Peptide-Resorcinarene Conjugates Obtained via Click Chemistry: Synthesis and Antimicrobial Activity
Abstract
1. Introduction
2. Results and Discussion
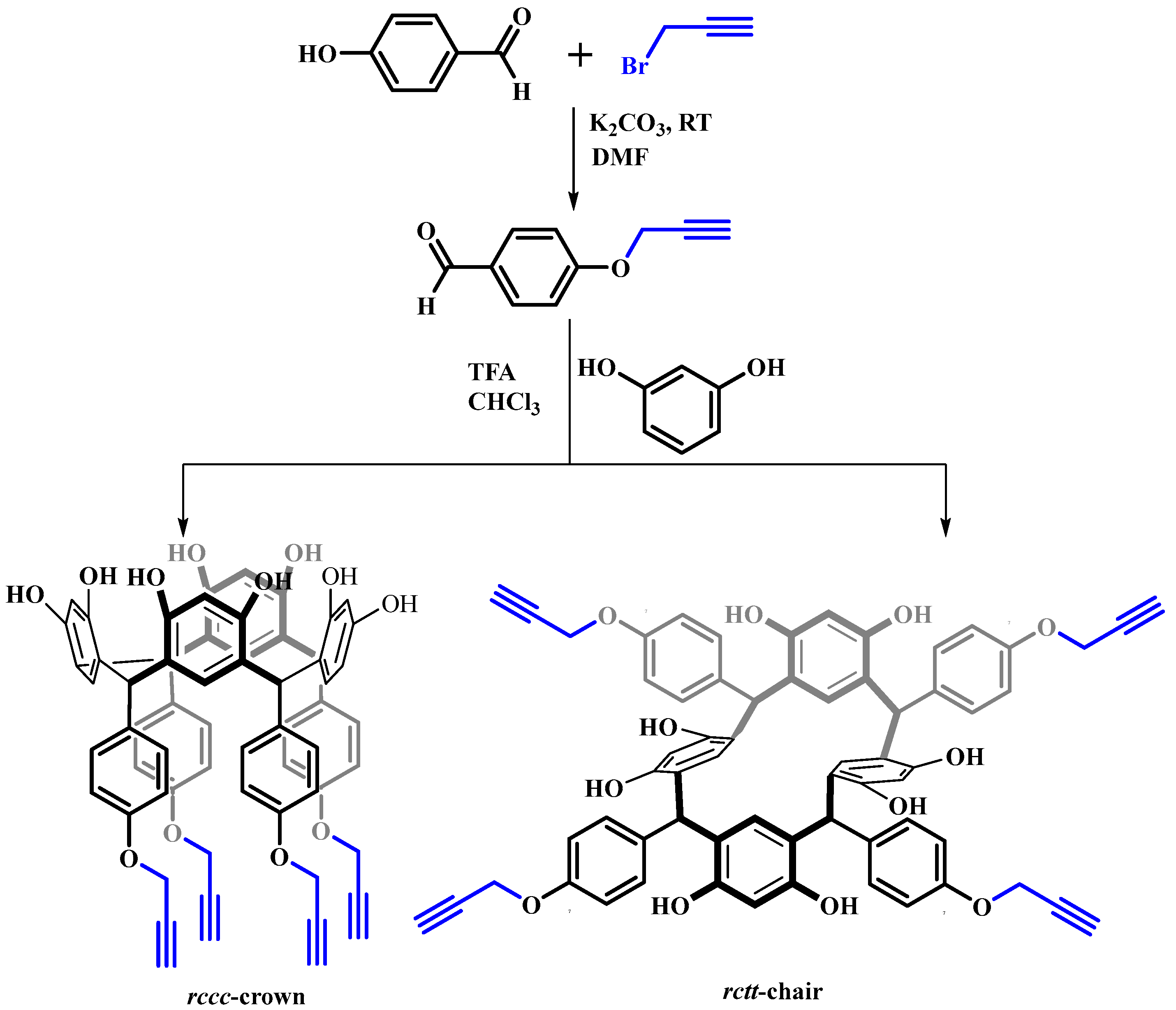
2.1. Synthesis and Characterization of Resorcinarene and Peptide Precursors
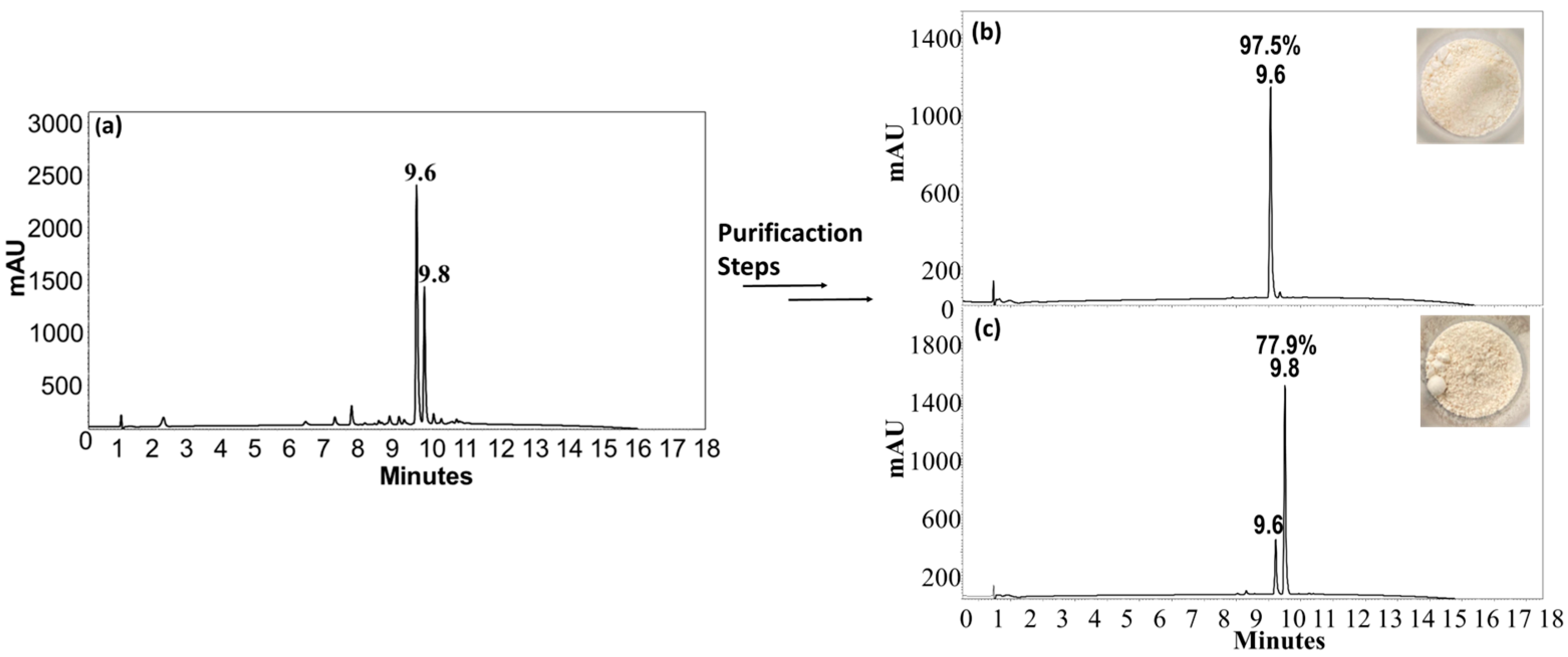
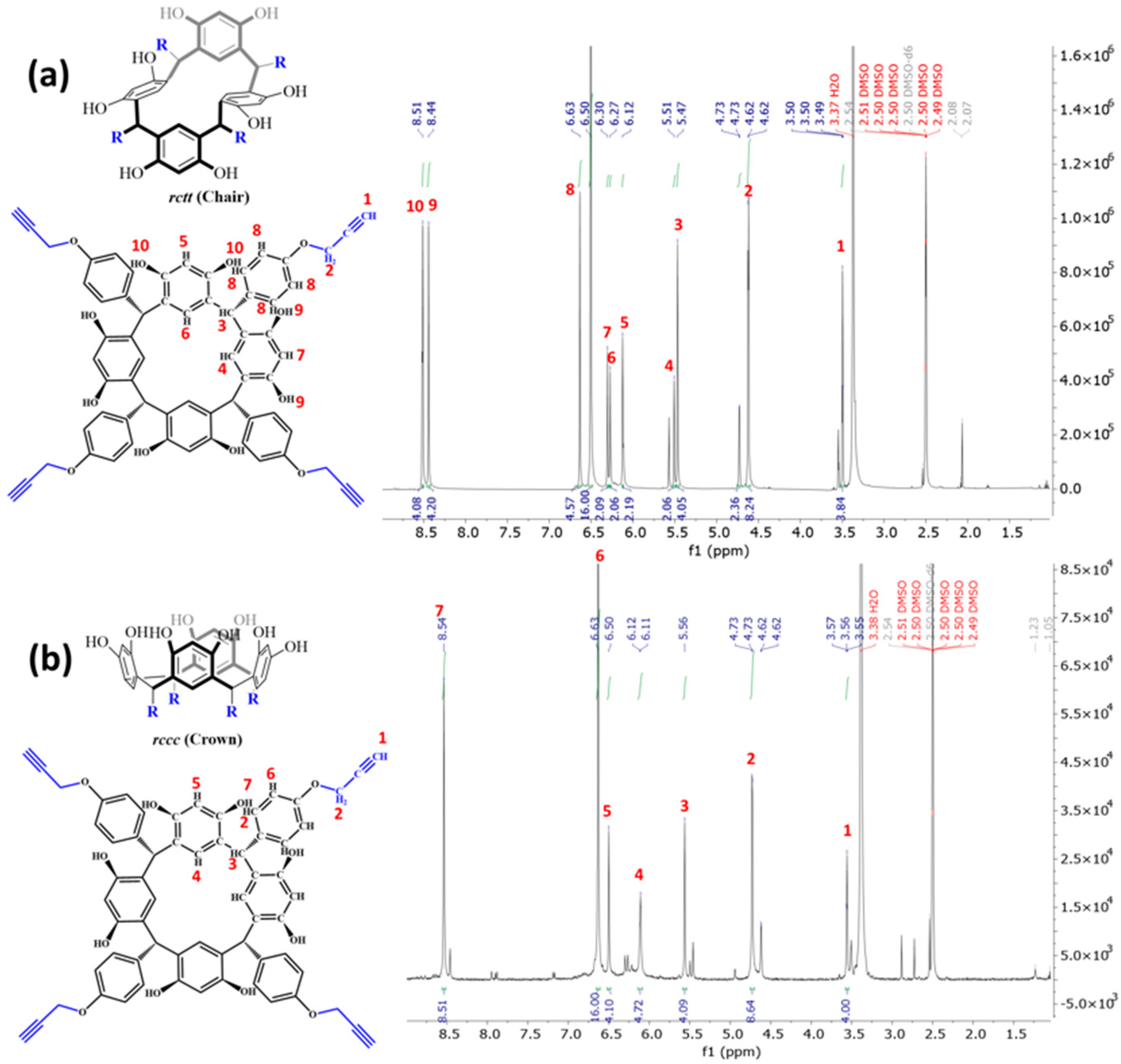
2.2. Synthesis and Characterization of Peptide-Resorcinarene Conjugates
2.3. Antibacterial/Antifungal Activity against Reference Strains and Clinical Isolates
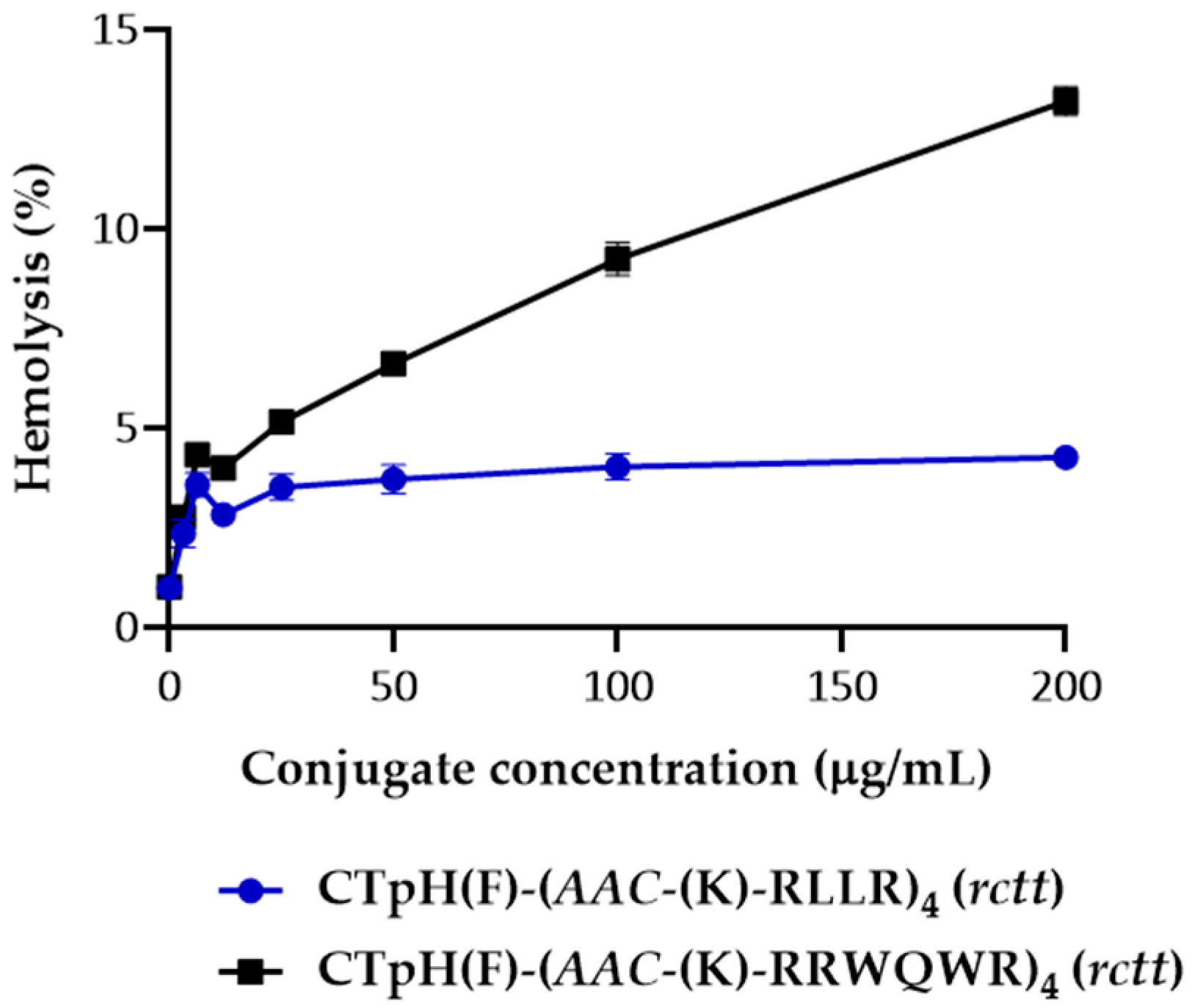
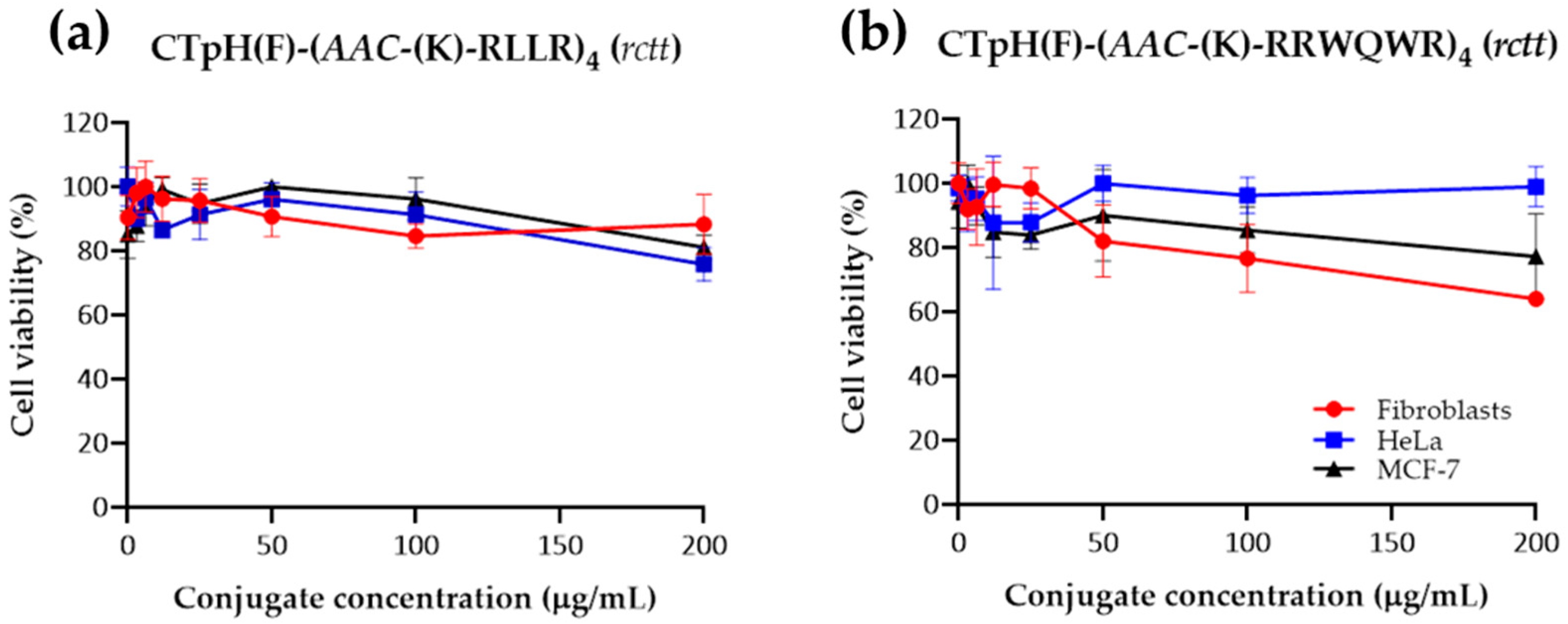
2.4. Cytotoxic/Hemolytic Activity
3. Materials and Methods
3.1. General Method
3.2. Peptide Synthesis
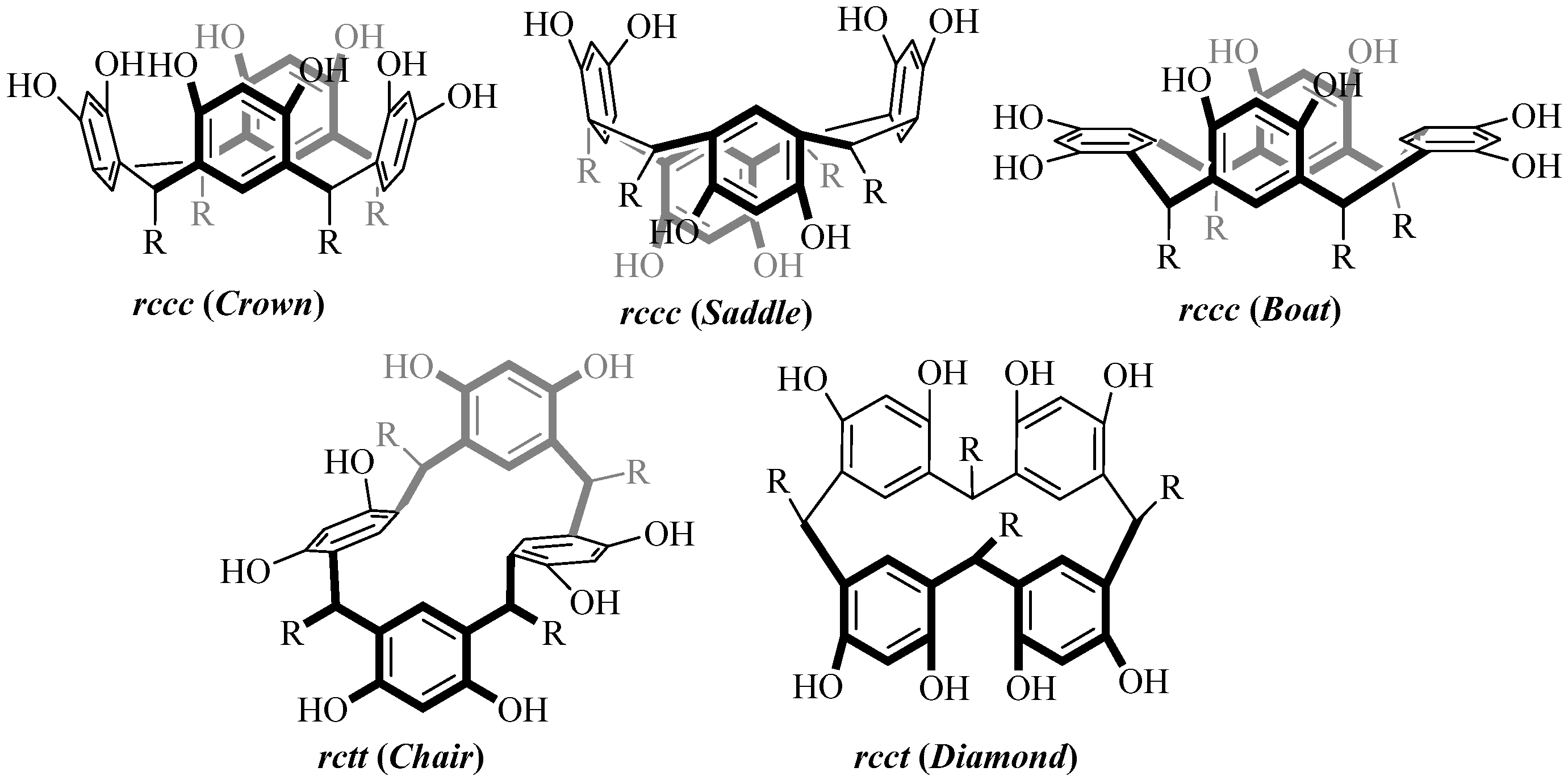
3.3. Synthesis of Resorcinarenes
3.3.1. Synthesis of Precursor 1
3.3.2. C-Tetra(4-(prop-2-yn-1-yloxy)phenyl)calix[4]resorcinarene
3.4. (Peptide)4-Resorcinarene Conjugate Synthesis (Click Chemistry)
3.5. Purification and Characterization
3.6. Activity Assays
3.6.1. Antibacterial Activity
3.6.2. Antifungal Activity
3.7. Time-Kill Curve
3.8. Cytotoxic Activity
3.8.1. Cell Culture
3.8.2. MTT Assay
3.8.3. Hemolysis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Antibiotic Resistance. Available online: http://www.who.int/mediacentre/factsheets/antibiotic-resistance/en/ (accessed on 15 February 2023).
- WHO. Prevention & AMP. Available online: http://www.who.int/antimicrobial-resistance/amr-aidememoire-may2016.pdf (accessed on 15 February 2023).
- WHO. Available online: http://www.who.int/antimicrobial-resistance/Microbes_and_Antimicrobials/en/ (accessed on 15 February 2023).
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 15 February 2023).
- Castañeda-Casimiro, J.; Ortega-Roque, J.A.; Marcela, A.; Aquino-Andrade, A.; Serafín-López, J.; Estrada-Parra, S.; Estrada, I. Péptidos Antimicrobianos: Péptidos Con Múltiples Funciones. Alerg. Asma Inmunol. 2009, 18, 16–29. [Google Scholar]
- Pineda-Castañeda, H.M.; Insuasty-Cepeda, D.S.; Niño-Ramírez, V.A.; Curtidor, H.; Rivera-Monroy, Z.J. Designing Short Peptides: A Sisyphean Task? Curr. Org. Chem. 2020, 24, 2448–2474. [Google Scholar] [CrossRef]
- Agrawal, Y.K.; Patadia, R.N. Studies on Resorcinarenes and Their Analytical Applications. Rev. Anal. Chem. 2006, 25, 155–239. [Google Scholar] [CrossRef]
- Shah, M.D.; Agrawal, Y. Calixarene: A New Architecture in the Analytical and Pharmaceutical Technology. J. Sci. Ind. Res. 2012, 71, 21–26. [Google Scholar]
- Jain, V.K.; Kanaiya, P.H. Chemistry of calix[4]resorcinarenes. Russ. Chem. Rev. 2011, 80, 75–102. [Google Scholar] [CrossRef]
- Puttreddy, R.; Beyeh, N.K.; Rissanen, K. Conformational changes in Cmethyl-resorcinarene pyridine: N-oxide inclusion complexes in the solid state. Cryst. Eng. Comm. 2016, 18, 4971–4976. [Google Scholar] [CrossRef]
- Moore, D.; Watson, G.W.; Gunnlaugsson, T.; Matthews, S.E. Selective formation of the rctt chair stereoisomers of octa-O-alkyl Resorcin[4]arenes using Brønsted acid catalysis. New J. Chem. 2008, 32, 994–1002. [Google Scholar] [CrossRef]
- Ziaja, P.; Krogul, A.; TS, P.; Litwinienko, G. Structure and stoichiometry of resorcinarene solvates as host-guest complexes—NMR, X-ray and thermoanalytical studies. Thermochim. Acta 2016, 623, 112–119. [Google Scholar] [CrossRef]
- Castillo-Aguirre, A.A.; Pérez-Redondo, A.; Maldonado, M. Influence of the hydrogen bond on the iteroselective O-alkylation of calix[4]resorcinarenes. J. Mol. Struct. 2020, 1202, 127402. [Google Scholar] [CrossRef]
- Pedro-Hernández, L.D.; Martínez-Klimova, E.; Cortez-Maya, S.; Mendoza-Cardozo, S.; Ramírez-Ápan, T.; Martínez-García, M. Synthesis, Characterization, and Nanomedical Applications of Conjugates between Resorcinarene-Dendrimers and Ibuprofen. Nanomaterials 2017, 7, 163. [Google Scholar] [CrossRef]
- Tang, H.; Guo, H.; Yang, F.; Zhu, S. Synthesis and mesomorphic properties of calix[4]resorcinarene-triphenylene oligomers. Liq. Cryst. 2017, 44, 1566–1574. [Google Scholar] [CrossRef]
- Reynolds, M.R.; Pick, F.S.; Hayward, J.J.; Trant, J.F. A Concise Synthesis of a Methyl Ester 2-Resorcinarene: A Chair-Conformation Macrocycle. Symmetry 2021, 13, 627. [Google Scholar] [CrossRef]
- Rashad, A.A. Click Chemistry for Cyclic Peptide Drug Design. Methods Mol. Biol. 2019, 2001, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Fuaad, A.A.H.; Azmi, F.; Skwarczynski, M.; Toth, I. Peptide Conjugation via CuAAC ‘click’ Chemistry. Molecules 2013, 18, 13148–13174. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of Azide-Alkyne Cycloaddition-Click Chemistry over the Recent Decade. Tetrahedron 2016, 72, 5257–5282. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Rivera-Monroy, Z.J.; Maldonado, M. Copper(I)-Catalyzed Alkyne–Azide Cycloaddition (CuAAC) “Click” Reaction: A Powerful Tool for Functionalizing Polyhydroxylated Platforms. ACS Omega 2023, 8, 3650–3666. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef]
- Baker, S.D.; Sparreboom, A.; Verweij, J. Clinical Pharmacokinetics of Docetaxel: Recent Developments. Clin. Pharm. 2006, 45, 235–252. [Google Scholar] [CrossRef]
- Starpharma. Starpharma to Commence DEP ® Cabazitaxel Phase 1/2 Trial; BioSpectrum: Singapore, 2018. [Google Scholar]
- INSTITUT PASTEUR. A Synthetic Glycopeptide for Anti-Tumor Immunotherapy: From Design to First Use in Human; INSTITUT PASTEUR: Paris, France, 2015. [Google Scholar]
- ClinicalTrials. Treatment of Non-Responding to Conventional Therapy Inoperable Liver Cancers by In Situ Introduction of ImDendrim (ImDendrim); National Library of Medicine: Bethesda, MD, USA, 2017. [Google Scholar]
- ClinicalTrials. A Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of OP-101 after Intravenous Administration in Healthy Volunteers; National Library of Medicine: Bethesda, MD, USA, 2018. [Google Scholar]
- Patel, P.; Patel, V.; Patel, P.M. Synthetic Strategy of Dendrimers: A Review. J. Indian Chem. Soc. 2022, 99, 100514. [Google Scholar] [CrossRef]
- Soomro, Z.H.; Cecioni, S.; Blanchard, H.; Praly, J.P.; Imberty, A.; Vidal, S.; Matthews, S.E. CuAAC synthesis of resorcin[4]arene-based glycoclusters as multivalent ligands of lectins of lectins. Org. Biomol. Chem. 2011, 9, 6587–6597. [Google Scholar] [CrossRef]
- Knyazeva, I.R.; Abdrafikova, D.K.; Mukhamedyanova, K.M.; Syakaev, V.V.; Gabidullin, B.M.; Gubaidullin, A.T.; Habicher, W.D.; Burilov, A.R.; Pudovik, M.A. Synthesis of novel highly functionalized triazole-linked calix[4]resorcinols via click reaction. Mendeleev Commun. 2017, 27, 556–558. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Maldonado, M.; Rivera-Monroy, Z.J. Efficient Separation of C-Tetramethylcalix[4]resorcinarene Conformers by Means of Reversed-Phase Solid-Phase Extraction. ACS Omega 2023, 8, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Schneggenburger, P.E.; Worbs, B.; Diederichsen, U. Azide Reduction during Peptide Cleavage from Solid Support—The Choice of Thioscavenger? J. Pept. Sci. 2010, 16, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Guataqui, K.; Márquez-Torres, M.; Pineda-Castañeda, H.M.; Vargas-Casanova, Y.; Ceballos-Garzon, A.; Rivera-Monroy, Z.J.; García-Castañeda, J.E.; Parra-Giraldo, C.M. Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II: Antifungal Activity against Reference Strains and Clinical Isolates of Candida spp. Antibiotics 2022, 11, 1561. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Bonilla-Velásquez, L.D.; Leal-Castro, A.L.; Fierro-Medina, R.; García-Castañeda, J.E.; Rivera-Monroy, Z.J. Use of Click Chemistry for Obtaining an Antimicrobial Chimeric Peptide Containing the LfcinB and Buforin II Minimal Antimicrobial Motifs. ChemistrySelect 2020, 5, 1655–1657. [Google Scholar] [CrossRef]
- Guerra, J.R.; Cárdenas, A.B.; Ochoa-Zarzosa, A.; Meza, J.L.; Pérez, A.U.; Fierro-Medina, R.; Monroy, Z.J.R.; Castañeda, J.E.G. The Tetrameric Peptide LfcinB (20–25)4 Derived from Bovine Lactoferricin Induces Apoptosis in the MCF-7 Breast Cancer Cell Line. RSC Adv. 2019, 9, 20497–20504. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, A.; Yilmaz, C.; Konus, M.; Koca, H.; Aydemir, S.; Oagaz, J.A. Synthesis and biological properties of novel1-methyl-2-(2-(prop-2-yn-1-yloxy)benzylidene) hydrazine analogues. Turk. J. Chem. 2018, 42, 306–316. [Google Scholar] [CrossRef]
- Li, X. Click to Join Peptides/Proteins Together. Chem. Asian J. 2011, 6, 2606–2616. [Google Scholar] [CrossRef]
- Avrutina, O.; Empting, M.; Fabritz, S.; Daneschdar, M.; Frauendorf, H.; Diederichsen, U.; Kolmar, H. Application of copper(I) catalyzed azide-alkyne[3+2] cycloaddition to the synthesis of template-assembled multivalent peptide conjugates. Org. Biomol. Chem. 2009, 7, 4177–4185. [Google Scholar] [CrossRef]
- Suárez, A. Reacciones de cicloadición 1,3-dipolares a alquinos catalizadas por cobre. An. Quím. 2012, 108, 306–313. [Google Scholar]
- Cockerill, F.; Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute. M27-A3. In Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts Approved Standard–Third Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Barry, A.L.; Craig, W.A.; Nadler, L.H.; Reller, L.B. M26-A: Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. Clin. Lab. Stand. Inst. 1999, 19, 56–78. [Google Scholar]
- Langan, T.; Rodgers, K.; Chou, R. Synchromization of Mammalian Cell Cultures by Serum Deprivation. Methods Cell Sci. 2017, 1524, 97–105. [Google Scholar] [CrossRef]



| Peptides a | RP-HPLC | MS | |||
|---|---|---|---|---|---|
| tR (min) | Purity b (%) | Theorical c [M+H]+ | m/z [M+H]+ | ||
| RLLR | 2.4 | 97.2 | 556.0 | 556.2 | |
| RRWQWR | 4.1 | 92.5 | 986.5 | 986.5 | |
| K(N3)-RLLR | 5.3 | 98.2 | 710.5 | 709.9 | |
| K(N3)-RRWQWR | 5.4 | 83.7 | 1139.6 | 1139.4 | |
| Alkynyl-resorcinarene | RP-HPLC | Yield (%) | MS | ||
| tR (min) | Purity b (%) | Theorical c [M+H]+ | m/z [M+H]+ | ||
| CTpH(F) (rctt) | 9.6 | 97.5 | 58.9 | 1009.3 | 1009.3 |
| CTpH(F) (rccc) | 9.8 | 77.9 | 20.8 | 1009.3 | 1008.7 |
| (Peptide)4-resorcinarene conjugates | RP-HPLC | Yield (%) | MS | ||
| tR (min) | Purity b (%) | Theorical c [M+H]+ | m/z [M+H]+ | ||
| CTpH(F)-(AAC-(K)-RLLR)4 (rctt) | 6.0 | 96.4 | 75.6 | 3847.2 | 3847.5 |
| CTpH(F)-(AAC-(K)-RLLR)4 (rccc) | 6.0 | 88.7 | 60.1 | 3847.2 | 3844.2 |
| CTpH(F)-(AAC-(K)-RRWQWR)4 (rctt) | 6.4 | 74.6 | 56.6 | 5570.4 | 5575.2 |
| Antibacterial Activity against ATCC Strains | |||
|---|---|---|---|
| Molecule | Sequence | MIC/MBC (µM) | |
| E. coli25922 | S. aureus25923 | ||
| Control Peptide | RLLR | >359/>359 | >359/>359 |
| RRWQWR | 203/203 | >203/>203 | |
| Conjugate | CTpH(F)-(AAC-(K)-RLLR)4 (rctt) | 13/26 | 13/Nd |
| CTpH(F)-(AAC-(K)-RLLR)4 (rccc) | 52/>52 | Nd | |
| CTpH(F)-(AAC-(K)-RRWQWR)4 (rctt) | 36/36 | Nd | |
| Conjugate CTpH(F)-(AAC-(K)-RLLR)4 (rctt) antimicrobial activity | |||
| Strain (classification) | Resistant to | MIC/MBC (µM) | |
| E. coli 1004 (S) | - | 26/>52 | |
| E. coli 129797 (R) | AM, SAM, CEF | 26/52 | |
| E. coli 301755 (M) | AM, SAM, CPE, CAZ, CAX, CIP, GEN, NIT, NOR, STX | 13/26 | |
| S. aureus 109095 (R) | P | 26/52 | |
| S. aureus 117719 (M) | P, TET | 52/52 | |
| S. aureus 124653 (M) | P, ERY, TET | 13/52 | |
| Strain (classification) | Resistant to | MIC/MFC (µM) | |
| C. albicans SC5314 (S) | - | 26/26 | |
| C. albicans 256 HUSI-PUJ (R) | FLC | 13/13 | |
| C. auris 435 (S) | FLC | 26/>26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Castañeda, H.M.; Maldonado-Villamil, M.; Parra-Giraldo, C.M.; Leal-Castro, A.L.; Fierro-Medina, R.; Rivera-Monroy, Z.J.; García-Castañeda, J.E. Peptide-Resorcinarene Conjugates Obtained via Click Chemistry: Synthesis and Antimicrobial Activity. Antibiotics 2023, 12, 773. https://doi.org/10.3390/antibiotics12040773
Pineda-Castañeda HM, Maldonado-Villamil M, Parra-Giraldo CM, Leal-Castro AL, Fierro-Medina R, Rivera-Monroy ZJ, García-Castañeda JE. Peptide-Resorcinarene Conjugates Obtained via Click Chemistry: Synthesis and Antimicrobial Activity. Antibiotics. 2023; 12(4):773. https://doi.org/10.3390/antibiotics12040773
Chicago/Turabian StylePineda-Castañeda, Héctor Manuel, Mauricio Maldonado-Villamil, Claudia Marcela Parra-Giraldo, Aura Lucía Leal-Castro, Ricardo Fierro-Medina, Zuly Jenny Rivera-Monroy, and Javier Eduardo García-Castañeda. 2023. "Peptide-Resorcinarene Conjugates Obtained via Click Chemistry: Synthesis and Antimicrobial Activity" Antibiotics 12, no. 4: 773. https://doi.org/10.3390/antibiotics12040773
APA StylePineda-Castañeda, H. M., Maldonado-Villamil, M., Parra-Giraldo, C. M., Leal-Castro, A. L., Fierro-Medina, R., Rivera-Monroy, Z. J., & García-Castañeda, J. E. (2023). Peptide-Resorcinarene Conjugates Obtained via Click Chemistry: Synthesis and Antimicrobial Activity. Antibiotics, 12(4), 773. https://doi.org/10.3390/antibiotics12040773







