Local Antibiotic Delivery Options in Prosthetic Joint Infection
Abstract
1. Introduction
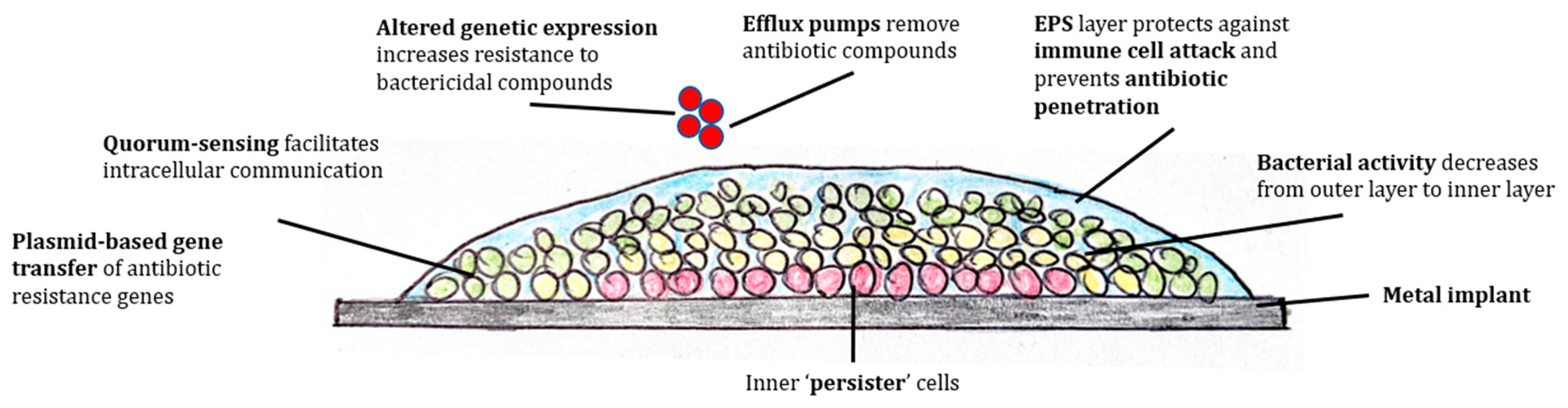
2. Biofilm Pathology
3. Local Antibiotics
3.1. Antibiotic Choice
3.2. Pharmacokinetics
3.3. Naked Antibiotics
3.4. Regular Intra-Articular Administration
4. Local Antibiotic Carriers
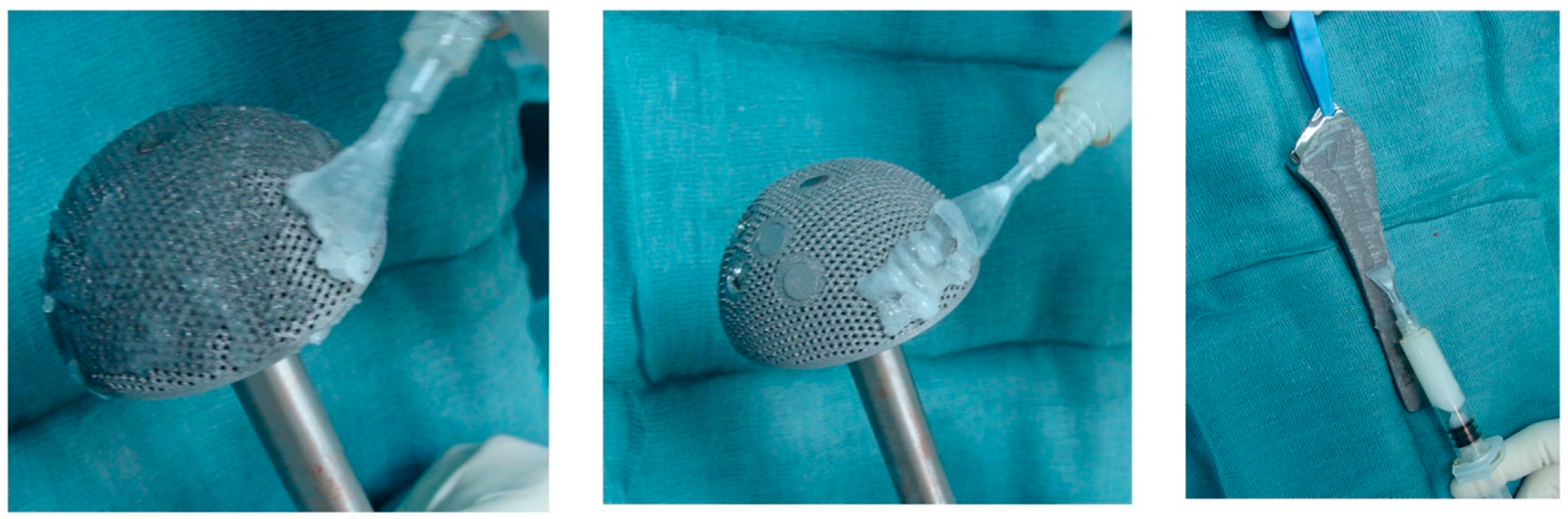
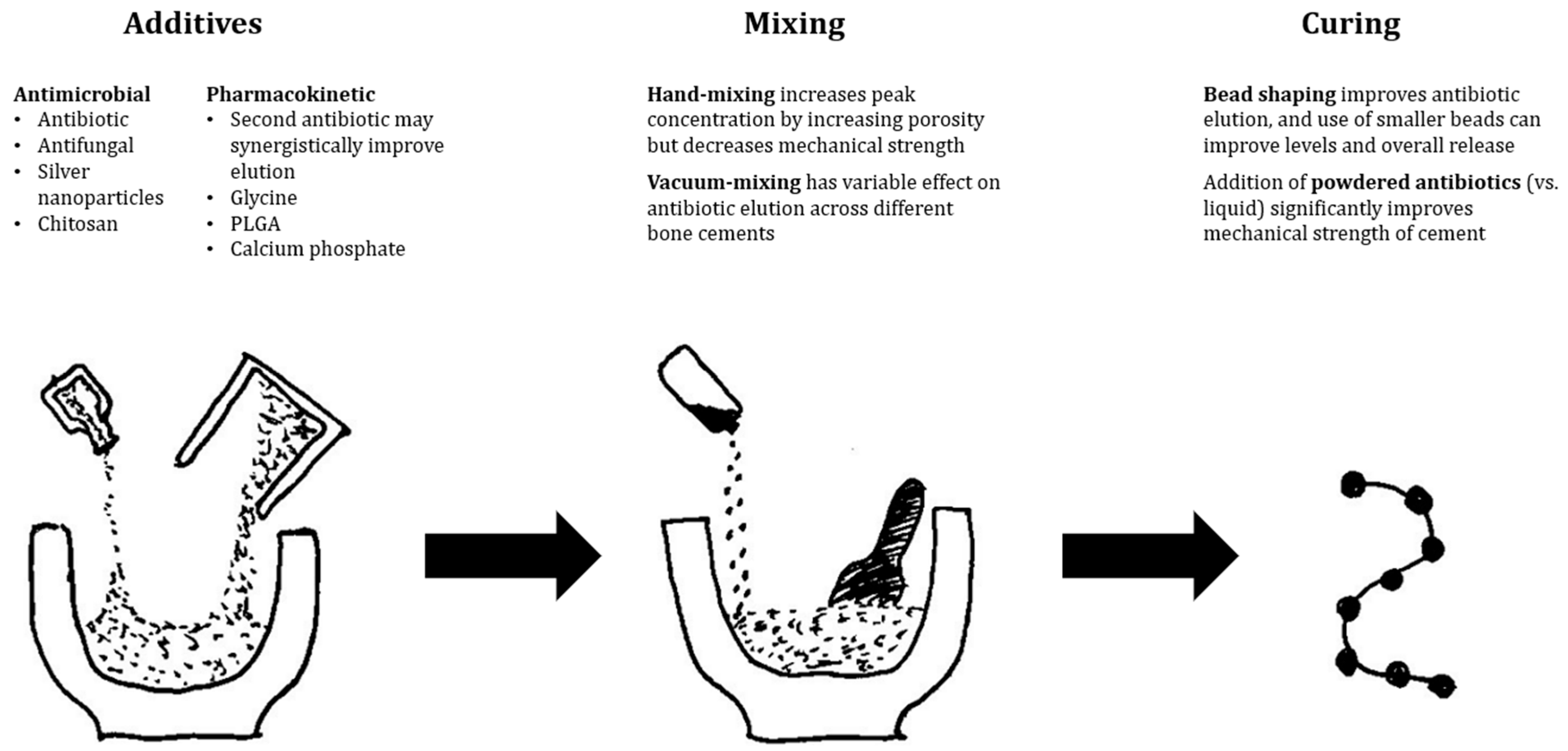
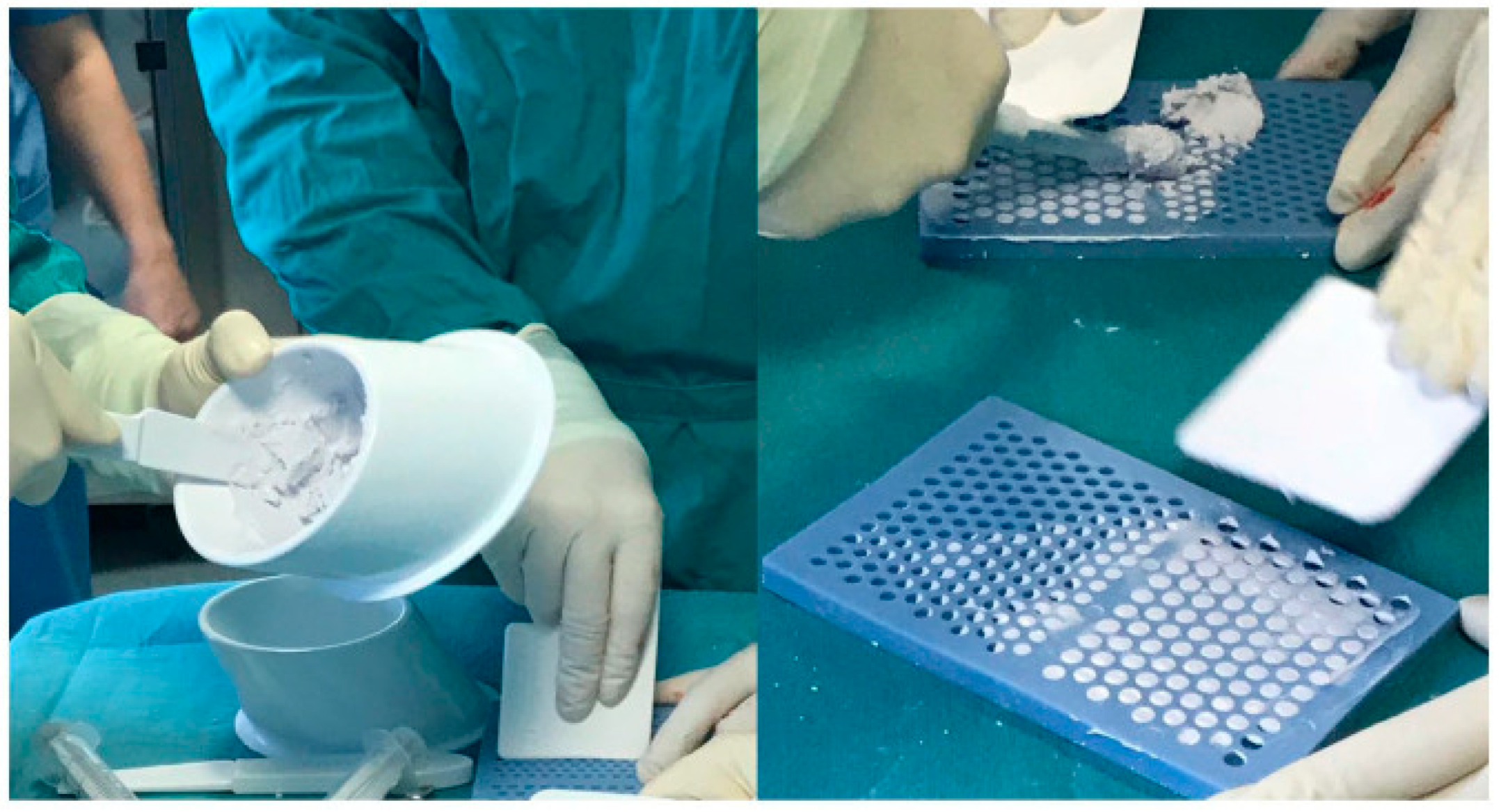
4.1. PMMA
4.2. Resorbable Carriers
4.3. Calcium Sulphate
4.4. Hydroxyapatite
4.5. Bioactive Glass
4.6. Hydrogels
4.7. Nanocarriers
5. The Future: Novel Antibiofilm Therapies
5.1. Bacteriophages
5.2. Ceragenins
5.3. Other Novel Options
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Matar, H.E.; Bloch, B.V.; Snape, S.E.; James, P.J. Outcomes of single- and two-stage revision total knee arthroplasty for chronic periprosthetic joint infection. Bone Jt. J. 2021, 103-B, 1373–1379. [Google Scholar] [CrossRef]
- AOANJRR. Hip, Knee & Shoulder Arthroplasty: 2021 Annual Report; AOA: Adelaide, Australia, 2021. [Google Scholar]
- Sinagra, Z.P.; Davis, J.S.; Lorimer, M.; de Steiger, R.N.; Graves, S.E.; Yates, P.; Manning, L. The accuracy of reporting of periprosthetic joint infection to the Australian Orthopaedic Association National Joint Replacement Registry. Bone Jt. Open 2022, 3, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Brochin, R.L.; Phan, K.; Poeran, J.; Zubizarreta, N.; Galatz, L.M.; Moucha, C.S. Trends in Periprosthetic Hip Infection and Associated Costs: A Population-Based Study Assessing the Impact of Hospital Factors Using National Data. J. Arthroplast. 2018, 33, S233–S238. [Google Scholar] [CrossRef] [PubMed]
- Lenguerrand, E.; Whitehouse, M.; Beswick, A.; Jones, S.A.; Porter, M.L.; Blom, A.W. Revision for prosthetic joint infection following hip arthroplasty. Bone Jt. Res. 2017, 6, 391–398. [Google Scholar] [CrossRef]
- Knebel, C.; Menzemer, J.; Pohlig, F.; Herschbach, P.; Burgkart, R.; Obermeier, A.; Von Eisenhart-Rothe, R.; Mühlhofer, H.M. Peri-Prosthetic Joint Infection of the Knee Causes High Levels of Psychosocial Distress: A Prospective Cohort Study. Surg. Infect. 2020, 21, 877–883. [Google Scholar] [CrossRef]
- Wildeman, P.; Rolfson, O.M.; Söderquist, B.M.; Wretenberg, P.M.; Lindgren, V.M. What Are the Long-term Outcomes of Mortality, Quality of Life, and Hip Function after Prosthetic Joint Infection of the Hip? A 10-year Follow-up from Sweden. Clin. Orthop. Relat. Res. 2021, 479, 2203–2213. [Google Scholar] [CrossRef]
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality During Total Hip Periprosthetic Joint Infection. J. Arthroplast. 2018, 34, S337–S342. [Google Scholar] [CrossRef] [PubMed]
- Lum, Z.C.; Natsuhara, K.M.; Shelton, T.J.; Giordani, M.; Pereira, G.C.; Meehan, J.P. Mortality During Total Knee Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 3783–3788. [Google Scholar] [CrossRef]
- Okafor, C.; Hodgkinson, B.; Nghiem, S.; Vertullo, C.; Byrnes, J. Cost of septic and aseptic revision total knee arthroplasty: A systematic review. BMC Musculoskelet. Disord. 2021, 22, 706. [Google Scholar] [CrossRef] [PubMed]
- Kallala, R.F.; Vanhegan, I.S.; Ibrahim, M.S.; Sarmah, S.; Haddad, F.S. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs. Bone Jt. J. 2015, 97-B, 197–201. [Google Scholar] [CrossRef]
- Garfield, K.; Noble, S.; Lenguerrand, E.; Whitehouse, M.R.; Sayers, A.; Reed, M.R.; Blom, A.W. What are the inpatient and day case costs following primary total hip replacement of patients treated for prosthetic joint infection: A matched cohort study using linked data from the National Joint Registry and Hospital Episode Statistics. BMC Med. 2020, 18, 335. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.; Dowsey, M.; Buising, K.; Liew, D.; Choong, P. Cost analysis of debridement and retention for management of prosthetic joint infection. Clin. Microbiol. Infect. 2013, 19, 181–186. [Google Scholar] [CrossRef]
- Merollini, K.; Crawford, R.W.; Graves, N. Surgical treatment approaches and reimbursement costs of surgical site infections post hip arthroplasty in Australia: A retrospective analysis. BMC Health Serv. Res. 2013, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.J.; Hevesi, M.; Visscher, S.L.; Ransom, J.E.; Lewallen, D.G.; Berry, D.J.; Kremers, H.M. Direct Inpatient Medical Costs of Operative Treatment of Periprosthetic Hip and Knee Infections Are Twofold Higher Than Those of Aseptic Revisions. J. Bone Jt. Surg. 2020, 103, 312–318. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Meinders, J.; Van Der Mei, H.; Busscher, H. Deposition Efficiency and Reversibility of Bacterial Adhesion under Flow. J. Colloid Interface Sci. 1995, 176, 329–341. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Genet. 2006, 5, 48–56. [Google Scholar] [CrossRef]
- Keren, I.; Shah, D.; Spoering, A.; Kaldalu, N.; Lewis, K. Specialized Persister Cells and the Mechanism of Multidrug Tolerance in Escherichia coli. J. Bacteriol. 2004, 186, 8172–8180. [Google Scholar] [CrossRef]
- Suci, P.A.; Mittelman, M.W.; Yu, F.P.; Geesey, G.G. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 1994, 38, 2125–2133. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.; Phillips, F.; Olliff, C. The effects of extracellular slime from Staphylococcus epidermidison phagocytic ingestion and killing. FEMS Immunol. Med. Microbiol. 1994, 9, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Morán, F.J.; García, C.; Pérez-Giraldo, C.; Hurtado, C.; Blanco, M.T.; Gómez-García, A.C. Phagocytosis and killing of slime-producing Staphylococcus epidermidis by polymorphonuclear leukocytes. Effects of sparfloxacin. Rev. Esp. Quimioter. 1998, 11, 52–57. [Google Scholar]
- Leid, J.G.; Willson, C.J.; Shirtliff, M.E.; Hassett, D.J.; Parsek, M.R.; Jeffers, A.K. The Exopolysaccharide Alginate Protects Pseudomonas aeruginosa Biofilm Bacteria from IFN-γ-Mediated Macrophage Killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus Biofilms Promote Horizontal Transfer of Antibiotic Resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mah, T.-F. Involvement of a Novel Efflux System in Biofilm-Specific Resistance to Antibiotics. J. Bacteriol. 2008, 190, 4447–4452. [Google Scholar] [CrossRef]
- Grobe, K.J.; Zahller, J.; Stewart, P. Role of dose concentration in biocide efficacy against Pseudomonas aeruginosa biofilms. J. Ind. Microbiol. Biotechnol. 2002, 29, 10–15. [Google Scholar] [CrossRef]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef]
- Organisation, W.H. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries. A Practical Toolkit; World Health Organisation: Geneva, Switzerland, 2019. [Google Scholar]
- Dagneaux, L.; Limberg, A.K.; Osmon, D.R.; Leung, N.; Berry, D.J.; Abdel, M.P. Acute Kidney Injury When Treating Periprosthetic Joint Infections After Total Knee Arthroplasties with Antibiotic-Loaded Spacers. J. Bone Jt. Surg. 2021, 103, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Badha, V.; Moore, R.; Heffernan, J.; Castaneda, P.; McLaren, A.; Overstreet, D. Determination of Tobramycin and Vancomycin Exposure Required to Eradicate Biofilms on Muscle and Bone Tissue In Vitro. J. Bone Jt. Infect. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Wong, M.T.; Sridharan, S.S.; Davison, E.M.; Ng, R.; Desy, N.M. Can Topical Vancomycin Prevent Periprosthetic Joint Infection in Hip and Knee Arthroplasty? A Systematic Review. Clin. Orthop. Relat. Res. 2021, 479, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Lin, X.; Kuang, X.; Teng, Z.; Lu, S. The application of topical vancomycin powder for the prevention of surgical site infections in primary total hip and knee arthroplasty: A meta-analysis. Orthop. Traumatol. Surg. Res. 2020, 107, 102741. [Google Scholar] [CrossRef]
- The Major Extremity Trauma Research Consortium (METRC); O’Toole, R.V.; Joshi, M.; Carlini, A.R.; Murray, C.K.; Allen, L.E.; Huang, Y.; Scharfstein, D.O.; O’Hara, N.N.; Gary, J.L.; et al. Effect of Intrawound Vancomycin Powder in Operatively Treated High-risk Tibia Fractures. JAMA Surg. 2021, 156, e207259. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-Y.; Herwaldt, L.A.; Blevins, A.E.; Cho, E.; Schweizer, M.L. Effectiveness of local vancomycin powder to decrease surgical site infections: A meta-analysis. Spine J. 2014, 14, 397–407. [Google Scholar] [CrossRef]
- Johnson, J.D.; Nessler, J.M.; Horazdovsky, R.D.; Vang, S.; Thomas, A.J.; Marston, S.B. Serum and Wound Vancomycin Levels After Intrawound Administration in Primary Total Joint Arthroplasty. J. Arthroplast. 2017, 32, 924–928. [Google Scholar] [CrossRef]
- Schneider, R.K.; Bramlage, L.R.; Mecklenburg, L.M.; Moore, R.M.; Gabel, A.A. Open drainage, intra-articular and systemic antibiotics in the treatment of septic arthritis/tenosynovitis in horses. Equine Veter. J. 1992, 24, 443–449. [Google Scholar] [CrossRef]
- Whiteside, L.A.; Roy, M.E. One-stage Revision With Catheter Infusion of Intraarticular Antibiotics Successfully Treats Infected THA. Clin. Orthop. Relat. Res. 2017, 475, 419–429. [Google Scholar] [CrossRef]
- Whiteside, L.A.; Peppers, M.; Nayfeh, T.A.; Roy, M.E. Methicillin-resistant Staphylococcus aureus in TKA Treated With Revision and Direct Intraarticular Antibiotic Infusion. Clin. Orthop. Relat. Res. 2011, 469, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, L.A.; Nayfeh, T.A.; LaZear, R.; Roy, M.E. Reinfected Revised TKA Resolves With an Aggressive Protocol and Antibiotic Infusion. Clin. Orthop. Relat. Res. 2012, 470, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Li, G.; Zhang, X.; Wang, Y.; Mu, W.; Cao, L. Effective treatment of single-stage revision using intra-articular antibiotic infusion for culture-negative prosthetic joint infection. Bone Jt. J. 2020, 102-B, 336–344. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, X.; Xu, B.; Guo, W.; Mu, W.; Cao, L. Single-Stage Revision for Chronic Fungal Periprosthetic Joint Infection: An Average of 5 Years of Follow-Up. J. Arthroplast. 2017, 32, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.E.; Peppers, M.P.; Whiteside, L.A.; LaZear, R.M. Vancomycin Concentration in Synovial Fluid: Direct Injection into the Knee vs. Intravenous Infusion. J. Arthroplast. 2014, 29, 564–568. [Google Scholar] [CrossRef]
- Wei, J.; Wen, Y.; Tong, K.; Wang, H.; Chen, L. Local Application of Vancomycin in One-Stage Revision of Prosthetic Joint Infection Caused by Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2021, 65, AAC0030321. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, H.W.; Engelbrecht, H. Depot effects of various antibiotics mixed with palacos resins. Chirurg 1970, 41, 511–515. [Google Scholar]
- Luu, A.; Syed, F.; Raman, G.; Bhalla, A.; Muldoon, E.; Hadley, S.; Smith, E.; Rao, M. Two-Stage Arthroplasty for Prosthetic Joint Infection. J. Arthroplast. 2013, 28, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Wilmes, P.; Schmitt, E.; Kelm, J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009, 80, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Wall, V.; Nguyen, T.-H.; Nguyen, N.; Tran, P. Controlling Antibiotic Release from Polymethylmethacrylate Bone Cement. Biomedicines 2021, 9, 26. [Google Scholar] [CrossRef]
- Zalavras, C.G.; Patzakis, M.J.; Holtom, P. Local Antibiotic Therapy in the Treatment of Open Fractures and Osteomyelitis. Clin. Orthop. Relat. Res. 2004, 427, 86–93. [Google Scholar] [CrossRef]
- E Kent, M.; Rapp, R.P.; Smith, K.M. Antibiotic Beads and Osteomyelitis: Here Today, What’s Coming Tomorrow? Orthopedics 2006, 29, 599–603. [Google Scholar] [CrossRef]
- van Vugt, T.A.G.; Arts, J.J.; Geurts, J.A.P. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.L.; Evans, R.P.; Blaha, J.D.; Calhoun, J.; Henry, S.L.; Patzakis, M.J. A comparison of gentamicin-impregnated polymethylmethacrylate bead implantation to conventional parenteral antibiotic therapy in infected total hip and knee arthroplasty. Clin. Orthop. Relat. Res. 1993, 295, 96–101. [Google Scholar] [CrossRef]
- Stockley, I.; Mockford, B.J.; Hoad-Reddick, A.; Norman, P. The use of two-stage exchange arthroplasty with depot antibiotics in the absence of long-term antibiotic therapy in infected total hip replacement. J. Bone Jt. Surg. 2008, 90, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Hoad-Reddick, D.A.; Evans, C.R.; Norman, P.; Stockley, I. Is there a role for extended antibiotic therapy in a two-stage revision of the infected knee arthroplasty? J. Bone Jt. Surg. 2005, 87, 171–174. [Google Scholar] [CrossRef]
- Hart, W.J.; Jones, R.S. Two-stage revision of infected total knee replacements using articulating cement spacers and short-term antibiotic therapy. J. Bone Jt. Surg. 2006, 88, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K.; Meyer, C. Antibiotic Elution from Hip and Knee Acrylic Bone Cement Spacers: A Systematic Review. BioMed Res. Int. 2017, 2017, 4657874. [Google Scholar] [CrossRef]
- Walenkamp, G.H.I.M. Antibiotic Loaded Cement: From Research to Clinical Evidence. In Infection and Local Treatment in Orthopedic Surgery; Springer: Berlin/Heidelberg, Germany, 2007; pp. 170–175. [Google Scholar]
- Dodds, S.; Smith, T.J.; Akid, R.; Stephenson, J.; Nichol, T.; Banerjee, R.D.; Stockley, I.; Townsend, R. Contrasting Effects of Physical Wear on Elution of Two Antibiotics from Orthopedic Cement. Antimicrob. Agents Chemother. 2012, 56, 1471–1475. [Google Scholar] [CrossRef]
- Walenkamp, G. Small PMMA beads improve gentamicin release. Acta Orthop. 1989, 60, 668–669. [Google Scholar] [CrossRef]
- Stevens, C.M.; Tetsworth, K.D.; Calhoun, J.H.; Mader, J.T. An articulated antibiotic spacer used for infected total knee arthroplasty: A comparative in vitro elution study of Simplex® and Palacos® bone cements. J. Orthop. Res. 2005, 23, 27–33. [Google Scholar] [CrossRef]
- van De Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van Der Mei, H.C.; Busscher, H.J. Gentamicin release from polymethylmethacrylate bone cements and Staphylococcus aureus biofilm formation. Acta Orthop. 2000, 71, 625–629. [Google Scholar] [CrossRef]
- Neut, D.; van de Belt, H.; van Horn, J.; van der Mei, H.; Busscher, H. The effect of mixing on gentamicin release from polymethylmethacrylate bone cements. Acta Orthop. 2003, 74, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, D.; Giorgi, G.; Fornara, P.; Bocchi, L.; Neri, L.; Ceffa, R.; Ghisellini, F.; Ritter, M.A. The in vitro elution characteristics of vancomycin combined with imipenem-cilastatin in acrylic bone–cements: A pharmacokinetic study. J. Arthroplast. 2002, 17, 619–626. [Google Scholar] [CrossRef]
- Bitsch, R.; Kretzer, J.; Vogt, S.; Büchner, H.; Thomsen, M.; Lehner, B. Increased antibiotic release and equivalent biomechanics of a spacer cement without hard radio contrast agents. Diagn. Microbiol. Infect. Dis. 2015, 83, 203–209. [Google Scholar] [CrossRef]
- Penner, M.J.; Duncan, C.P.; Masri, B.A. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J. Arthroplast. 1999, 14, 209–214. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A.C.; Nelson, C.L.; McLaren, S.G.; DeClerk, G.R. The Effect of Glycine Filler on the Elution Rate of Gentamicin from Acrylic Bone Cement. Clin. Orthop. Relat. Res. 2004, 427, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Frew, N.M.; Cannon, T.; Nichol, T.; Smith, T.; Stockley, I. Comparison of the elution properties of commercially available gentamicin and bone cement containing vancomycin with ‘home-made’ preparations. Bone Jt. J. 2017, 99-B, 73–77. [Google Scholar] [CrossRef]
- Meyer, J.; Piller, G.; Spiegel, C.A.; Hetzel, S.; Squire, M. Vacuum-Mixing Significantly Changes Antibiotic Elution Characteristics of Commercially Available Antibiotic-Impregnated Bone Cements. J. Bone Jt. Surg. 2011, 93, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Minelli, E.B.; Della Bora, T.; Benini, A. Different microbial biofilm formation on polymethylmethacrylate (PMMA) bone cement loaded with gentamicin and vancomycin. Anaerobe 2011, 17, 380–383. [Google Scholar] [CrossRef]
- McLaren, A.C.; Nugent, M.; Economopoulos, K.; Kaul, H.; Vernon, B.L.; McLemore, R. Hand-mixed and Premixed Antibiotic-loaded Bone Cement Have Similar Homogeneity. Clin. Orthop. Relat. Res. 2009, 467, 1693–1698. [Google Scholar] [CrossRef]
- Samara, E.; Moriarty, T.F.; Decosterd, L.; Richards, R.G.; Gautier, E.; Wahl, P. Antibiotic stability over six weeks in aqueous solution at body temperature with and without heat treatment that mimics the curing of bone cement. Bone Jt. Res. 2017, 6, 296–306. [Google Scholar] [CrossRef]
- Edelstein, A.I.; Okroj, K.T.; Rogers, T.; Della Valle, C.J.; Sporer, S.M. Systemic Absorption of Antibiotics From Antibiotic-Loaded Cement Spacers for the Treatment of Periprosthetic Joint Infection. J. Arthroplast. 2018, 33, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Huang, R.; Joshi, A.; Parvizi, J.; Hozack, W.J. Renal Impairment Following Total Joint Arthroplasty: Who Is at Risk? J. Arthroplast. 2010, 25, 49–53. [Google Scholar] [CrossRef]
- Duey, R.E.; Chong, A.C.M.; McQueen, D.A.; Womack, J.L.; Song, Z.; Steinberger, T.A.; Wooley, P.H. Mechanical properties and elution characteristics of polymethylmethacrylate bone cement impregnated with antibiotics for various surface area and volume constructs. Iowa Orthop. J. 2012, 32, 104–115. [Google Scholar] [PubMed]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements: A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Buttaro, M.; Valentini, R.; Piccaluga, F. Persistent infection associated with residual cement after resection arthroplasty of the hip. Acta Orthop. 2004, 75, 427–429. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Y.; Ni, H.; Li, M.; Zhang, C.; Qi, B.; Wei, M.; Wang, T.; Xu, Y. Antibiotic-loaded calcium sulfate in clinical treatment of chronic osteomyelitis: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 104. [Google Scholar] [CrossRef]
- Tarar, M.Y.; Khalid, A.; Usman, M.; Javed, K.; Shah, N.; Abbas, M.W. Wound Leakage With the Use of Calcium Sulphate Beads in Prosthetic Joint Surgeries: A Systematic Review. Cureus 2021, 13, e19650. [Google Scholar] [CrossRef]
- Abosala, A.; Ali, M. The Use of Calcium Sulphate beads in Periprosthetic Joint Infection, a systematic review. J. Bone Jt. Infect. 2020, 5, 43–49. [Google Scholar] [CrossRef]
- Howlin, R.P.; Brayford, M.J.; Webb, J.S.; Cooper, J.J.; Aiken, S.S.; Stoodley, P. Antibiotic-Loaded Synthetic Calcium Sulfate Beads for Prevention of Bacterial Colonization and Biofilm Formation in Periprosthetic Infections. Antimicrob. Agents Chemother. 2015, 59, 111–120. [Google Scholar] [CrossRef]
- Heuberger, R.; Wahl, P.; Krieg, J.; Gautier, E. Low in vitro third-body wear on total hip prostheses induced by calcium sulphate used for local antibiotic therapy. Eur. Cells Mater. 2014, 28, 246–257. [Google Scholar] [CrossRef] [PubMed]
- McPherson, F.E.; Dipane, B.M.; Sherif, S. Dissolvable Antibiotic Beads in Treatment of Periprosthetic Joint Infection and Revision Arthroplasty—The Use of Synthetic Pure Calcium Sulfate (Stimulan®) Impregnated with Vancomycin & Tobramycin. Reconstr. Rev. 2013, 3, 32–43. [Google Scholar] [CrossRef]
- Kallala, R.; Harris, W.E.; Ibrahim, M.; Dipane, M.; McPherson, E. Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty. Bone Jt. Res. 2018, 7, 570–579. [Google Scholar] [CrossRef]
- Tarar, M.Y.; Toe, K.K.Z.; Javed, K.; Shah, N.; Khalid, A. The Risk of Iatrogenic Hypercalcemia in Patients Undergoing Calcium Sulphate Beads Implantation in Prosthetic Joint Surgery: A Systematic Review. Cureus 2021, 13, e18777. [Google Scholar] [CrossRef] [PubMed]
- Tarity, T.D.; Xiang, W.; Jones, C.W.; Gkiatas, I.; Nocon, A.; Selemon, N.A.; Carli, A.; Sculco, P.K. Do Antibiotic-Loaded Calcium Sulfate Beads Improve Outcomes After Debridement, Antibiotics, and Implant Retention? A Matched Cohort Study. Arthroplast. Today 2022, 14, 90–95. [Google Scholar] [CrossRef]
- Flierl, M.A.; Culp, B.M.; Okroj, K.T.; Springer, B.D.; Levine, B.R.; Della Valle, C.J. Poor Outcomes of Irrigation and Debridement in Acute Periprosthetic Joint Infection With Antibiotic-Impregnated Calcium Sulfate Beads. J. Arthroplast. 2017, 32, 2505–2507. [Google Scholar] [CrossRef]
- Reinisch, K.; Schläppi, M.; Meier, C.; Wahl, P. Local antibiotic treatment with calcium sulfate as carrier material improves the outcome of debridement, antibiotics, and implant retention procedures for periprosthetic joint infections after hip arthroplasty—A retrospective study. J. Bone Jt. Infect. 2022, 7, 11–21. [Google Scholar] [CrossRef]
- Lum, Z.; Pereira, G.C. Local bio-absorbable antibiotic delivery in calcium sulfate beads in hip and knee arthroplasty. J. Orthop. 2018, 15, 676–678. [Google Scholar] [CrossRef]
- Ene, R.; Nica, M.; Ene, D.; Cursaru, A.; Cirstoiu, C. Review of calcium-sulphate-based ceramics and synthetic bone substitutes used for antibiotic delivery in PJI and osteomyelitis treatment. EFORT Open Rev. 2021, 6, 297–304. [Google Scholar] [CrossRef]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tone, S.; Naito, Y.; Wakabayashi, H.; Sudo, A. Use of antibiotic-impregnated hydroxyapatite for infection following total knee arthroplasty. Mod. Rheumatol. 2021, 31, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.A.; Ferguson, J.Y.; Lau, A.C.K.; Diefenbeck, M.; Scarborough, M.; Ramsden, A.J.; Atkins, B.L. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite. Bone Jt. J. 2016, 98-B, 1289–1296. [Google Scholar] [CrossRef]
- Bidossi, A.; Bottagisio, M.; Logoluso, N.; De Vecchi, E. In Vitro Evaluation of Gentamicin or Vancomycin Containing Bone Graft Substitute in the Prevention of Orthopedic Implant-Related Infections. Int. J. Mol. Sci. 2020, 21, 9250. [Google Scholar] [CrossRef]
- Zampelis, V.; Tägil, M.; Lidgren, L.; Isaksson, H.; Atroshi, I.; Wang, J.-S. The effect of a biphasic injectable bone substitute on the interface strength in a rabbit knee prosthesis model. J. Orthop. Surg. Res. 2013, 8, 25. [Google Scholar] [CrossRef]
- Colding-Rasmussen, T.; Horstmann, P.; Petersen, M.M.; Hettwer, W. Antibiotic Elution Characteristics and Pharmacokinetics of Gentamicin and Vancomycin from a Mineral Antibiotic Carrier: An in vivo Evaluation of 32 Clinical Cases. J. Bone Jt. Infect. 2018, 3, 234–240. [Google Scholar] [CrossRef]
- Logoluso, N.; Drago, L.; Gallazzi, E.; George, D.A.; Morelli, I.; Romanò, C.L. Calcium-Based, Antibiotic-Loaded Bone Substitute as an Implant Coating: A Pilot Clinical Study. J. Bone Jt. Infect. 2016, 1, 59–64. [Google Scholar] [CrossRef]
- McNally, M.A.; Ferguson, J.Y.; Scarborough, M.; Ramsden, A.; Stubbs, D.A.; Atkins, B.L. Mid- to long-term results of single-stage surgery for patients with chronic osteomyelitis using a bioabsorbable gentamicin-loaded ceramic carrier. Bone Jt. J. 2022, 104-B, 1095–1100. [Google Scholar] [CrossRef]
- Sakellariou, V.I.; Savvidou, O.; Markopoulos, C.; Drakou, A.; Mavrogenis, A.F.; Papagelopoulos, P.J. Combination of Calcium Hydroxyapatite Antibiotic Carrier with Cement Spacers in Peri-Prosthetic Knee Infections. Surg. Infect. 2015, 16, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, N.; Hyvönen, P.; Nyyssönen, M.; Kirjavainen, M.; Kankare, J.; Gullichsen, E.; Salo, J. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Bal, B.S.; Huang, W. Review: Emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Mater. Sci. Eng. C 2014, 41, 224–231. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef]
- Bortolin, M.; De Vecchi, E.; Romanò, C.L.; Toscano, M.; Mattina, R.; Drago, L. Antibiofilm agents against MDR bacterial strains: Is bioactive glass BAG-S53P4 also effective? J. Antimicrob. Chemother. 2015, 71, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Romanò, D.; De Vecchi, E.; Vassena, C.; Logoluso, N.; Mattina, R.; Romano, C.L. Bioactive glass BAG-S53P4 for the adjunctive treatment of chronic osteomyelitis of the long bones: An in vitroand prospective clinical study. BMC Infect. Dis. 2013, 13, 584. [Google Scholar] [CrossRef]
- Pérez-Tanoira, R.; García-Pedrazuela, M.; Hyyrynen, T.; Soininen, A.; Aarnisalo, A.; Nieminen, M.; Tiainen, V.-M.; Konttinen, Y.T.; Kinnari, T. Effect of S53P4 bone substitute on staphylococcal adhesion and biofilm formation on other implant materials in normal and hypoxic conditions. J. Mater. Sci. Mater. Med. 2015, 26, 239. [Google Scholar] [CrossRef]
- Cunha, M.T.; Murça, M.A.; Nigro, S.; Klautau, G.B.; Salles, M.J.C. In vitro antibacterial activity of bioactive glass S53P4 on multiresistant pathogens causing osteomyelitis and prosthetic joint infection. BMC Infect. Dis. 2018, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Majumdar, S.; Krishnamurthy, S. Bioactive glass: A multifunctional delivery system. J. Control Release 2021, 335, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Olmo, L.; Ros-Nicolás, M.J.; Clavel-Sainz, M.; Vicente-Ortega, V.; Alcaraz-Baños, M.; Lax-Pérez, A.; Arcos, D.; Ragel, C.V.; Vallet-Regí, M. Biocompatibility andin vivogentamicin release from bioactive sol-gel glass implants. J. BioMed Mater. Res. 2002, 61, 458–465. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, X.; Jia, W.; Zhang, C.; Huang, W.; Wang, J. Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate glass releasing vancomycin. J. Control Release 2009, 139, 118–126. [Google Scholar] [CrossRef]
- Jia, W.-T.; Zhang, X.; Luo, S.-H.; Liu, X.; Huang, W.-H.; Rahaman, M.N.; Day, D.E.; Zhang, C.-Q.; Xie, Z.-P.; Wang, J.-Q. Novel borate glass/chitosan composite as a delivery vehicle for teicoplanin in the treatment of chronic osteomyelitis. Acta Biomater. 2010, 6, 812–819. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, W.; Gu, Y.; Xiao, W.; Liu, X.; Wang, D.; Zhang, C.; Huang, W.; Rahaman, M.N.; Day, D.E.; et al. Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials 2010, 31, 5865–5874. [Google Scholar] [CrossRef]
- Domingues, R.; Cortés, M.; Gomes, T.; Diniz, H.; Freitas, C.; Gomes, J.; Faria, A.M.; Sinisterra, R. Bioactive glass as a drug delivery system of tetracycline and tetracycline associated with β-cyclodextrin. Biomaterials 2004, 25, 327–333. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, C.-J.; Cui, X.; Gu, Y.-F.; Jia, W.-T.; Rahaman, M.N.; Wang, Y.; Huang, W.-H.; Zhang, C.-Q. A Novel Injectable Borate Bioactive Glass Cement as an Antibiotic Delivery Vehicle for Treating Osteomyelitis. PLoS ONE 2014, 9, e85472. [Google Scholar] [CrossRef]
- Jia, W.-T.; Fu, Q.; Huang, W.-H.; Zhang, C.-Q.; Rahaman, M.N. Comparison of Borate Bioactive Glass and Calcium Sulfate as Implants for the Local Delivery of Teicoplanin in the Treatment of Methicillin-Resistant Staphylococcus aureus-Induced Osteomyelitis in a Rabbit Model. Antimicrob. Agents Chemother. 2015, 59, 7571–7580. [Google Scholar] [CrossRef]
- Lindfors, N.; Geurts, J.; Drago, L.; Arts, J.J.; Juutilainen, V.; Hyvönen, P.; Suda, A.J.; Domenico, A.; Artiaco, S.; Alizadeh, C.; et al. Antibacterial Bioactive Glass, S53P4, for Chronic Bone Infections—A Multinational Study. In A Modern Approach to Biofilm-Related Orthopaedic Implant Infections: Advances in Microbiology, Infectious Diseases and Public Health Volume 5; Drago, L., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 81–92. [Google Scholar]
- Romanò, C.L.; Logoluso, N.; Meani, E.; Romanò, D.; De Vecchi, E.; Vassena, C.; Drago, L. A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis. Bone Jt. J. 2014, 96-B, 845–850. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, J.; Efrimescu, C.; Sheehan, E.; Niall, D. Through the looking glass; bioactive glass S53P4 (BonAlive®) in the treatment of chronic osteomyelitis. Ir. J. Med Sci. 2013, 182, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.J.; Huynh, R.; Jarbo, K.; McLemore, R.Y.; Vernon, B.L. In situforming, resorbable graft copolymer hydrogels providing controlled drug release. J. BioMed Mater. Res. Part A 2012, 101A, 1437–1446. [Google Scholar] [CrossRef]
- Censi, R.; Casadidio, C.; Dubbini, A.; Cortese, M.; Scuri, S.; Grappasonni, I.; Golob, S.; Vojnovic, D.; Sabbieti, M.G.; Agas, D.; et al. Thermosensitive hybrid hydrogels for the controlled release of bioactive vancomycin in the treatment of orthopaedic implant infections. Eur. J. Pharm. Biopharm. 2019, 142, 322–333. [Google Scholar] [CrossRef]
- Lai, P.-L.; Hong, D.-W.; Ku, K.-L.; Lai, Z.-T.; Chu, I.-M. Novel thermosensitive hydrogels based on methoxy polyethylene glycol-co-poly(lactic acid-co-aromatic anhydride) for cefazolin delivery. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Tsikopoulos, K.; Bidossi, A.; Drago, L.; Petrenyov, D.; Givissis, P.; Mavridis, D.; Papaioannidou, P. Is Implant Coating With Tyrosol- and Antibiotic-loaded Hydrogel Effective in Reducing Cutibacterium (Propionibacterium) acnes Biofilm Formation? A Preliminary In Vitro Study. Clin. Orthop. Relat. Res. 2019, 477, 1736–1746. [Google Scholar] [CrossRef]
- Kim, H.; Chang, R.; Morales, S.; Chan, H.-K. Bacteriophage-Delivering Hydrogels: Current Progress in Combating Antibiotic Resistant Bacterial Infection. Antibiotics 2021, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.J.; Badha, V.S.; Heffernan, J.M.; Childers, E.P.; Moore, R.C.; Vernon, B.L.; McLaren, A.C. Temperature-responsive PNDJ hydrogels provide high and sustained antimicrobial concentrations in surgical sites. Drug Deliv. Transl. Res. 2019, 9, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Yu, Y.-H.; Lee, D.; Chou, Y.-C.; Wu, C.-K.; Lu, C.-J.; Liu, S.-J. Pharmaceutical-eluting hybrid degradable hydrogel/microparticle loaded sacs for finger joint interpositional arthroplasty. Biomater. Adv. 2022, 137, 212846. [Google Scholar] [CrossRef]
- De Meo, D.; Ceccarelli, G.; Iaiani, G.; Torto, F.L.; Ribuffo, D.; Persiani, P.; Villani, C. Clinical Application of Antibacterial Hydrogel and Coating in Orthopaedic and Traumatology Surgery. Gels 2021, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Zagra, L.; Gallazzi, E.; Romanò, D.; Scarponi, S.; Romanò, C. Two-stage cementless hip revision for peri-prosthetic infection with an antibacterial hydrogel coating: Results of a comparative series. Int. Orthop. 2018, 43, 111–115. [Google Scholar] [CrossRef]
- Capuano, N.; Logoluso, N.; Gallazzi, E.; Drago, L.; Romanò, C.L. One-stage exchange with antibacterial hydrogel coated implants provides similar results to two-stage revision, without the coating, for the treatment of peri-prosthetic infection. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 3362–3367. [Google Scholar] [CrossRef]
- Thakrar, R.R.; Horriat, S.; Kayani, B.; Haddad, F.S. Indications for a single-stage exchange arthroplasty for chronic prosthetic joint infection. Bone Jt. J. 2019, 101-B, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; Van Der Straeten, C.; Scarponi, S.; Drago, L. Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection After Joint Arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y.; Su, L.; Yang, G.; Ren, Y.; Zhang, M.; Jing, H.; Zhang, X.; Bayston, R.; van der Mei, H.C.; Busscher, H.J.; et al. Self-targeting of zwitterion-based platforms for nano-antimicrobials and nanocarriers. J. Mater. Chem. B 2022, 10, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, L.; Tang, K.; Chen, S.; Xu, Y.; Liao, H.; Niu, C. IR780 Based Sonotherapeutic Nanoparticles to Combat Multidrug-Resistant Bacterial Infections. Front. Chem. 2022, 10, 840598. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, T.; Lin, F.-C.; Zhang, B.; Zink, J.I. Supramolecular Assemblies of Heterogeneous Mesoporous Silica Nanoparticles to Co-deliver Antimicrobial Peptides and Antibiotics for Synergistic Eradication of Pathogenic Biofilms. ACS Nano 2020, 14, 5926–5937. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Wu, S.; Sun, Y.; Ren, J.; Qu, X. Self-Propelled Active Photothermal Nanoswimmer for Deep-Layered Elimination of Biofilm In Vivo. Nano Lett. 2020, 20, 7350–7358. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Winkler, T.; Müller, M.; Perka, C.; Trampuz, A. Bacteriophages as Adjuvant to Antibiotics for the Treatment of Periprosthetic Joint Infection Caused by Multidrug-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 64, e00924-19. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Leboucher, G.; Fevre, C.; Herry, Y.; Conrad, A.; Josse, J.; Batailler, C.; Chidiac, C.; Medina, M.; Lustig, S.; et al. Salvage Debridement, Antibiotics and Implant Retention (“DAIR”) With Local Injection of a Selected Cocktail of Bacteriophages: Is It an Option for an Elderly Patient With Relapsing Staphylococcus aureus Prosthetic-Joint Infection? Open Forum Infect. Dis. 2018, 5, ofy269. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Batailler, C.; Souche, A.; Cassino, C.; Chidiac, C.; Perpoint, T.; le Corvaisier, C.; Josse, J.; Gaillard, R.; Roger, J.; et al. Arthroscopic “Debridement and Implant Retention” With Local Administration of Exebacase (Lysin CF-301) Followed by Suppressive Tedizolid as Salvage Therapy in Elderly Patients for Relapsing Multidrug-Resistant, S. epidermidis Prosthetic Knee Infection. Front. Med. 2021, 8, 550853. [Google Scholar] [CrossRef]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gustave, C.-A.; Lustig, S.; Malatray, M.; Fevre, C.; Josse, J.; Petitjean, C.; Chidiac, C.; et al. Phage Therapy as Adjuvant to Conservative Surgery and Antibiotics to Salvage Patients With Relapsing, S. aureus Prosthetic Knee Infection. Front. Med. 2020, 7, 570572. [Google Scholar] [CrossRef]
- Ferry, T.; Kolenda, C.; Briot, T.; Souche, A.; Lustig, S.; Josse, J.; Batailler, C.; Pirot, F.; Medina, M.; Leboucher, G.; et al. Past and Future of Phage Therapy and Phage-Derived Proteins in Patients with Bone and Joint Infection. Viruses 2021, 13, 2414. [Google Scholar] [CrossRef]
- Akanda, Z.Z.; Taha, M.; Abdelbary, H. Current review-The rise of bacteriophage as a unique therapeutic platform in treating peri-prosthetic joint infections. J. Orthop. Res. 2017, 36, 1051–1060. [Google Scholar] [CrossRef]
- Clarke, A.L.; De Soir, S.; Jones, J.D. The Safety and Efficacy of Phage Therapy for Bone and Joint Infections: A Systematic Review. Antibiotics 2020, 9, 795. [Google Scholar] [CrossRef]
- Fabijan, A.P.; Lin, R.C.; Ho, J.; Maddocks, S.; Zakour, N.L.b.; Iredell, J.R. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef]
- Ferry, T.; Batailler, C.; Brosset, S.; Kolenda, C.; Goutelle, S.; Sappey-Marinier, E.; Josse, J.; Laurent, F.; Lustig, S.; On Behalf of the Lyon BJI Study Group. Medical innovations to maintain the function in patients with chronic PJI for whom explantation is not desirable: A pathophysiology-, multidisciplinary-, and experience-based approach. Sicot-J 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Gibb, B.P.; Hadjiargyrou, M. Bacteriophage therapy for bone and joint infections. Bone Jt. J. 2021, 103-B, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gaillard, R.; Gustave, C.-A.; Lustig, S.; Fevre, C.; Petitjean, C.; Leboucher, G.; Laurent, F.; et al. Case Report: Arthroscopic “Debridement Antibiotics and Implant Retention” With Local Injection of Personalized Phage Therapy to Salvage a Relapsing Pseudomonas Aeruginosa Prosthetic Knee Infection. Front. Med. 2021, 8, 569159. [Google Scholar] [CrossRef] [PubMed]
- Patey, O.; McCallin, S.; Mazure, H.; Liddle, M.; Smithyman, A.; Dublanchet, A. Clinical Indications and Compassionate Use of Phage Therapy: Personal Experience and Literature Review with a Focus on Osteoarticular Infections. Viruses 2018, 11, 18. [Google Scholar] [CrossRef]
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M.; et al. Phage Therapy for Limb-threatening Prosthetic KneeKlebsiella pneumoniaeInfection: Case Report and In Vitro Characterization of Anti-biofilm Activity. Clin. Infect. Dis. 2020, 73, e144–e151. [Google Scholar] [CrossRef]
- Mitchell, G.; Silvis, M.R.; Talkington, K.C.; Budzik, J.M.; Dodd, C.E.; Paluba, J.M.; Oki, E.A.; Trotta, K.L.; Licht, D.J.; Jimenez-Morales, D.; et al. Ceragenins and Antimicrobial Peptides Kill Bacteria through Distinct Mechanisms. Mbio 2022, 13, e02726-2. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, S.J.; Skłodowski, K.; Piktel, E.; Suprewicz, Ł.; Fiedoruk, K.; Daniluk, T.; Wolak, P.; Savage, P.B.; Bucki, R. NDM-1 Carbapenemase-Producing Enterobacteriaceae are Highly Susceptible to Ceragenins CSA-13, CSA-44, and CSA-131. Infect. Drug Resist. 2020, 13, 3277–3294. [Google Scholar] [CrossRef]
- Bucki, R.; Niemirowicz, K.; Wnorowska, U.; Byfield, F.J.; Piktel, E.; Wątek, M.; Janmey, P.A.; Savage, P.B. Bactericidal Activity of Ceragenin CSA-13 in Cell Culture and in an Animal Model of Peritoneal Infection. Antimicrob. Agents Chemother. 2015, 59, 6274–6282. [Google Scholar] [CrossRef]
- Mills, R.J.; Boyling, A.; Cheng, T.L.; Peacock, L.; Savage, P.B.; Tägil, M.; Little, D.G.; Schindeler, A. CSA-90 reduces periprosthetic joint infection in a novel rat model challenged with local and systemic Staphylococcus aureus. J. Orthop. Res. 2020, 38, 2065–2073. [Google Scholar] [CrossRef]
- Wnorowska, U.; Piktel, E.; Deptuła, P.; Wollny, T.; Król, G.; Głuszek, K.; Durnaś, B.; Pogoda, K.; Savage, P.B.; Bucki, R. Ceragenin CSA-13 displays high antibacterial efficiency in a mouse model of urinary tract infection. Sci. Rep. 2022, 12, 19164. [Google Scholar] [CrossRef]
- Spałek, J.; Daniluk, T.; Godlewski, A.; Deptuła, P.; Wnorowska, U.; Ziembicka, D.; Cieśluk, M.; Fiedoruk, K.; Ciborowski, M.; Krętowski, A.; et al. Assessment of Ceragenins in Prevention of Damage to Voice Prostheses Caused by Candida Biofilm Formation. Pathogens 2021, 10, 1371. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.M.; Rovig, J.; Bateman, J.; Holden, B.S.; Modelzelewski, T.; Gueorguieva, I.; von Dyck, M.; Bracken, R.; Genberg, C.; Deng, S.; et al. Preclinical testing of a broad-spectrum antimicrobial endotracheal tube coated with an innate immune synthetic mimic. J. Antimicrob. Chemother. 2017, 73, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Medicine, U.S.N.L.o. Ceragenin Coated Endotracheal Tubes for the Prevention of Ventilator Associated Pneumonia (CEASEVAP); National Institute of Health: Bethesda, Maryland, United States of America, 2023. [Google Scholar]
- Li, P.; Gao, Z.; Tan, Z.; Xiao, J.; Wei, L.; Chen, Y. New developments in anti-biofilm intervention towards effective management of orthopedic device related infections (ODRI’s). Biofouling 2021, 37, 1–35. [Google Scholar] [CrossRef]
- Dudareva, M.; Kümin, M.; Vach, W.; Kaier, K.; Ferguson, J.; McNally, M.; Scarborough, M. Short or Long Antibiotic Regimes in Orthopaedics (SOLARIO): A randomised controlled open-label non-inferiority trial of duration of systemic antibiotics in adults with orthopaedic infection treated operatively with local antibiotic therapy. Trials 2019, 20, 693. [Google Scholar] [CrossRef]
- Baeza, J.; Cury, M.B.; Fleischman, A.; Ferrando, A.; Fuertes, M.; Goswami, K.; Lidgren, L.; Linke, P.; Manrique, J.; Makar, G.; et al. General Assembly, Prevention, Local Antimicrobials: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2018, 34, S75–S84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steadman, W.; Chapman, P.R.; Schuetz, M.; Schmutz, B.; Trampuz, A.; Tetsworth, K. Local Antibiotic Delivery Options in Prosthetic Joint Infection. Antibiotics 2023, 12, 752. https://doi.org/10.3390/antibiotics12040752
Steadman W, Chapman PR, Schuetz M, Schmutz B, Trampuz A, Tetsworth K. Local Antibiotic Delivery Options in Prosthetic Joint Infection. Antibiotics. 2023; 12(4):752. https://doi.org/10.3390/antibiotics12040752
Chicago/Turabian StyleSteadman, William, Paul R. Chapman, Michael Schuetz, Beat Schmutz, Andrej Trampuz, and Kevin Tetsworth. 2023. "Local Antibiotic Delivery Options in Prosthetic Joint Infection" Antibiotics 12, no. 4: 752. https://doi.org/10.3390/antibiotics12040752
APA StyleSteadman, W., Chapman, P. R., Schuetz, M., Schmutz, B., Trampuz, A., & Tetsworth, K. (2023). Local Antibiotic Delivery Options in Prosthetic Joint Infection. Antibiotics, 12(4), 752. https://doi.org/10.3390/antibiotics12040752






