Kleinman, K.; Septimus, E.J.; Varma, N.; Blanchard, J.; Poland, R.E.; Coady, M.H.; Yokoe, D.S.; et al. CDC Prevention Epicenters Program. The Impact of Coronavirus Disease 2019 (COVID-19) on Healthcare-Associated Infections. Clin. Infect. Dis. 2022, 74, 1748–1754. [Google Scholar] [CrossRef]
- Giuffrè, M.; Ricchizzi, E.; Accogli, M.; Barbanti, F.; Monaco, M.; Pimentel de Araujo, F.; Farina, C.; Fazii, P.; Mattei, R.; Sarti, M.; et al. Colonization by multidrug-resistant organisms in long-term care facilities in Italy: A point-prevalence study. Clin. Microbiol. Infect. 2017, 23, 961–967. [Google Scholar] [CrossRef]
- Tomczyk, S.; Taylor, A.; Brown, A.; de Kraker, M.E.A.; El-Saed, A.; Alshamrani, M.; Hendriksen, R.S.; Jacob, M.; Löfmark, S.; Perovic, O.; et al. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: A global survey. J. Antimicrob. Chemother. 2021, 76, 3045–3058. [Google Scholar] [CrossRef]
- Claud, E.C. Neonatal Necrotizing Enterocolitis-Inflammation and Intestinal Immaturity. Anti Inflamm. Anti Allergy Agents Med. Chem. 2009, 8, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Milic, M.; Siljic, M.; Cirkovic, V.; Jovicevic, M.; Perovic, V.; Markovic, M.; Martic, J.; Stanojevic, M.; Mijac, V. Colonization with Multidrug-Resistant Bacteria in the First Week of Life among Hospitalized Preterm Neonates in Serbia: Risk Factors and Outcomes. Microorganisms 2021, 9, 2613. [Google Scholar] [CrossRef] [PubMed]
- Al-Humaidan, O.S.; El-Kersh, T.A.; Al-Akeel, R.A. Risk factors of nasal carriage of Staphylococcus aureus and methicillin-resistant S. aureus among health care staff in a teaching hospital in central Saudi Arabia. Saudi Med. J. 2015, 36, 1084–1090. [Google Scholar] [CrossRef]
- Cipolla, D.; Giuffrè, M.; Mammina, C.; Corsello, G. Prevention of nosocomial infections and surveillance of emerging resistances in NICU. J. Matern. Neonatal Med. 2011, 24 (Suppl. S1), 23–26. [Google Scholar] [CrossRef] [PubMed]
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Banerjee, R.; Schwenk, H. Antibiotic Stewardship for the Neonatologist and Perinatologist. Clin. Perinatol. 2021, 48, 379–391. [Google Scholar] [CrossRef]
- Das, P.; Singh, A.K.; Pal, T.; Dasgupta, S.; Ramamurthy, T.; Basu, S. Colonization of the gut with Gram-negative bacilli, its association with neonatal sepsis and its clinical relevance in a developing country. J. Med. Microbiol. 2011, 60 Pt 11, 1651–1660. [Google Scholar] [CrossRef]
- Ramirez, C.B.; Cantey, J.B. Antibiotic Resistance in the Neonatal Intensive Care Unit. Neoreviews 2019, 20, e135–e144. [Google Scholar] [CrossRef]
- Pinheiro, F.R.; Rozza-de-Menezes, R.E.; Blum, M.C.; Pereira, R.F.A.; Rocha, J.A.; Guedes Pinto, M.C.F.; Penna, B.A.; Riley, L.W.; Agular-Alves, F. Evaluation of changes in antimicrobial susceptibility in bacteria infecting children and their mothers in pediatric, neonatal-intensive care unit, and gynecology/obstetrics wards of a quaternary referral hospital during the COVID-19 pandemic. Front. Microbiol. 2023, 14, 1096223. [Google Scholar] [CrossRef]
- Thoma, R.; Seneghini, M.; Seiffert, S.N.; Vuichard Gysin, D.; Scanferla, G.; Haller, S.; Flury, D.; Boggian, K.; Kleger, G.R.; Filipovic, M.; et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist. Infect. Control 2022, 11, 12. [Google Scholar] [CrossRef]
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodríguez-Baňo. Impact of COVID-19 pandemic on multidrug resistant gram positive and gram negative pathogens: A systematic review. J. Infect. Public Health 2023, 16, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, B.; Pelit, S.; Bulut, M.E.; Aktaş, E. Trend in Antibiotic Resistance of Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli and Klebsiella Pneumoniae Bloodstream Infections. Sisli Etfal Hastan. Tip Bul. 2019, 53, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Martischang, R.; François, P.; Cherkaoui, A.; Gaïa, N.; Renzi, G.; Agostinho, A.; Perez, M.; Graf, C.E.; Harbarth, S. Epidemiology of ESBL-producing Escherichia coli from repeated prevalence studies over 11 years in a long-term-care facility. Antimicrob. Resist. Infect. Control 2021, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Folgori, L.; Bernaschi, P.; Piga, S.; Carletti, M.; Cunha, F.P.; Lara, P.H.R.; de Castro Peixoto, N.C.; Guimarăes, B.G.A.; Sharland, M.; Araujo da Silva, A.R.; et al. Healthcare-associated infections in pediatric and neonatal intensive care units: Impact of underlying risk factors and antimicrobial resistance on 30-day case-fatality in Italy and Brazil. Infect. Control Hosp. Epidemiol. 2016, 37, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Patel, K.M.; Léger, M.M.; Short, B.; Sprague, B.M.; Kalu, N.; Campos, J.M. Risk of resistant infections with Enterobacteriaceae in hospitalized neonates. Pediatr. Infect. Dis. J. 2002, 21, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Lukac, P.J.; Bonomo, R.A.; Logan, L.K. Extended-spectrum β-lactamaseproducing Enterobacteriaceae in children: Old foe, emerging threat. Clin. Infect. Dis. 2015, 60, 1389–1397. [Google Scholar] [CrossRef]
- Dutta, S.; Kumar, P.; Paulpandian, R.; Saini, S.S.; Sreenivasan, P.; Mukhopadhyay, K.; Sundaram, V.; Kumar, J.; Ray, P. Relationship Between COVID-19 Lockdown and Epidemiology of Neonatal Sepsis. Pediatr. Infect. Dis. J. 2022, 41, 482–489. [Google Scholar] [CrossRef]
- Shbaklo, N.; Corcione, S.; Vicentini, C.; Giordano, S.; Fiorentino, D.; Bianco, G.; Cattel, F.; Cavallo, R.; Zotti, C.M.; De Rosa, F.G. An Observational Study of MDR Hospital-Acquired Infections and Antibiotic Use during COVID-19 Pandemic: A Call for Antimicrobial Stewardship Programs. Antibiotics 2022, 11, 695. [Google Scholar] [CrossRef]
- Saporito, L.; Graziano, G.; Mescolo, F.; Amodio, E.; Insinga, V.; Rinaudo, G.; Aleo, A.; Bonura, C.; Vitaliti, M.; Corsello, G.; et al. Efficacy of a coordinated strategy for containment of multidrug-resistant Gram-negative bacteria carriage in a Neonatal Intensive Care Unit in the context of an active surveillance program. Antimicrob. Resist. Infect. Control 2021, 10, 30. [Google Scholar] [CrossRef]
- Witt, L.S.; Howard-Anderson, J.R.; Jacob, J.T.; Gottlieb, L.B. The impact of COVID-19 on multidrug-resistant organisms causing healthcare-associated infections: A narrative review. JAC Antimicrob. Resist. 2022, 5, dlac130. [Google Scholar] [CrossRef]
- Cheikh, A.; Belefquih, B.; Chajai, Y.; Cheikhaoui, Y.; El Hassani, A.; Benouda, A. Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) colonization as a risk factor for developing ESBL infections in pediatric cardiac surgery patients: “retrospective cohort study”. BMC Infect. Dis. 2017, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Dezza, F.C.; Arcari, G.; Alessi, F.; Valeri, S.; Curtolo, A.; Sacco, F.; Ceccarelli, G.; Raponi, G.; Alessandri, F.; Mastroianni, C.M.; et al. Clinical Impact of COVID-19 on Multi-Drug-Resistant Gram-Negative Bacilli Bloodstream Infections in intensive care Unit Setting: Two Pandemics Compared. Antibiotics 2022, 11, 926. [Google Scholar] [CrossRef]
- Meschiari, M.; Onorato, L.; Bacca, E.; Orlando, G.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Santoro, A.; Sarti, M.; et al. Long-Term Impact of the COVID-19 Pandemic on In-Hospital Antibiotic Consumption and Antibiotic Resistance: A Time Series Analysis (2015–2021). Antibiotics 2022, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Ceparano, M.; Sciurti, A.; Isonne, C.; Baccolini, V.; Migliara, G.; Marzuillo, C.; Natale, F.; Terrin, G.; Villari, P.; The Collaborating Group. Incidence of Healthcare-Associated Infections in a Neonatal Intensive Care Unit before and during the COVID-19 Pandemic: A Four-Year Retrospective Cohort Study. J. Clin. Med. 2023, 12, 2621. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Barrett, R.E.; Fleiss, N.; Hansen, C.; Campbell, M.M.; Rychalsky, M.; Murdzek, C.; Krechevsky, K.; Abbott, M.; Allegra, T.; Blazevich, B.; et al. Reducing MRSA Infection in a New NICU During the COVID-19 Pandemic. Pediatrics 2023, 151, e2022057033. [Google Scholar] [CrossRef]
- The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book. Available online: https://www.who.int/publications/i/item (accessed on 1 September 2023).
- De Giglio, O.; Diella, G.; Lopuzzo, M.; Triggiano, F.; Calia, C.; Pousis, C.; Fasano, F.; Caggiano, G.; Calabrese, G.; Rafaschieri, V.; et al. Impact of lockdown on the microbiological status of the hospital water network during COVID-19 pandemic. Environ. Res. 2020, 191, 110231. [Google Scholar] [CrossRef]
- EUCAST: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://eucast.org/clinical_breakpoints/ (accessed on 3 August 2023).
- Giuffrè, M.; Geraci, D.M.; Bonura, C.; Saporito, L.; Graziano, G.; Insinga, V.; Aleo, A.; Vecchio, D.; Mammina, C. The Increasing Challenge of Multidrug-Resistant Gram-Negative Bacilli: Results of a 5-Year Active Surveillance Program in a Neonatal Intensive Care Unit. Medicine 2016, 95, e3016. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2017. 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2017 (accessed on 3 August 2023).
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk difusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baňo, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20 (Suppl. S1), 1–55. [Google Scholar] [CrossRef]
- Corbella, M.; Caltagirone, M.; Gaiarsa, S.; Mariani, B.; Sassera, D.; Bitar, I.; Muzzi, A.; Migliavacca, R.; Scudeller, L.; Stronati, M.; et al. Characterization of an outbreak of extended-spectrum β-lactamase producing klebsiella pneumoniae in a neonatal intensive care unit in Italy. Microb. Drug Resist. 2018, 24, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Goering, R.V.; Tenover, F.C. Epidemiological interpretation of chromosomal macro-restriction fragment patterns analyzed by pulsed-feld gel electrophoresis. J. Clin. Microbiol. 1997, 35, 2432–2433. [Google Scholar] [CrossRef] [PubMed]
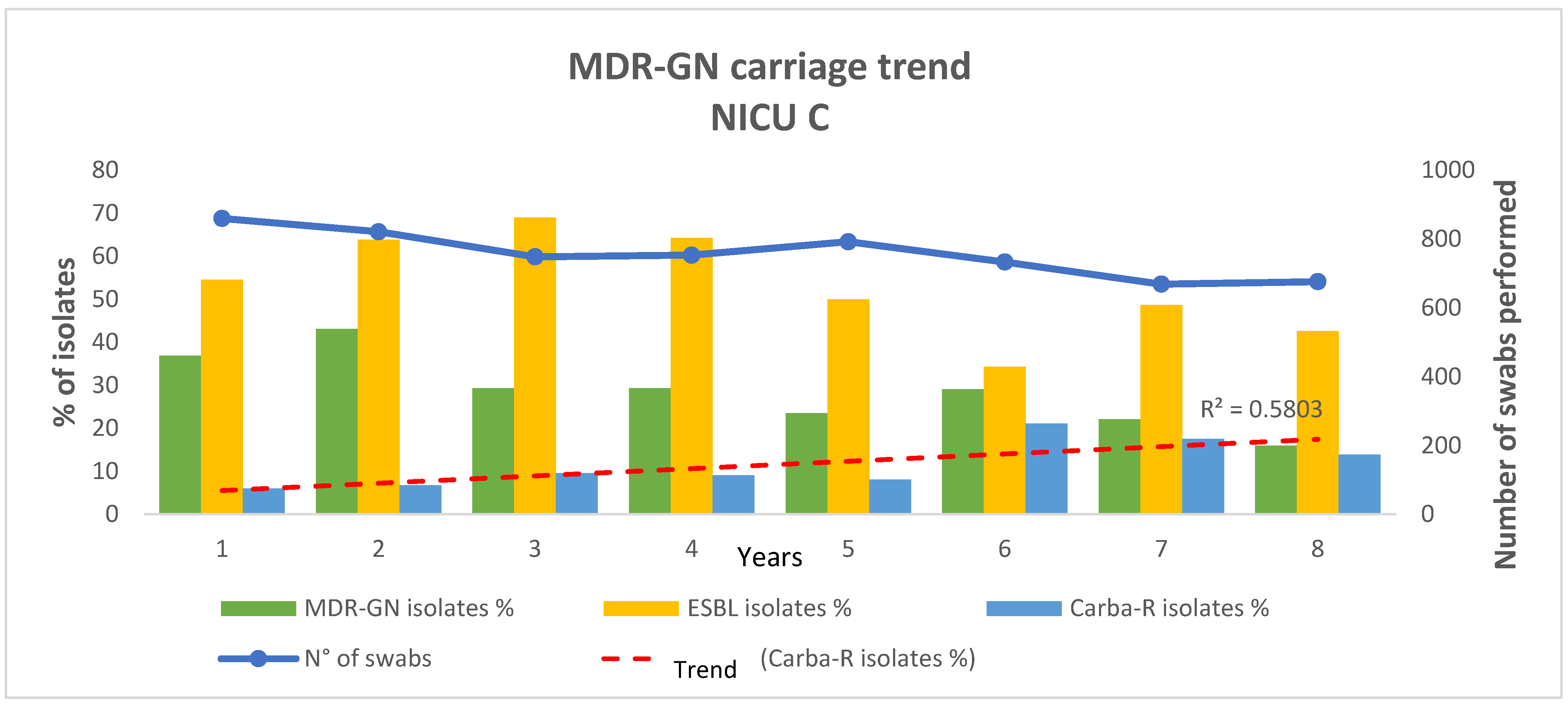
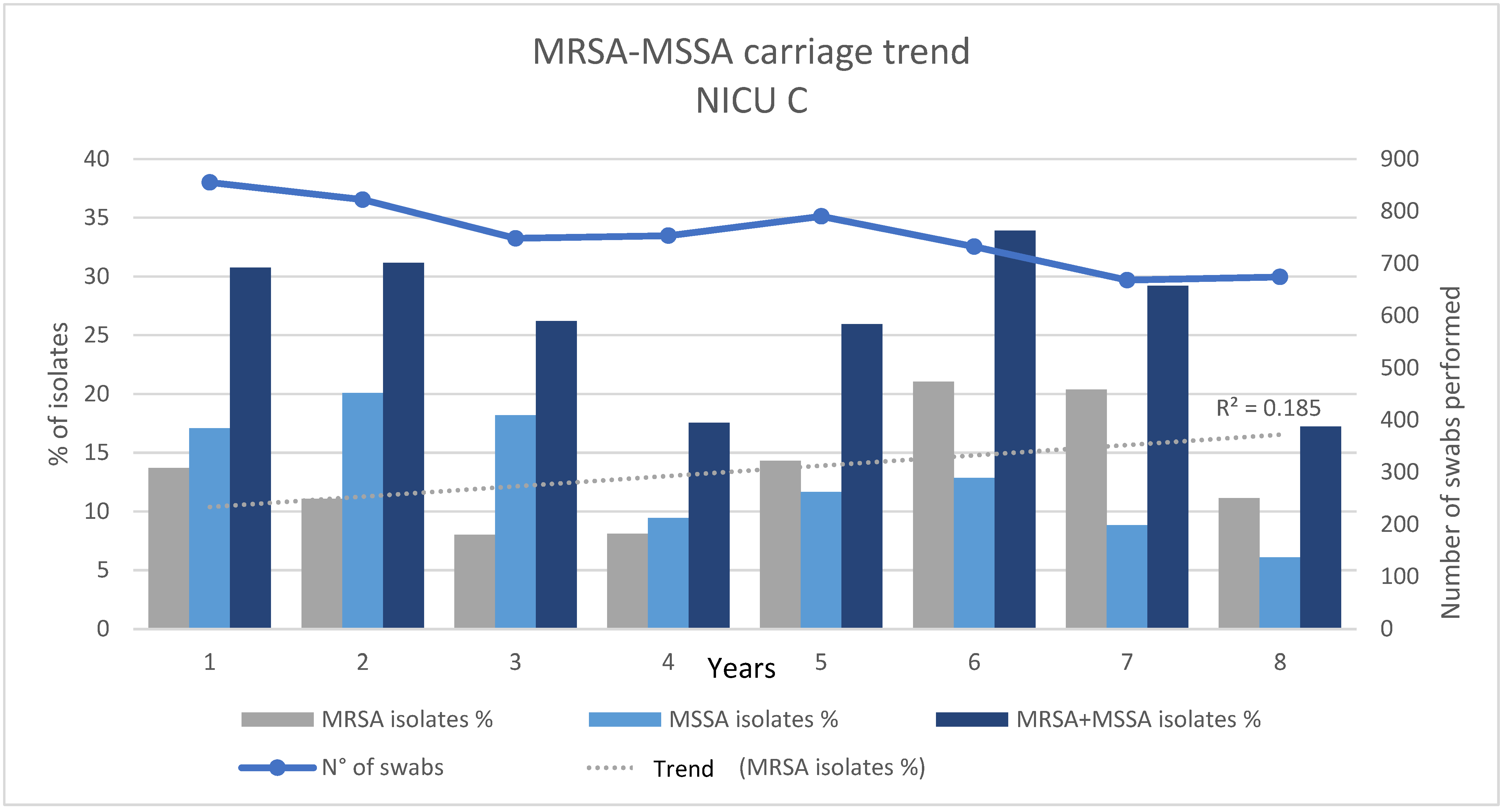
| NICU C | |||
|---|---|---|---|
| March 2014–February 2019 “Pre—Pandemic Period” | March 2020–February 2022 “Pandemic Period” | ||
| Number of rectal swabs | 4707 | 1345 | |
| MDR-GNB | Number of MDR-GNB positive swabs | 1386 | 241 |
| Number of MDR-GNB isolates | 1510 | 256 | |
| % (MDR-GNB isolates/Number of rectal swabs) | 32.08 | 19.03 | |
| ESBL+ | Number of ESBL+ isolates | 858 | 118 |
| % (ESBL+ isolates/Number of MDR-GNB isolates) | 56.82 | 46.09 | |
| % (EBSL+ isolates/Number of rectal swabs) | 18.23 | 8.77 | |
| CARBA-R+ | Number of CARBA-R+ isolates | 144 | 41 |
| % (CARBA-R+ isolates/Number of MDR-GNB isolates) | 9.54 | 16.02 | |
| % (CARBA-R+ isolates/Number of rectal swabs) | 3.06 | 3.05 | |
| Number of nasal swabs | 4700 | 1342 | |
| MRSA/MSSA | Number of MRSA isolates | 596 | 211 |
| % (MRSA isolates/Number of nasal swabs) | 12.68 | 15.72 | |
| Number of MSSA isolates | 704 | 100 | |
| % (MSSA isolates/Number of nasal swabs) | 14.98 | 7.45 | |
| Number of MRSA and MSSA isolates | 1300 | 311 | |
| % (MRSA and MSSA isolates/Number of nasal swabs) | 27.66 | 23.17 | |
| NICU C | |||
|---|---|---|---|
| p-Value March 2014–February 2019 vs. March 2020–February 2021 | adjOR–95% C.I. | ||
| MDR-GNB | Number of MDR-GNB positive swabs/Number of rectal swabs | p < 0.0001 | 1.91 (1.61–2.23) |
| Number of MDR-GNB isolates/Number of rectal swabs | p < 0.0001 | 2.01 (1.73–2.33) | |
| ESBL+ | Number of EBSL+ isolates/Number of rectal swabs | p < 0.0001 | 2.32 (1.89–2.84) |
| Number of ESBL+ isolates/Number of MDR-GNB isolates | p < 0.05 | 1.54 (1.18–2.01) | |
| CARBA-R+ | Number of CARBA-R+ isolates/Number of rectal swabs | p = 0.96 | |
| Number of CARBA-R+ isolates/Number of MDR-GNB isolates | p < 0.05 | 0.55 (0.38–0.80) | |
| MRSA/MSSA | Number of MRSA isolates/Number of nasal swabs | p < 0.01 | 0.78 (0.66–0.92) |
| Number of MSSA isolates/Number of nasal swabs | p < 0.0001 | 2.20 (1.77–2.76) | |
| Number of MRSA + MSSA isolates/Number of nasal swabs | p < 0.01 | 1.27 (1.09–1.46) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graziano, G.; Notarbartolo, V.; Priano, W.; Maida, C.M.; Insinga, V.; Rinaudo, G.; Russo, A.; Palermo, R.; Vitale, F.; Giuffrè, M. Surveillance of Multidrug-Resistant Pathogens in Neonatal Intensive Care Units of Palermo, Italy, during SARS-CoV-2 Pandemic. Antibiotics 2023, 12, 1457. https://doi.org/10.3390/antibiotics12091457
Graziano G, Notarbartolo V, Priano W, Maida CM, Insinga V, Rinaudo G, Russo A, Palermo R, Vitale F, Giuffrè M. Surveillance of Multidrug-Resistant Pathogens in Neonatal Intensive Care Units of Palermo, Italy, during SARS-CoV-2 Pandemic. Antibiotics. 2023; 12(9):1457. https://doi.org/10.3390/antibiotics12091457
Chicago/Turabian StyleGraziano, Giorgio, Veronica Notarbartolo, Walter Priano, Carmelo Massimo Maida, Vincenzo Insinga, Grazia Rinaudo, Arianna Russo, Roberta Palermo, Francesco Vitale, and Mario Giuffrè. 2023. "Surveillance of Multidrug-Resistant Pathogens in Neonatal Intensive Care Units of Palermo, Italy, during SARS-CoV-2 Pandemic" Antibiotics 12, no. 9: 1457. https://doi.org/10.3390/antibiotics12091457
APA StyleGraziano, G., Notarbartolo, V., Priano, W., Maida, C. M., Insinga, V., Rinaudo, G., Russo, A., Palermo, R., Vitale, F., & Giuffrè, M. (2023). Surveillance of Multidrug-Resistant Pathogens in Neonatal Intensive Care Units of Palermo, Italy, during SARS-CoV-2 Pandemic. Antibiotics, 12(9), 1457. https://doi.org/10.3390/antibiotics12091457






