Evaluation of Antimicrobial Resistance Profiles of Bacteria Isolated from Biofilm in Meat Processing Units
Abstract
1. Introduction
2. Results
2.1. Total Aerobic Colony Count Level
2.2. Enterobacteriaceae Level
2.3. Identification and Antimicrobial Resistance Profiles
3. Discussions
4. Materials and Methods
4.1. Sample Collection and Bacterial Isolation
4.2. Antimicrobial Susceptibility Testing
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rega, M.; Andriani, L.; Cavallo, S.; Bonilauri, P.; Bonardi, S.; Conter, M.; Carmosino, I.; Bacci, C. Antimicrobial Resistant E. coli in Pork and Wild Boar Meat: A Risk to Consumers. Foods 2022, 11, 3662. [Google Scholar] [CrossRef]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies Against Multidrug-Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 15 November 2022).
- European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Available online: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/european-surveillance-veterinary-antimicrobial-consumption-esvac (accessed on 5 January 2023).
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Gerlach, R. An overview of biofilm formation–combating strategies and mechanisms of action of antibiofilm agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, T.M.; Chow, L.; Asher, A.J.; Waldron, L.S.; Gillings, M.R. Evolution of class 1 integrons: Mobilization and dispersal via foodborne bacteria. PLoS ONE 2017, 12, e0179169. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry-A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bauermeister, L.J.; Hill, G.N.; Singh, M.; Bilgili, S.F.; McKee, S.R. Efficacy of various antimicrobials on reduction of Salmonella and Campylobacter and quality attributes of ground chicken obtained from poultry parts treated in a postchill decontamination tank. J. Food Prot. 2014, 77, 1882–1888. [Google Scholar] [CrossRef]
- Chylkova, T.; Cadena, M.; Ferreiro, A.; Pitesky, M. Susceptibility of Salmonella biofilm and planktonic bacteria to common disinfectant agents used in poultry processing. J. Food Prot. 2017, 80, 1072–1079. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galie, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Morente, E.O.; Fernandez-Fuentes, M.A.; Burgos, M.J.G.; Abriouel, H.; Pulido, R.P.; Galvez, A. Biocide tolerance in bacteria. Int. J. Food Microbiol. 2013, 162, 13–25. [Google Scholar] [CrossRef]
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Piras, F.; Fois, F.; Consolati, S.G.; Mazza, R.; Mazzette, R. Influence of temperature, source, and serotype on biofilm formation of Salmonella enterica isolates from pig slaughterhouses. J. Food Prot. 2015, 78, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Barco, L.; Roccato, A.; Ricci, A. Escherichia coli and Enterobacteriaceae counts on poultry carcasses along the slaughterline: A systematic review and meta-analysis. Food Control 2016, 60, 269–280. [Google Scholar] [CrossRef]
- Monger, X.C.; Gilbert, A.A.; Saucier, L.; Vincent, A.T. Antibiotic Resistance: From Pig to Meat. Antibiotics 2021, 10, 1209. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1441/2007 of 5 December 2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:322:0012:0029:EN:PDF (accessed on 1 November 2022).
- Dan, S.D.; Tabaran, A.; Mihaiu, L.; Mihaiu, M. Antibiotic susceptibility and prevalence of foodborne pathogens in poultry meat in Romania. J. Infect. Dev. Ctries. 2015, 9, 035–041. [Google Scholar] [CrossRef] [PubMed]
- Forgaciu, A.; Tabaran, A.; Colobatiu, L.; Mihaiu, R.; Dan, S.D.; Mihaiu, M. Concerning Increase in Antimicrobial Resistance Patterns of Pathogenic Strains of Salmonella Isolated in Poultry Meat Products. Antibiotics 2022, 11, 1469. [Google Scholar] [CrossRef]
- Zaulet, M.; Dumitrache, R.; Tanasuica, R.; Nichita, C.; Kevorkian, S.E.M.; Buburuzan, L. Prevalence of some foodborne pathogens in meat products in Romania. Rom. Biotechnol. Lett. 2016, 21, 11949–11958. [Google Scholar]
- Gaspar, C.M.; Cocora, Z.M.; Brudiu, I.; Lazarescu, C.F.; Popovici, R.A.; Tigmeanu, C.V.; Tibru, I. Absorbent food PADS from meat packages–potential source of contamination. Rev. De Chim. 2019, 70, 784–789. [Google Scholar] [CrossRef]
- Imre, K.; Herman, V.; Morar, A. Scientific achievements in the study of the occurrence and antimicrobial susceptibility profile of major foodborne pathogenic bacteria in foods and food processing environments in Romania: Review of the last decade. BioMed Res. Int. 2020, 5134764. [Google Scholar] [CrossRef]
- Institutul Național de Statistică. Available online: http://statistici.insse.ro:8077/tempo-online/#/pages/tables/insse-table (accessed on 21 March 2023).
- Barco, L.; Belluco, S.; Roccato, A.; Ricci, A. A systematic review of studies on Escherichia coli and Enterobacteriaceae on beef carcasses at the slaughterhouse. Int. J. Food Microbiol. 2015, 207, 30–39. [Google Scholar] [CrossRef]
- Nakamura, A.; Takahashi, H.; Koike, F.; Kuda, T.; Kobayashi, M. Transition of microbial contamination on the surface of carcass during the cattle slaughter process. Food Microbiol. 2023, 112, 104245. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Moussa, I.; Abalkhail, A.; Ibrahem, M.; Rawway, M. Pseudomonas species prevalence, protein analysis, and antibiotic resistance: An evolving public health challenge. AMB Express 2022, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Brinas, L.; Zarazaga, M.; Sáenz, Y.; Ruiz-Larrea, F.; Torres, C. β-Lactamases in ampicillin-resistant Escherichia coli isolates from foods, humans, and healthy animals. Antimicrob. Agents Chemother. 2002, 46, 3156–3163. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chang, J.; Zhang, W.; Lin, J.; Yin, J.; Yin, Y.; Su, Y. Analysis of Escherichia coli Resistance to Ampicillin. Research Square 2023. preprint (Version 1). [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2021 Zoonoses Report; The European Union: Maastricht, The Netherlands, 2021. [Google Scholar]
- Giedraitiene, A.; Pereckaite, L.; Bredelyte-Gruodiene, E.; Virgailis, M.; Ciapiene, I.; Tatarunas, V. CTX-M-producing Escherichia coli strains: Resistance to temocillin, fosfomycin, nitrofurantoin and biofilm formation. Future Microbiol. 2022, 17, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Reffuveille, F.; Fuente-Núñez Cde, L.; Fairfull-Smith, K.E.; Hancock, R.E. Potentiation of ciprofloxacin action against Gram-negative bacterial biofilms by a nitroxide. Pathog. Dis. 2015, 73, ftv016. [Google Scholar] [CrossRef]
- Verderosa, A.D.; de la Fuente-Núñez, C.; Mansour, S.C.; Cao, J.; Lu, T.K.; Hancock, R.E.W. Ciprofloxacin-nitroxide hybrids with potential for biofilm control. Eur. J. Med. Chem. 2017, 138, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Pavlickova, S.; Klancnik, A.; Dolezalova, M.; Mozina, S.S.; Holko, I. Antibiotic resistance, virulence factors and biofilm formation ability in Escherichia coli strains isolated from chicken meat and wildlife in the Czech Republic. J. Environ. Sci. Health Part B 2017, 52, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Rasschaert, G.; Van Elst, D.; Colson, L.; Herman, L.; de Carvalho Ferreira, H.C.; Dewulf, J.; Decrop, J.; Meirlaen, J.; Heyndrickx, M.; Daeseleire, E. Antibiotic Residues and Antibiotic-Resistant Bacteria in Pig Slurry Used to Fertilize Agricultural Fields. Antibiotics 2020, 9, 34. [Google Scholar] [CrossRef]
- Barilli, E.; Vismarra, A.; Villa, Z.; Bonilauri, P.; Bacci, C.E.S.L.E. ESβL E. coli isolated in pig’s chain: Genetic analysis associated to the phenotype and biofilm synthesis evaluation. Int. J. Food Microbiol. 2019, 289, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.A.; Nero, L.A.; Silva, L.C.; d’Ovidio, L.; Monteiro, F.A.; Tamanini, R. Listeria monocytogenes: Occurrence in beef and identification of the main contamination points in processing plants. Meat Sci. 2007, 76, 591–596. [Google Scholar] [CrossRef] [PubMed]
- ISO 17604:2015; Microbiology of the Food Chain—Carcass Sampling for Microbiological Analysis. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 4833:2003; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Microorganisms—Colony-Count Technique at 30 Degrees C. International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-bromo-4-chloro-3-indolyl Beta-D-Glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 13720:2010; Meat and Meat Products—Enumeration of Presumptive Pseudomonas spp. International Organization for Standardization: Geneva, Switzerland, 2010.
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Monnet, D.L. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Morar, A.; Ban-Cucerzan, A.; Herman, V.; Tîrziu, E.; Sallam, K.I.; Abd-Elghany, S.M.; Imre, K. Multidrug Resistant Coagulase-Positive Staphylococcus aureus and Their Enterotoxins Detection in Traditional Cheeses Marketed in Banat Region, Romania. Antibiotics 2021, 10, 1458. [Google Scholar] [CrossRef]
- Imre, K.; Ban-Cucerzan, A.; Herman, V.; Sallam, K.I.; Cristina, R.T.; Abd-Elghany, S.M.; Morar, D.; Popa, S.A.; Imre, M.; Morar, A. Occurrence, Pathogenic Potential and Antimicrobial Resistance of Escherichia coli Isolated from Raw Milk Cheese Commercialized in Banat Region, Romania. Antibiotics 2022, 11, 721. [Google Scholar] [CrossRef] [PubMed]
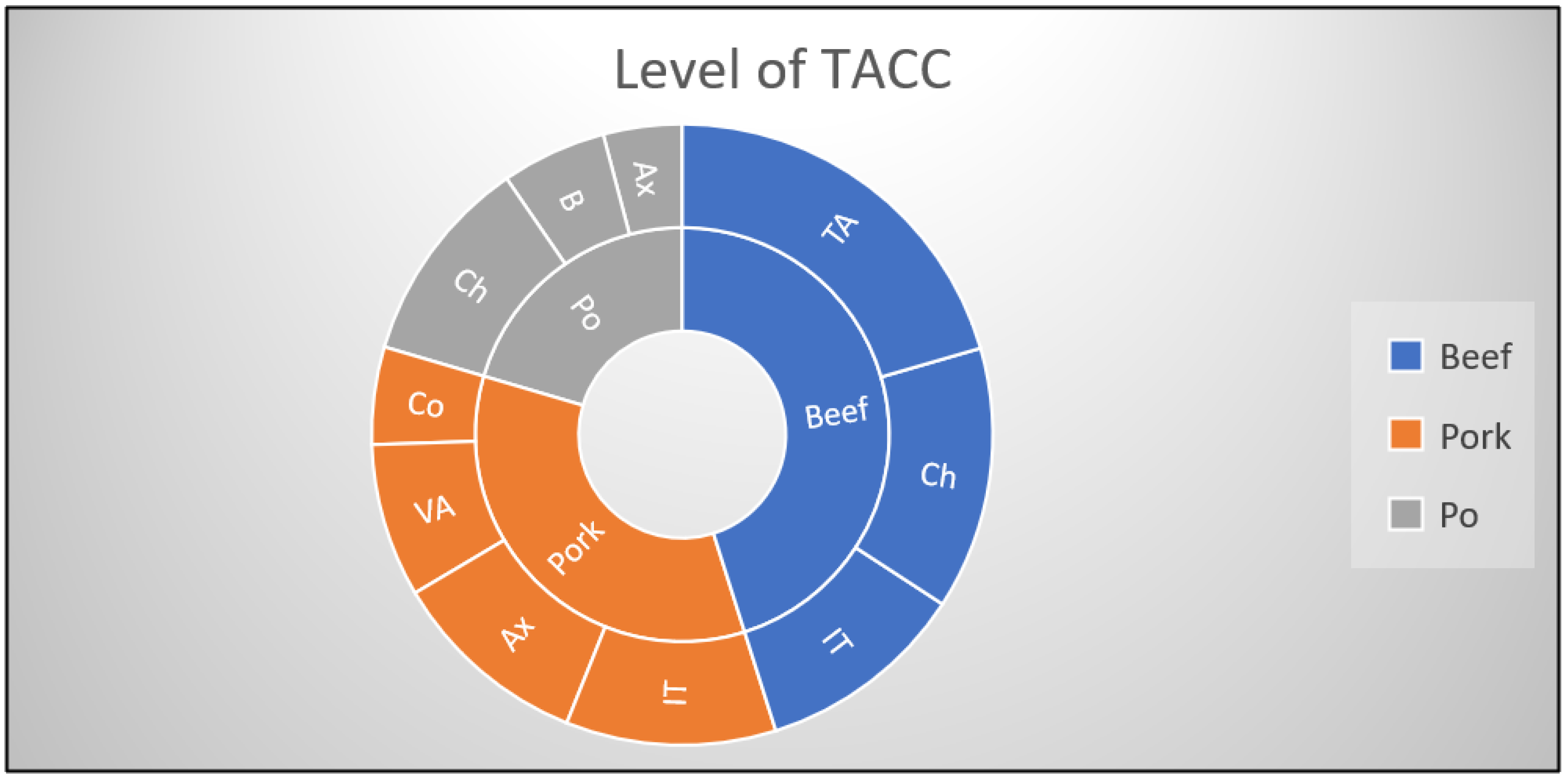
| Origin of Carcass | No. and (%) of Samples with Different Levels of TACC (log CFU/cm2) | No. and (%) of Samples with Different Levels of Enterobacteriaceae (log CFU/cm2) | |||
|---|---|---|---|---|---|
| Below the Regulatory Limits | Above the Regulatory Limits | Below the Regulatory Limits | Above the Regulatory Limits [17] | ||
| <5.0 log CFU/cm2 | >5.0 log CFU/cm2 | <1.5 log CFU/cm2 | >1.5 log <2.5 log CFU/cm2 | >2.5 log CFU/cm2 | |
| Beef (n = 35) | 30 (85.7) | 5 (14.2) | 20 (57.1) | 10 (28.5) | 5 (14.2) |
| Pork (n = 50) | 45 (90) | 5 (10) | 25 (50) | 5 (10) | 20 (40) |
| Poultry (n = 45) | 45 (100) | - | - | 15 (30) | 30 (60) |
| TOTAL (n = 130) | 120 (92.3) | 10 (7.6) | 45 (34.6) | 30 (23) | 55 (42.3) |
| Origin of Carcass | No. of Samples Containing | |||
|---|---|---|---|---|
| E. coli (%) | Enterobacter (%) | Serratia (%) | Pseudomonas (%) | |
| Beef (n = 35) | - | 5 (14.2) | 10 (28.5) | 10 (28.5) |
| Pork (n = 50) | 30 (60) | 20 (40) | 15 (30) | 10 (20) |
| Poultry (n = 45) | 10 (22.2) | - | 15 (33.3) | - |
| TOTAL (n = 130) | 40 (30.7) | 25 (19.2) | 40 (30.7) | 20 (15.3) |
| Origin of Samples | Vitek Card Used | |||
|---|---|---|---|---|
| AST-GN27 | AST-N093 | |||
| E. coli | E. coli | P. aeruginosa | P. putida | |
| Beef (n = 10) | 6 | - | 2 | 2 |
| Pork (n = 32) | 26 | 2 | 2 | 2 |
| Poultry (n = 10) | 10 | - | - | - |
| TOTAL (n = 52) | 42 | 2 | 4 | 4 |
| Antimicrobial | Susceptibility Test Result of 42 E. coli Strains (n/%) | |||
|---|---|---|---|---|
| Class | Agent | MIC Range µL/mL | R | S |
| β-lactams | AMP | ≥32 | 32 (76.1) | 10 (23.8) |
| AMC | 8 | 26 (61.9) | 16 (38.1) | |
| PIP | ≥128 | 8 (19) | 34 (80.9) | |
| TZP | ≤4 | - | 42 (100) | |
| cephalosporins | CFZ | ≤4 | 30 (71.4) | 12 (28.5) |
| CXT | ≤4 | 20 (47.6) | 22 (52.3) | |
| CTX | ≤1 | 4 (9.5) | 38 (90.4) | |
| CAZ | ≤1 | 2 (4.7) | 40 (95.2) | |
| CPM | ≤1 | 2 (4.7) | 40 (95.2) | |
| carbapenems | IMP | ≤1 | - | 42 (100) |
| aminoglycosides | AMK | ≤2 | 2 (4.7) | 40 (95.2) |
| GEN | ≤1 | 2 (4.7) | 40 (95.2) | |
| fluoroquinolones | CIP | ≤0.25 | 2 (4.7) | 40 (95.2) |
| NOR | 2 | 8 (19) | 34 (80.9) | |
| tetracyclines | TET | ≥16 | 16 (38.1) | 26 (61.9) |
| nitrofuran derivative | NIT | ≥16 | 22 (52.3) | 20 (47.6) |
| sulfonamides | SXT | ≤20 | 5 (11.9) | 37 (88.1) |
| Antimicrobial | Susceptibility Test Result of 10 Strains (n/%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Class | Agent | MIC Range µL/mL | P. aeruginosa | P. putida | E. coli | |||
| R | S | R | S | R | S | |||
| β-lactams | PIP | 32 | - | 4 | 4 | 2 | - | |
| TZP | 32 | 4 | 2 | 2 | - | 2 | ||
| TIC | 16 | 4 | - | 4 | - | 2 | - | |
| TIM | 16 | 4 | - | 4 | - | 2 | - | |
| cephalosporins | CAZ | 4 | - | 4 | 4 | 2 | - | |
| CPM | 2 | - | 4 | - | 4 | 2 | - | |
| carbapenems | IMP | 4 | - | 4 | - | 4 | - | 2 |
| MEM | ≤0.25 | - | 4 | - | 4 | - | 2 | |
| aminoglycosides | AMK | 8 | - | 4 | - | 4 | - | 2 |
| GEN | 2 | - | 4 | - | 4 | - | 2 | |
| ISP | 8 | - | 4 | - | 4 | - | 2 | |
| TOB | ≤1 | - | 4 | - | 4 | - | 2 | |
| fluoroquinolones | CIP | ≤0.25 | - | 4 | - | 4 | - | 2 |
| PEF | 1 | N.A. | N.A. | 2 | - | |||
| tetracyclines | MIN | 4 | N.A. | N.A. | - | 2 | ||
| sulfonamides | SXT | ≥320 | N.A. | N.A. | 2 | - | ||
| monobactams | AZM | 16 | 4 | - | 4 | - | 2 | - |
| polymyxins | COL | ≤0.5 | - | 4 | - | 4 | - | 2 |
| Crt. No. | Origin | No. of Isolates | Vitek Card Used | No. of Classes with Resistance | Resistance to Antimicrobial Profile | Classes with Resistance |
|---|---|---|---|---|---|---|
| 1. | Pork | 2 | AST-GN27 | 4 | AMP, AMC, PIP, CFZ, CXT, CTX, CAZ, CPM, NOR, NIT | β-lactams, cephalosporins, fluoroquinolones, nitrofuran derivative |
| 2. | Pork | 2 | AST-N093 | 5 | TIC, TIM, PIP, CAZ, CPM, PEF, SXT, AZM | β-lactams, cephalosporins, fluoroquinolones, sulfonamides, monobactams |
| 3. | Pork | 2 | AST-GN27 | 4 | AMP, AMC, CFZ, CXT, CTX, NIT, SXT | β-lactams, cephalosporins, nitrofuran derivative, sulfonamides |
| 4. | Pork | 2 | AST-GN27 | 5 | AMP, PIP, CFZ, NOR, TET, SXT | β-lactams, cephalosporins, nitrofuran derivatives, tetracyclines, sulfonamides |
| 5. | Pork | 2 | AST-GN27 | 4 | AMP, AMC, CFZ, CXT, TET, NIT | β-lactams, cephalosporins, nitrofuran derivative, tetracyclines |
| 6. | Beef | 2 | ||||
| 7. | Pork | 2 | AST-GN27 | 3 | AMP, AMC, CFZ, CXT, AMK, GEN | β-lactam, cephalosporins, aminoglycosides |
| 8. | Pork | 4 | AST-GN27 | 3 | AMP, AMC, CFZ, CXT, NIT | β-lactams, cephalosporins, nitrofuran derivative |
| 9. | Beef | 2 | ||||
| 10. | Poultry | 2 | ||||
| 11. | Poultry | 2 | AST-GN27 | 3 | AMP, PIP, CIP, NOR, TET | β-lactams, fluoroquinolones, tetracyclines |
| 12. | Poultry | 4 | AST-GN27 | 3 | AMP, AMC, CFZ, NIT | β-lactams, cephalosporins, nitrofuran derivative |
| 13. | Pork | 2 | AST-GN27 | 3 | AMP, PIP, NOR, TET | β-lactams, nitrofuran derivatives, tetracyclines |
| 14. | Poultry | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban-Cucerzan, A.; Morar, A.; Tîrziu, E.; Imre, K. Evaluation of Antimicrobial Resistance Profiles of Bacteria Isolated from Biofilm in Meat Processing Units. Antibiotics 2023, 12, 1408. https://doi.org/10.3390/antibiotics12091408
Ban-Cucerzan A, Morar A, Tîrziu E, Imre K. Evaluation of Antimicrobial Resistance Profiles of Bacteria Isolated from Biofilm in Meat Processing Units. Antibiotics. 2023; 12(9):1408. https://doi.org/10.3390/antibiotics12091408
Chicago/Turabian StyleBan-Cucerzan, Alexandra, Adriana Morar, Emil Tîrziu, and Kálmán Imre. 2023. "Evaluation of Antimicrobial Resistance Profiles of Bacteria Isolated from Biofilm in Meat Processing Units" Antibiotics 12, no. 9: 1408. https://doi.org/10.3390/antibiotics12091408
APA StyleBan-Cucerzan, A., Morar, A., Tîrziu, E., & Imre, K. (2023). Evaluation of Antimicrobial Resistance Profiles of Bacteria Isolated from Biofilm in Meat Processing Units. Antibiotics, 12(9), 1408. https://doi.org/10.3390/antibiotics12091408









