Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin–Tazobactam or Meropenem and Microbiological Outcome among Urologic Patients with Documented Gram-Negative Infections
Abstract
:1. Introduction
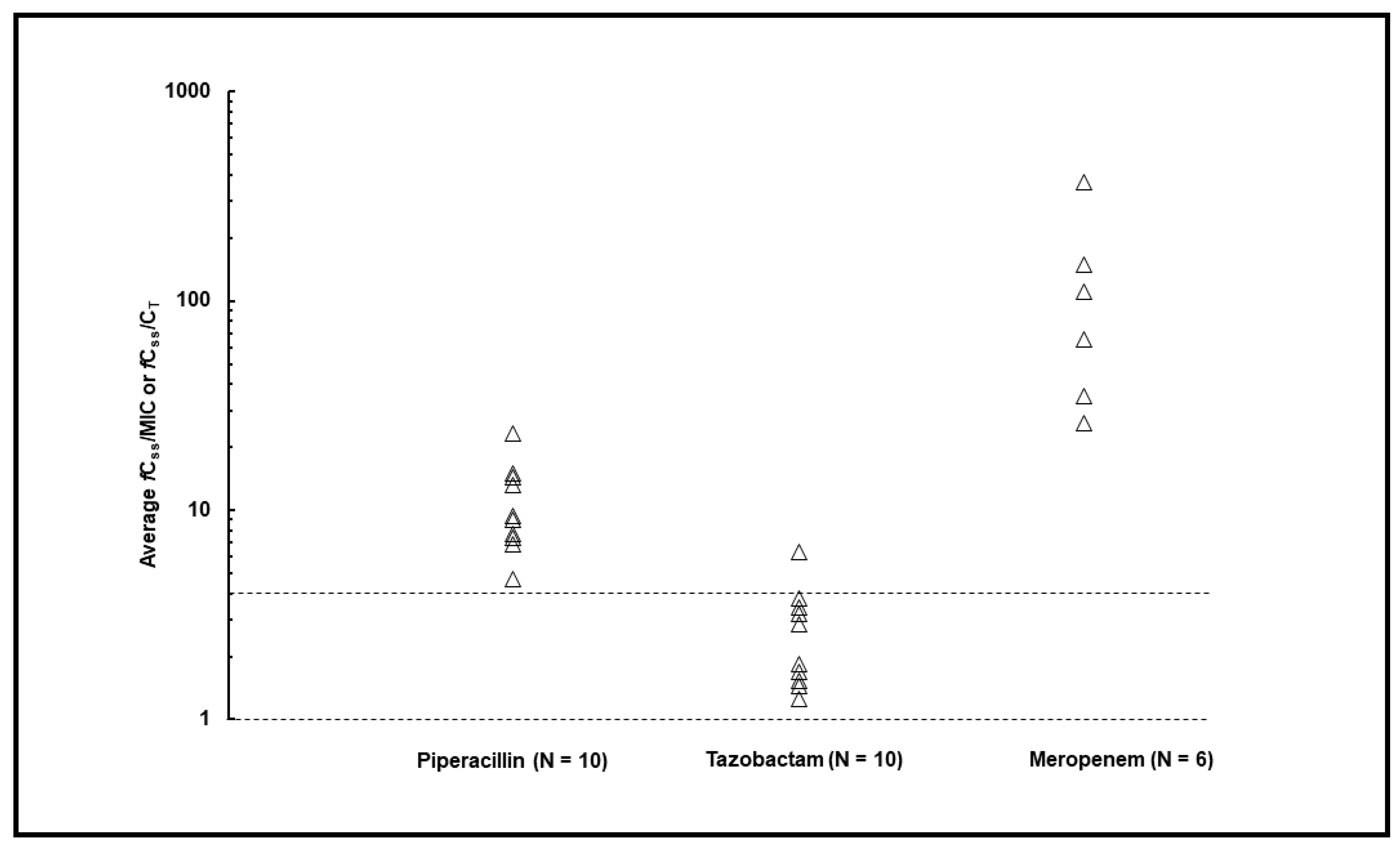
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Collection
4.3. Beta-Lactam Administration and Sampling Procedure
4.4. Relationship between Beta-Lactam PK/PD Target and Microbiological Outcome
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medina-Polo, J.; Naber, K.G.; Bjerklund Johansen, T.E. Healthcare-Associated Urinary Tract Infections in Urology. GMS Infect. Dis. 2021, 9, Doc05. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Harris, P.N.A.; Roberts, M.J.; Tambyah, P.A.; Schembri, M.A.; Pezzani, M.D.; Williamson, D.A.; Paterson, D.L. The Emerging Threat of Multidrug-Resistant Gram-Negative Bacteria in Urology. Nat. Rev. Urol. 2015, 12, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, L.; Luo, S.; Hu, D.; Zhao, X.; Zhao, G.; Tang, W.; Guo, Y. Analysis of Characteristics, Pathogens and Drug Resistance of Urinary Tract Infection Associated with Long-Term Indwelling Double-J Stent. Infect. Drug Resist. 2023, 16, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Hrbacek, J.; Cermak, P.; Zachoval, R. Current Antibiotic Resistance Trends of Uropathogens in Central Europe: Survey from a Tertiary Hospital Urology Department 2011–2019. Antibiotics 2020, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Guven, S.; Mert, A. Infections in Urology: Slow Progress Reflected in Clinical Practice. World J. Urol. 2020, 38, 2667–2668. [Google Scholar] [CrossRef]
- Kandil, H.; Cramp, E.; Vaghela, T. Trends in Antibiotic Resistance in Urologic Practice. Eur. Urol. Focus. 2016, 2, 363–373. [Google Scholar] [CrossRef]
- Giannella, M.; Malosso, P.; Scudeller, L.; Bussini, L.; Rebuffi, C.; Gatti, M.; Bartoletti, M.; Ianniruberto, S.; Pancaldi, L.; Pascale, R.; et al. Quality of Care Indicators in the Management of Bloodstream Infections Caused by Enterobacteriaceae (MAMBOO-E Study): State of the Art and Research Agenda. Int. J. Antimicrob. Agents 2021, 57, 106320. [Google Scholar] [CrossRef]
- Tam, V.H.; Chang, K.-T.; Zhou, J.; Ledesma, K.R.; Phe, K.; Gao, S.; Van Bambeke, F.; Sánchez-Díaz, A.M.; Zamorano, L.; Oliver, A.; et al. Determining β-Lactam Exposure Threshold to Suppress Resistance Development in Gram-Negative Bacteria. J. Antimicrob. Chemother. 2017, 72, 1421–1428. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Alexander, K.M.; Manigaba, K.; Shoulders, B.R.; Felton, T.W.; Mathew, S.K.; Peloquin, C.A. Beta-Lactam Target Attainment and Associated Outcomes in Patients with Bloodstream Infections. Int. J. Antimicrob. Agents 2023, 61, 106727. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Pai Mangalore, R.; Ashok, A.; Lee, S.J.; Romero, L.; Peel, T.N.; Udy, A.A.; Peleg, A.Y. Beta-Lactam Antibiotic Therapeutic Drug Monitoring in Critically Ill Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022, 75, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Codina, M.; Bozkir, H.Ö.; Jorda, A.; Zeitlinger, M. Individualized Antimicrobial Dose Optimization: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Microbiol. Infect. 2023, 29, 845–857. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic Drug Monitoring-Based Dose Optimisation of Piperacillin and Meropenem: A Randomised Controlled Trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Roberts, M.S.; Tiong, I.S.; Gardner, J.H.; Lehman, S.; Peake, S.L.; Hahn, U.; Warner, M.S.; Roberts, J.A. Can Therapeutic Drug Monitoring Optimize Exposure to Piperacillin in Febrile Neutropenic Patients with Haematological Malignancies? A Randomized Controlled Trial. J. Antimicrob. Chemother. 2015, 70, 2369–2375. [Google Scholar] [CrossRef]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of Therapeutic Drug Monitoring-Based Dose Optimization of Piperacillin/Tazobactam on Sepsis-Related Organ Dysfunction in Patients with Sepsis: A Randomized Controlled Trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.G.; Gatti, M.; Bonifazi, F.; Caramelli, F.; Castelli, A.; Cavo, M.; Cescon, M.; Corvaglia, L.T.; Lanari, M.; Marinelli, S.; et al. Impact of a Newly Established Expert Clinical Pharmacological Advice Program Based on TDM Results in Tailoring Antimicrobial Therapies Hospital-Wide in a Tertiary University Hospital: Findings after the First-Year of Implementation. Int. J. Antimicrob. Agents 2023, 62, 106884. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus Short-Term Intravenous Infusion of Antipseudomonal β-Lactams for Patients with Sepsis: A Systematic Review and Meta-Analysis of Randomised Trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Liu, J.; O’Donnell, J.N.; Dulhunty, J.M.; Abdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged Infusion Piperacillin-Tazobactam Decreases Mortality and Improves Outcomes in Severely Ill Patients: Results of a Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.-H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. A Meta-Analysis of Individual Patient Data from Randomized Trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Croom, K.; Adomakoh, N. Continuous Infusion of Beta-Lactam Antibiotics: Narrative Review of Systematic Reviews, and Implications for Outpatient Parenteral Antibiotic Therapy. Expert. Rev. Anti Infect. Ther. 2023, 21, 375–385. [Google Scholar] [CrossRef]
- Gatti, M.; Pea, F. Continuous versus Intermittent Infusion of Antibiotics in Gram-Negative Multidrug-Resistant Infections. Curr. Opin. Infect. Dis. 2021, 34, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; Louart, B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Kalimeris, G.D.; Triarides, N.A.; Falagas, M.E. An Update on Adverse Drug Reactions Related to β-Lactam Antibiotics. Expert. Opin. Drug Saf. 2018, 17, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Abodakpi, H.; Chang, K.-T.; Gao, S.; Sánchez-Díaz, A.M.; Cantón, R.; Tam, V.H. Optimal Piperacillin-Tazobactam Dosing Strategies against Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e01906-18. [Google Scholar] [CrossRef]
- Gatti, M.; Tam, V.H.; Gaibani, P.; Cojutti, P.G.; Viale, P.; Pea, F. A Novel Method to Evaluate Ceftazidime/Avibactam Therapy in Patients with Carbapenemase-Producing Enterobactericeae (CPE) Bloodstream Infections. Int. J. Antimicrob. Agents 2023, 61, 106760. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Merlau, P.R.; Hudson, C.S.; Kline, E.G.; Eales, B.M.; Smith, J.; Sofjan, A.K.; Shields, R.K. Optimal Ceftazidime/Avibactam Dosing Exposure against KPC-Producing Klebsiella Pneumoniae. J. Antimicrob. Chemother. 2022, 77, 3130–3137. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Borchardt, R.A.; Tzizik, D. Update on Surgical Site Infections: The New CDC Guidelines. JAAPA 2018, 31, 52–54. [Google Scholar] [CrossRef]
- Carlier, M.; Stove, V.; Verstraete, A.G.; De Waele, J.J. Stability of Generic Brands of Meropenem Reconstituted in Isotonic Saline. Minerva Anestesiol. 2015, 81, 283–287. [Google Scholar] [PubMed]
- Franceschi, L.; Cojutti, P.; Baraldo, M.; Pea, F. Stability of Generic Meropenem Solutions for Administration by Continuous Infusion at Normal and Elevated Temperatures. Ther. Drug Monit. 2014, 36, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, S.; Barton, S.; Whitney, L.; Swinden, J.; Nabhani-Gebara, S. Stability of Meropenem After Reconstitution for Administration by Prolonged Infusion. Hosp. Pharm. 2019, 54, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert Clinical Pharmacological Advice May Make an Antimicrobial TDM Program for Emerging Candidates More Clinically Useful in Tailoring Therapy of Critically Ill Patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. The Pharmacology of Meropenem, a New Carbapenem Antibiotic. Clin. Infect. Dis. 1997, 24 (Suppl. S2), S266–S275. [Google Scholar] [CrossRef] [PubMed]
- Sanz Codina, M.; Gatti, M.; Troisi, C.; Fornaro, G.; Pasquini, Z.; Trapani, F.; Zanoni, A.; Caramelli, F.; Viale, P.; Pea, F. Relationship between Pharmacokinetic/Pharmacodynamic Target Attainment and Microbiological Outcome in Critically Ill COVID-19 Patients with Documented Gram-Negative Superinfections Treated with TDM-Guided Continuous-Infusion Meropenem. Pharmaceutics 2022, 14, 1585. [Google Scholar] [CrossRef]
- Sörgel, F.; Kinzig, M. The Chemistry, Pharmacokinetics and Tissue Distribution of Piperacillin/Tazobactam. J. Antimicrob. Chemother. 1993, 31 (Suppl. SA), 39–60. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Pascale, R.; Cojutti, P.G.; Rinaldi, M.; Ambretti, S.; Conti, M.; Tedeschi, S.; Giannella, M.; Viale, P.; Pea, F. A Descriptive Pharmacokinetic/Pharmacodynamic Analysis of Continuous Infusion Ceftazidime/Avibactam in a Case Series of Renal Critically Ill Patients Treated for Documented Carbapenem-Resistant Gram-Negative Bloodstream Infections and/or Ventilator-Associated Pneumonia. Int. J. Antimicrob. Agents 2022, 61, 106699. [Google Scholar] [CrossRef]
- EUCAST-European Committee on Antimicrobial Susceptibility Testing. European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 12.0, Valid from 2022-01-01. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 15 July 2023).
| Demographics and Clinical Variables | Patients (N = 16) |
|---|---|
| Patient demographics | |
| Age (years) (median (IQR)) | 70.5 (65.25–74.0) |
| Gender (male/female) (n (%)) | 11/5 (68.75/31.25) |
| Body weight (Kg) (median (IQR)) | 71.5 (64.25–81.25) |
| Body mass index (Kg/m2) (median (IQR)) | 25.2 (23.7–27.7) |
| Charlson Comorbidity Index (median (IQR)) | 5 (3.75–8) |
| Average baseline creatinine clearance (mL/min/1.73 m2) (median (IQR)) | 41.0 (28.0–74.25) |
| Average baseline serum albumin levels (mg/dL) (median (IQR)) | 3.06 (2.99–3.30) |
| Average baseline white blood cell count (×103 µL) (median (IQR)) | 11.51 (8.48–23.00) |
| Average baseline serum CRP levels (mg/dL) (median (IQR)) | 21.65 (14.21–26.21) |
| Average baseline serum PCT levels (ng/mL) (median (IQR)) | 7.00 (1.08–64.43) |
| Surgical intervention (n (%)) | 11 (68.75) |
| Site of infection (n (%)) | |
| cUTI | 6 (37.5) |
| cUTI + BSI | 4 (25.0) |
| BSI | 4 (25.0) |
| SSI | 1 (6.25) |
| NSTI | 1 (6.25) |
| Isolated gram-negative pathogens 1 (n (%)) | |
| Escherichia coli | 7 (36.7) |
| Pseudomonas aeruginosa | 4 (21.1) |
| Klebsiella pneumoniae | 3 (15.8) |
| Enterobacter cloacae | 2 (10.5) |
| Klebsiella oxytoca | 1 (5.3) |
| Serratia marcescens | 1 (5.3) |
| Bacteroides fragilis | 1 (5.3) |
| Beta-lactam treatment | |
| Meropenem (n (%)) | 6 (37.5) |
| Piperacillin–tazobactam (n (%)) | 10 (62.5) |
| Beta-lactam TDM | |
| Meropenem dose (mg/day) (median (IQR)) | 2000 (2000–2000) |
| Piperacillin–tazobactam dose (mg/day) (median (IQR)) | 13,500 (9000–13,500) |
| Meropenem fCss (mg/L) (median (IQR)) | 11.4 (7.0–23.0) |
| Piperacillin fCss (mg/L) (median (IQR)) | 55.8 (35.4–80.4) |
| Tazobactam fCss (mg/L) (median (IQR)) | 7.4 (5.2–12.7) |
| Meropenem PK/PD target attainment | |
| fCss/MIC > 4 (n (%)) | 6 (100.0) |
| fCss/MIC = 1–4 (n (%)) | 0 (0.0) |
| fCss or Cmin/MIC < 1 (n (%)) | 0 (0.0) |
| Piperacillin–tazobactam joint PK/PD target attainment | |
| fCss/MIC > 4 (n (%)) | 10 (100.0) |
| fCss/MIC = 1–4 (n (%)) | 0 (0.0) |
| fCss or Cmin/MIC < 1 (n (%)) | 0 (0.0) |
| Expert clinical pharmacological advice | |
| Overall ECPAs | 30 |
| No. of TDM-guided ECPA per patient (median (IQR)) | 2 (1–2) |
| No. of dosage confirmed (n (%)) | 16 (53.4) |
| No. of dosage increases (n (%)) | 1 (3.3) |
| No. of dosage decreases (n (%)) | 13 (43.3) |
| First TDM assessment within desired range (n (%)) | 5 (31.25) |
| First TDM increase (n (%)) | 1 (6.25) |
| First TDM decrease (n (%)) | 10 (62.5) |
| Microbiological outcome | |
| Microbiological eradication (n (%)) | 14 (87.5) |
| Microbiological failure (n (%)) | 2 (12.5) |
| Resistance development (n (%)) | 2 (12.5) |
| Clinical cure (n (%)) | 13 (81.25) |
| ID Case | Age/Sex | CCI | Hospital Admission Diagnosis | Average CLCr (mL/min/ 1.73 m2) | Type of Infection | Pathogen | MIC (mg/L) | Beta-Lactam Initial Dosing | fCss/MIC Ratio | fCss/CT Ratio | Joint PK/PD Target | ECPA Recommendation at First TDM | Microbiological Eradication | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 82/M | 8 | Bladder cancer | 30.3 | cUTI | P. aeruginosa | 8 | PIP-TZB 13.5 g/day CI | 7.35 | 3.18 | Optimal | Decrease | Yes | Cured |
| #2 | 67/M | 2 | Inflammation of renal cyst | 69.7 | cUTI | E. coli | 8 | PIP-TZB 18 g/day CI | 7.68 | 1.45 | Optimal | Confirm | Yes | Cured |
| #3 | 48/F | 3 | Hydronephrosis | 18 | cUTI + BSI | E. coli | 4 | PIP-TZB 13.5 g/day CI | 15.07 | 2.85 | Optimal | Decrease | Yes | Cured |
| #4 | 68/M | 8 | Macrohematuria | 42 | BSI | ESBL-producing E. coli | 8 | PIP-TZB 13.5 g/day CI | 9.41 | 3.79 | Optimal | Decrease | Yes | Cured |
| #5 | 73/M | 5 | Hydronephrosis | 37 | cUTI | E. coli | 4 | PIP-TZB 13.5 g/day CI | 9.02 | 1.25 | Optimal | Confirm | Yes | Cured |
| #6 | 74/M | 5 | Hydronephrosis | 20 | cUTI + BSI | P. aeruginosa | 8 | PIP-TZB 13.5 g/day CI | 14.30 | 3.43 | Optimal | Decrease | Yes | Failed |
| #7 | 48/M | 3 | Renal transplant | 31 | cUTI + BSI | K. pneumoniae | 4 | PIP-TZB 9 g/day CI | 4.72 | 1.54 | Optimal | Confirm | Relapse BSI ESBL-producing K. pneumoniae | Failed |
| #8 | 60/M | 4 | Fournier gangrene | 16.5 | NSTI | K. oxytoca | 8 | PIP-TZB 13.5 g/day CI | 6.87 | 1.83 | Optimal | Decrease | Yes | Cured |
| #9 | 55/F | 1 | Hydronephrosis | 40 | BSI | ESBL-producing E. coli | 8 | PIP-TZB 18 g/day CI | 23.30 | 6.31 | Optimal | Decrease | Yes | Cured |
| #10 | 82/M | 12 | Bladder cancer | 71 | BSI | ESBL-producing K. pneumoniae | 4 | PIP-TZB 18 g/day CI | 13.22 | 1.68 | Optimal | Decrease | Yes | Cured |
| ID Case | Age/Sex | CCI | Hospital Admission Diagnosis | Average CLCr (mL/min/ 1.73 m2) | Type of Infection | Pathogen | MIC (mg/L) | Beta-Lactam Initial Dosing | fCss/MIC Ratio | PK/PD Target | ECPA Recommendation at First TDM | Microbiological Eradication | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | 74/M | 5 | Hydronephrosis | 46 | cUTI + BSI | AmpC-producing E. cloacae | 0.125 | MER 500 mg q6h CI | 111.48 | Optimal | Confirm | Yes | Cured |
| #2 | 68/F | 6 | Bladder cancer | 111.5 | SSI | B. fragilis | 0.125 | MER 500 mg q6h CI | 65.46 | Optimal | Confirm | Yes | Cured |
| #3 | 69/M | 8 | Bladder cancer | 110.5 | cUTI | E. coli | 0.125 | MER 500 mg q6h CI | 35.12 | Optimal | Increase | Yes | Cured |
| #4 | 72/F | 9 | Bladder cancer | 21 | cUTI | AmpC-producing S. marcescens | 1 | MER 500 mg q6h CI | 26.17 | Optimal | Decrease | Relapse BSI KPC-producing S. marcescens | Failed |
| #5 | 73/M | 7 | Bladder cancer | 97 | cUTI | P. aeruginosa/ESBL-producing E. coli | 0.125 | MER 1000 mg q6h CI | 370.77 | Optimal | Decrease | Yes | Cured |
| #6 | 75/F | 5 | Bladder cancer | 84 | BSI | ESBL-producing K. pneumoniae | 0.125 | MER 500 mg q6h CI | 149.04 | Optimal | Decrease | Yes | Cured |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berrino, P.M.; Gatti, M.; Rinaldi, M.; Brunocilla, E.; Viale, P.; Pea, F. Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin–Tazobactam or Meropenem and Microbiological Outcome among Urologic Patients with Documented Gram-Negative Infections. Antibiotics 2023, 12, 1388. https://doi.org/10.3390/antibiotics12091388
Berrino PM, Gatti M, Rinaldi M, Brunocilla E, Viale P, Pea F. Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin–Tazobactam or Meropenem and Microbiological Outcome among Urologic Patients with Documented Gram-Negative Infections. Antibiotics. 2023; 12(9):1388. https://doi.org/10.3390/antibiotics12091388
Chicago/Turabian StyleBerrino, Pasquale Maria, Milo Gatti, Matteo Rinaldi, Eugenio Brunocilla, Pierluigi Viale, and Federico Pea. 2023. "Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin–Tazobactam or Meropenem and Microbiological Outcome among Urologic Patients with Documented Gram-Negative Infections" Antibiotics 12, no. 9: 1388. https://doi.org/10.3390/antibiotics12091388
APA StyleBerrino, P. M., Gatti, M., Rinaldi, M., Brunocilla, E., Viale, P., & Pea, F. (2023). Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin–Tazobactam or Meropenem and Microbiological Outcome among Urologic Patients with Documented Gram-Negative Infections. Antibiotics, 12(9), 1388. https://doi.org/10.3390/antibiotics12091388







