Ceft-to-Ceft Study: Real-Life Experience with Ceftaroline and Ceftobiprole in Treatment of the Principal Infectious Syndromes in a Spanish Multicenter Hospital Cohort
Abstract
:1. Introduction
2. Results
2.1. Cohort Description
2.2. Microbiological Isolation
2.3. Health Outcomes
Infective Endocarditis
2.4. Adverse Effects
2.5. Mortality Risk Factors by Antibiotic
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Treatment Description
4.3. Variables
4.4. Definitions
4.5. Sample Size
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Antimicrobial Resistance Surveillance in Europe, 2022–2020 Data. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data (accessed on 6 August 2023).
- Farrell, D.J.; Castanheira, M.; Mendes, R.E.; Sader, H.S.; Jones, R.N. In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: A review of published studies and the AWARE Surveillance Program (2008–2010). Clin. Infect. Dis. 2012, 55 (Suppl. S3), 206–214. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; Lee, W.S.; Ko, W.C.; Hsueh, P.R. In vitro susceptibility of ceftaroline against clinically important Gram-positive cocci, Haemophilus species and Klebsiella pneumoniae in Taiwan: Results from the Antimicrobial Testing Leadership and Surveillance (ATLAS) in 2012–2018. J. Microbiol. Immunol. Infect. 2021, 54, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Laudano, J.B. Ceftaroline fosamil: A new broad-spectrum cephalosporin. J. Antimicrob. Chemother. 2011, 66 (Suppl. S3), 11–18. [Google Scholar] [CrossRef] [PubMed]
- Taboada, M.; Melnick, D.; Iaconis, J.P.; Sun, F.; Zhong, N.S.; File, T.M.; Llorens, L.; Friedland, H.D.; Wilson, D. Ceftaroline fosamil versus ceftriaxone for the treatment of community-acquired pneumonia: Individual patient data meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2016, 71, 862–870. [Google Scholar] [CrossRef]
- Corey, G.R.; Wilcox, M.H.; Talbot, G.H.; Thye, D.; Friedland, D.; Baculik, T. CANVAS 1: The first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections on behalf of the CANVAS 1 investigators. J. Antimicrob. Chemother. 2010, 65, 41–51. [Google Scholar] [CrossRef]
- Athans, V.; Kenney, R.M.; Wong, J.; Davis, S.L. Outpatient use of ceftaroline fosamil versus vancomycin for osteoarticular infection: A matched cohort study. J. Antimicrob. Chemother. 2016, 71, 3568–3574. [Google Scholar] [CrossRef]
- Pani, A.; Colombo, F.; Agnelli, F.; Frantellizzi, V.; Baratta, F.; Pastori, D.; Scaglione, F. Off-label use of ceftaroline fosamil: A systematic review. Int. J. Antimicrob. Agents 2019, 54, 562–571. [Google Scholar] [CrossRef]
- Cosimi, R.A.; Beik, N.; Kubiak, D.W.; Johnson, J.A. Ceftaroline for Severe Methicillin-Resistant Staphylococcus aureus Infections: A Systematic Review. Open Forum Infect. Dis. 2017, 4, ofx084. [Google Scholar] [CrossRef]
- Pérez-Crespo, P.M.M.; Cortés, L.E.L. Ceftobiprole: A clinical view. Rev. Española Quimioter. 2021, 34, 32. [Google Scholar] [CrossRef]
- Lupia, T.; Pallotto, C.; Corcione, S.; Boglione, L.; De Rosa, F.G. Ceftobiprole Perspective: Current and Potential Future Indications. Antibiotics 2021, 10, 170. [Google Scholar] [CrossRef]
- Rouse, M.S.; Steckelberg, J.M.; Patel, R. In vitro activity of ceftobiprole, daptomycin, linezolid, and vancomycin against methicillin-resistant staphylococci associated with endocarditis and bone and joint infection. Diagn. Microbiol. Infect. Dis. 2007, 58, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Abbanat, D.; Shang, W.; Amsler, K.; Santoro, C.; Baum, E.; Crespo-Carbone, S.; Lynch, A.S. Evaluation of the in vitro activities of ceftobiprole and comparators in staphylococcal colony or microtitre plate biofilm assays. Int. J. Antimicrob. Agents 2014, 43, 32–39. [Google Scholar] [CrossRef]
- Nicholson, S.C.; Welte, T.; File, T.M., Jr.; Strauss, R.S.; Michiels, B.; Kaul, P.; Balis, D.; Arbit, D.; Amsler, K.; Noel, G.J. A randomised, double-blind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation. Int. J. Antimicrob. Agents 2012, 39, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.S.; Rodriguez, A.H.; Chuang, Y.C.; Marjanek, Z.; Pareigis, A.J.; Reis, G.; Scheeren, T.W.; Sánchez, A.S.; Zhou, X.; Saulay, M.; et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin. Infect. Dis. 2014, 59, 51–61. [Google Scholar] [CrossRef]
- Overcash, J.S.; Kim, C.; Keech, R.; Gumenchuk, I.; Ninov, B.; Gonzalez-Rojas, Y.; Waters, M.; Simeonov, S.; Engelhardt, M.; Saulay, M.; et al. Ceftobiprole Compared with Vancomycin Plus Aztreonam in the Treatment of Acute Bacterial Skin and Skin Structure Infections: Results of a Phase 3, Randomized, Double-blind Trial (TARGET). Clin. Infect. Dis. 2021, 73, e1507–e1517. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef]
- Berger, M.L.; Sox, H.; Willke, R.J.; Brixner, D.L.; Eichler, H.G.; Goettsch, W.; Madigan, D.; Makady, A.; Schneeweiss, S.; Tarricone, R.; et al. Good Practices for Real-World Data Studies of Treatment and/or Comparative Effectiveness: Recommendations from the Joint ISPOR-ISPE Special Task Force on Real-World Evidence in Health Care Decision Making. Value Health 2017, 20, 1003–1008. [Google Scholar] [CrossRef]
- Geriak, M.; Haddad, F.; Rizvi, K.; Rose, W.; Kullar, R.; LaPlante, K.; Yu, M.; Vasina, L.; Ouellette, K.; Zervos, M.; et al. Clinical Data on Daptomycin plus Ceftaroline versus Standard of Care Monotherapy in the Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2019, 63, e02483-18. [Google Scholar] [CrossRef]
- Barber, K.E.; Werth, B.J.; Ireland, C.E.; Stone, N.E.; Nonejuie, P.; Sakoulas, G.; Pogliano, J.; Rybak, M.J. Potent synergy of ceftobiprole plus daptomycin against multiple strains of Staphylococcus aureus with various resistance phenotypes. J. Antimicrob. Chemother. 2014, 69, 3006–3010. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Ramani, A.; Udeani, G.; Evans, J.; Jandourek, A.; Cole, P.; Smith, A.; David Friedland, H. Contemporary use of ceftaroline fosamil for the treatment of community-acquired bacterial pneumonia: CAPTURE study experience. J. Chemother. 2014, 26, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Udeani, G.; Cole, P.; Friedland, H.D. Ceftaroline fosamil for the treatment of hospital-acquired pneumonia and ventilator-associated pneumonia. Hosp. Pract. 2015, 43, 144–149. [Google Scholar] [CrossRef]
- Destache, C.J.; Guervil, D.J.; Kaye, K.S. Ceftaroline fosamil for the treatment of Gram-positive endocarditis: CAPTURE study experience. Int. J. Antimicrob. Agents 2019, 53, 644–649. [Google Scholar] [CrossRef]
- Camazón, N.V.; Cediel, G.; Aragón, R.N.; Mateu, L.; Llibre, C.; Sopena, N.; Gual, F.; Ferrer, E.; Quesada, M.D.; Berastegui, E.; et al. Mortalidad a corto y largo plazo de pacientes con indicación quirúrgica no intervenidos en el curso de la endocarditis infecciosa izquierda. Rev. Esp. Cardiol. 2020, 73, 734–740. [Google Scholar] [CrossRef]
- Lan, S.H.; Lee, H.Z.; Lai, C.C.; Chang, S.P.; Lu, L.C.; Hung, S.H.; Lin, W.T. Clinical efficacy and safety of ceftobiprole in the treatment of acute bacterial skin and skin structure infection: A systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti-Infect. Ther. 2022, 20, 95–102. [Google Scholar] [CrossRef]
- Holland, T.L.; Cosgrove, S.E.; Doernberg, S.B.; Jenkins, T.C.; Turner, N.A.; Boucher, H.W.; Pavlov, O.; Titov, I.; Kosulnykov, S.; Atanasov, B.; et al. Ceftobiprole for Treatment of Complicated Staphylococcus aureus Bacteremia. N. Engl. J. Med. 2023, 389, 1390–1401. [Google Scholar] [CrossRef]
- Zevtera (Ceftobiprole). Product Monograph; AVIR Pharma Inc.: Blainville, QC, Canada, 2021; Available online: https://www.avirpharma.com/pdf/Product-Monograph-Zevtera.pdf (accessed on 6 August 2023).
- Bellut, H.; Arrayago, M.; Amara, M.; Roujansky, A.; Micaelo, M.; Bruneel, F.; Bedos, J.P. Real-life use of ceftobiprole for severe infections in a French intensive care unit. Infect. Dis. Now 2023, 27, 104790. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, K.S.; Hsann, Y.M.; Lim, V.; Ong, B.C. The effect of comorbidity and age on hospital mortality and length of stay in patients with sepsis. J. Crit. Care 2010, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Ryoo, S.M.; Shin, T.G.; Park, Y.S.; Jo, Y.H.; Lim, T.H.; Chung, S.P.; Choi, S.H.; Suh, G.J.; Kim, W.Y.; et al. Prognostic factors for late death in septic shock survivors: A multi-center, prospective, registry-based observational study. Intern. Emerg. Med. 2022, 17, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Tenorio, C.; Gálvez, J.; Martínez-Marcos, F.J.; Plata-Ciezar, A.; De La Torre-Lima, J.; López-Cortés, L.E.; Noureddine, M.; Reguera, J.M.; Vinuesa, D.; García, M.V.; et al. Clinical and prognostic differences between methicillin-resistant and methicillin-susceptible Staphylococcus aureus infective endocarditis. BMC Infect. Dis. 2020, 20, 160. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Ostermann, H.; Racketa, J.; Medina, J.; McBride, K.; Garau, J.; REACH study group. Early versus later response to treatment in patients with community-acquired pneumonia: Analysis of the REACH study. Respir. Res. 2014, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Low, D.E. Ceftaroline fosamil in the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections. Drugs 2012, 72, 1473–1493. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Andini, R.; Mazza, M.C.; Sangiovanni, F.; Bertolino, L.; Ursi, M.P.; Paradiso, L.; Karruli, A.; Esposito, C.; Murino, P.; et al. Real-life experience with ceftobiprole in a tertiary-care hospital. J. Glob. Antimicrob. Resist. 2020, 22, 386–390. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Kosar, J.; Baxter, M.; Dhami, R.; Borgia, S.; Irfan, N.; MacDonald, K.S.; Dow, G.; Lagacé-Wiens, P.; Dube, M.; et al. Real-life experience with ceftobiprole in Canada: Results from the CLEAR (Canadian Leadership on Antimicrobial Real-life usage) registry. J. Glob. Antimicrob. Resist. 2021, 24, 335–339. [Google Scholar] [CrossRef]
- Sullivan, E.L.; Turner, R.B.; O’Neal, H.R., Jr.; Crum-Cianflone, N.F. Ceftaroline-Associated Neutropenia: Case Series and Literature Review of Incidence, Risk Factors, and Outcomes. Open Forum Infect. Dis. 2019, 6, 168. [Google Scholar] [CrossRef]
- Zampino, R.; Gallo, R.; Salemme, A.; Marrazzo, T.; Iossa, D.; Karruli, A.; Andini, R.; Esitini, D.; Moretto, S.M.; De Gregorio, F.; et al. Clinical results with the use of ceftaroline and ceftobiprole: Real-life experience in a tertiary care hospital. Int. J. Antimicrob. Agents 2023, 62, 106883. [Google Scholar] [CrossRef]
- Charlson, M.E.; Charlson, R.E.; Peterson, J.C.; Marinopoulos, S.S.; Briggs, W.M.; Hollenberg, J.P. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J. Clin. Epidemiol. 2008, 61, 1234–1240. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 13.0; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2023. [Google Scholar]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; Musher, D.M.; Evans, S.E.; Cruz, C.D.; Crothers, K.A.; Hage, C.A.; Aliberti, S.; Anzueto, A.; Arancibia, F.; Arnold, F.; et al. Treatment of Community-Acquired Pneumonia in Immunocompromised Adults: A Consensus Statement Regarding Initial Strategies. Chest 2020, 158, 1896–1911. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Herreros, J.M.; Cobo-Reinoso, J.; Pujol-Rojo, M.; Rodríguez-Baño, J.; Salavert-Lletí, M. Guía para el diagnóstico y tratamiento del paciente con bacteriemia. Guías de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC). Enferm. Infecc. Microbiol. Clin. 2007, 25, 111–130. [Google Scholar] [CrossRef]
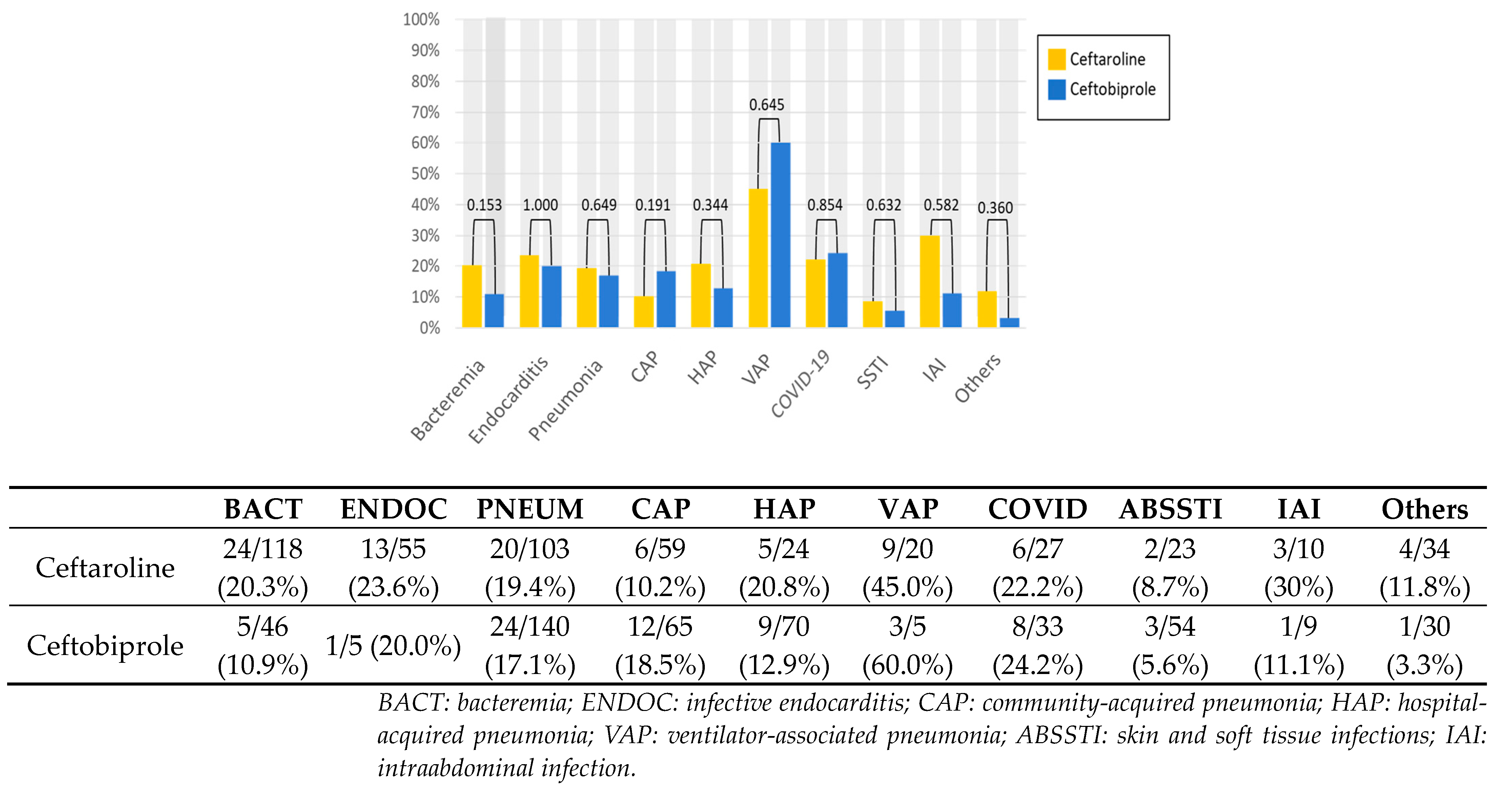

| Ceftaroline (Cohort N = 227) | Ceftobiprole (Cohort N = 212) | p * | OR (95%CI) | |
|---|---|---|---|---|
| Age, mean (years) (± SD) | 63.02 (±13.45) | 66.40 (±15.44) | 0.015 | 1.1 (1.001–1.05) |
| Charlson index, mean (IQR) | 4 (2–6) | 4 (2–6) | 0.290 | |
| Men, n (%) | 140 (61.7) | 126 (59.4) | 0.631 | |
| Sepsis or septic shock, n (%) | 86 (37.9) | 62 (29.2) | 0.056 | |
| 38 (16.7) | 53 (25.0) | 0.033 | 6.7 (1.96–22.9) |
| 48 (21.1) | 8 (4.2) | 0.0001 | 0.27 (0.09–0.81) |
| Acquisition of infection, n (%) | ||||
| 109 (48.0) | 91 (42.9) | 0.284 | |
| 118 (52.0) | 121 (57.1) | ||
| Inpatient department, n (%) | 191 (84.1) | 169 (79.7) | 0.228 | |
| Medical departments, n (%) | 119 (62.3) | 134 (78.1) | 0.234 | |
| Surgical department, n (%) | 36 (18.8) | 35 (20.7) | 0.853 | |
| OPAT, n (%) | 0 (0.0) | 8 (3.8) | 0.003 | 0 (0–0) |
| Cardiovascular risk factors, n (%) | 168 (74.0) | 104 (49.1) | 0.0001 | 0.66 (0.19–2.3) |
| 123 (54.2) | 91 (42.9) | 0.018 | 1.4 (0.45–3.9) |
| 82 (36.1) | 55 (25.9) | 0.021 | 1.04 (0.5–2.2) |
| 80 (35.2) | 31 (14.6) | 0.0001 | 0.38 (0.16–0.87) |
| 21 (9.3) | 1 (0.5) | 0.0001 | 0 (0–0) |
| 30 (13.2) | 9 (4.2) | 0.001 | 0.41 (0.12–1.42) |
| 18 (7.9) | 6 (2.8) | 0.019 | 0.52 (0.11–2.4) |
| Cardiovascular diseases, n (%) | 90 (39.6) | 68 (32.1) | 0.099 | |
| 33 (14.5) | 21 (9.9) | 0.140 | |
| 34 (15.0) | 33 (15.6) | 0.864 | |
| 43 (18.9) | 9 (4.2) | 0.0001 | 0.26 (0.08–0.82) |
| 37 (16.3) | 18 (8.5) | 0.014 | 1.2 (0.45–3.35) |
| Respiratory diseases, n (%) | 56 (24.7) | 45 (21.2) | 0.392 | |
| 32 (14.1) | 36 (17.0) | 0.404 | |
| 10 (4.4) | 11 (5.2) | 0.701 | |
| Cirrhosis—chronic liver disease, n (%) | 21 (9.3) | 23 (10.8) | 0.577 | |
| Chronic kidney disease, n (%) | 35 (15.4) | 27 (12.7) | 0.420 | |
| 7 (3.1) | 6 (2.8) | 0.876 | |
| Active solid malignancy, n (%) | 10 (4.4) | 15 (7.1) | 0.152 | |
| 6 (2.6) | 15 (7.1) | 0.030 | 2.9 (0.9–8.8) |
| 4 (1.8) | 0 (0.0) | 0.090 | |
| Active hematologic disease, n (%) | 15 (6.6) | 23 (10.8) | 0.114 | |
| Neurological disease, n (%) | 30 (13.2) | 37 (17.5) | 0.217 | |
| 6 (2.6) | 11 (5.2) | 0.167 | |
| 19 (8.4) | 15 (7.1) | 0.612 | |
| 3 (1.3) | 3 (1.4) | 1.000 | |
| Immunocompromised, n (%) | 60 (26.4) | 61 (28.8) | 0.583 | |
| 45 (19.8) | 35 (16.6) | 0.381 | |
| Solid organ transplantation, n (%) | 23 (10.1) | 5 (2.4) | 0.001 | |
| HIV infection, n (%) | 4 (1.8) | 7 (3.3) | 0.302 | |
| Antibiotic therapy | ||||
| Total dose of antibiotic (mg), median (IQR) | 11,700 (6600–19,800) | 11,000 (6250–15,000) | 0.015 | |
| Duration of antibiotic therapy (days), median (IQR) | 8 (5–14) | 7 (5–10) | 0.023 | |
| Treatment regimen, n (%) | ||||
| 25 (11.0) | 115 (54.2) | 0.0001 | 0.13 (0.06–0.28) |
| 202 (89.0) | 97 (45.8) | ||
| Susceptibility-guided treatment, n (%) | 131 (57.7) | 63 (29.7) | 0.0001 | 0.35 (0.18–0.69) |
| Empirical treatment, n (%) | 96 (42.3) | 149 (70.3) | 0.104 | |
| Antibiotic prescription, n (%) | ||||
| 40 (17.6) | 75 (35.4) | 0.0001 | 0.35 (0.18–0.69) |
| 187 (82.4) | 137 (64.6) | ||
| Duration of previous antibiotic treatment (days), median (IQR) | 6 (3–10) | 3 (2–6.50) | 0.0001 | 1 (1–1) |
| Reason for switching, n (%) | ||||
| 152 (81.3) | 92 (67.2) | 0.004 | 1.3 (0.32–5.4) |
| 22 (11.8) | 39 (28.5) | 0.0001 | 6.3 (1.3–30.5) |
| 13 (7.0) | 6 (4.4) | 0.330 |
| Ceftaroline (Cohort N = 227) | Ceftobiprole (Cohort N = 212) | p * | |
|---|---|---|---|
| Number of infective foci, median (IQR) | 1 (1–1) | 1 (1–1) | 0.007 |
| Bloodstream infections, n (%) | 118 (51.9) | 46 (21.7) | 0.0001 |
| 31 (13.7) | 34 (16) | 0.867 |
| 27 (11.9) | 7 (3.3) | 0.0001 |
| Infective endocarditis, n (%) | 55 (24.2) | 5 (2.4) | 0.0001 |
| Respiratory tract infections, n (%) | 103 (45.4) | 140 (66) | 0.006 |
| 59 (25.9) | 65 (30.7) | 0.780 |
| 24 (10.6) | 70 (33) | 0.0001 |
| 20 (8.8) | 5 (2.4) | 0.001 |
| 27 (11.9) | 34 (16) | 0.545 |
| Soft tissue and skin infection, n (%) | 23 (10.1) | 54 (25.4) | 0.0001 |
| 4 (1.8) | 10 (4.7) | |
| 6 (2.6) | 5 (2.4) | |
| 4 (1.8) | 7 (3.3) | |
| 1 (0.4) | 20 (9.4) | |
| 2 (0.7) | 7 (3.3) | |
| 6 (2.6) | 5 (2.4) | |
| Urinary tract infection, n (%) | 6 (2.6) | 10 (4.7) | 0.277 |
| 3 (1.3) | 3 (1.4) | |
| 3 (1.3) | 5 (2.4) | |
| 0 (0.0) | 2 (0.9) | |
| Central nervous system infection, n (%) | 9 (3.9) | 8 (3.8) | 0.719 |
| 3 (1.3) | 1 (0.5) | |
| 0 (0.0) | 2 (0.9) | |
| 2 (0.7) | 3 (1.4) | |
| 4 (1.8) | 2 (0.9) | |
| Intraabdominal infection, n (%) | 10 (4.4) | 9 (4.2) | 0.724 |
| Bone and joint infection, n (%) | 13 (5.7) | 11 (5.2) | 0.580 |
| 6 (2.6) | 5 (2.4) | |
| 7 (3.1) | 1 (0.5) | |
| 0 (0.0) | 5 (2.4) | |
| Spondylodiscitis, n (%) | 9 (3.9) | 4 (1.9) | 0.133 |
| Fever without focus, n (%) | 8 (3.5) | 0 (0.0) | 0.003 |
| Other type of infection, n (%) | 6 (2.6) | 4 (1.9) | 0.468 |
| Positive Microbial Samples | Ceftaroline (Cohort N = 150) | Ceftobiprole (Cohort N = 139) | p * |
|---|---|---|---|
| Microbial profile of isolates, n (%) | |||
| 129 (86.0) | 86 (61.9) | 0.0001 |
| 21 (14.0) | 53 (38.1) | |
| Gram-positive cocci, n (%) | 139 (92.7) | 90 (64.7) | 0.0001 |
| MR Staphylococcus aureus | 39 (26.0) | 25 (18.0) | 0.101 |
| MS Staphylococcus aureus | 31 (20.7) | 21 (15.1) | 0.219 |
| MR CoNS | 40 (26.7) | 14 (10.1) | 0.0001 |
| 31 (20.7) | 12 (8.6) | |
| 3 (2.0) | 1 (0.7) | |
| 4 (1.3) | 1 (0.7) | |
| 1 (0.7) | 0 (0.0) | |
| 1 (0.7) | 0 (0.0) | |
| MS CoNS | 9 (6.0) | 6 (4.3) | 0.519 |
| 3 (2.0) | 3 (2.2) | |
| 2 (1.3) | 1 (0.7) | |
| 0 (0.0) | 1 (0.7) | |
| 3 (2.0) | 0 (0.0) | |
| 0 (0.0) | 1 (0.7) | |
| 1 (0.7) | 1 (0.7) | |
| Enterococcus faecium | 0 (0.0) | 1 (0.7) | 0.481 |
| Enterococcus faecalis | 0 (0.0) | 10 (7.2) | 0.001 |
| Streptococcus pneumoniae | 16 (10.7) | 6 (4.3) | 0.042 |
| Other Streptococcus species | 4 (2.7) | 4 (3.6) | 0.742 |
| 1 (0.7) | 0 (0.0) | |
| 1 (0.7) | 3 (2.2) | |
| 1 (0.7) | 0 (0.0) | |
| 0 (0.0) | 1 (0.7) | |
| 1 (0.7) | 0 (0.0) | |
| Rothia spp. | 0 (0.0) | 2 (1.4) | 0.230 |
| Gram-positive bacilli, n (%) | 2 (1.3) | 1 (0.7) | 1.000 |
| 1 (0.7) | 0 (0.0) | |
| 1 (0.7) | 0 (0.0) | |
| 0 (0.0) | 1 (0.7) | |
| Gram-negative bacilli, n (%) | 9 (6.0) | 48 (19.7) | 0.0001 |
| Enterobacterales | 8 (5.3) | 12 (8.6) | 0.188 |
| 5 (3.3) | 4 (2.9) | |
| 2 (1.3) | 5 (3.6) | |
| 0 (0.0) | 1 (0.7) | |
| 0 (0.0) | 1 (0.7) | |
| 1 (0.7) | 0 (0.0) | |
| 0 (0.0) | 1 (0.7) | |
| Pseudomonas aeruginosa | 0 (0.0) | 32 (23.0) | 0.0001 |
| Other Gram-negative bacilli | 1 (0.7) | 4 (2.9) | 0.199 |
| 0 (0.0) | 2 (1.3) | |
| 0 (0.0) | 1 (0.7) | |
| 1 (0.7) | 1 (0.7) |
| Ceftaroline (N = 227) | Ceftobiprole (N = 212) | p * | |
|---|---|---|---|
| Length of hospital stay (days), median (IQR) | 36 (19–60) | 19.5 (12–30.7) | 0.0001 |
| Readmission and recurrence, n (%) | |||
| 3 (1.3) | 10 (4.7) | 0.036 |
| 0 (0.0) | 3 (1.4) | 0.072 |
| Mortality, n (%) | |||
| 77 (33.9) | 45 (21.2) | 0.003 |
| 43 (18.9) | 25 (11.8) | 0.039 |
| Infection-related mortality, n (%) | |||
| 30 (13.2) | 17 (8.0) | 0.078 |
| 11 (4.8) | 7 (3.3) | 0.415 |
| 2 (0.9) | 1 (0.5) | 1.000 |
| Ceftaroline (Cohort N = 55) | Ceftobiprole (Cohort N = 5) | |
|---|---|---|
| Age, mean (years) (± SD) | 69.33 (±11.62) | 67.20 (±2.39) |
| Charlson index, mean (IQR) | 5 (3–6) | 4 (4–4.50) |
| Number of infective foci, median (IQR) | 1 (1–1) | 2 (1–2) |
| Definition (modified Duke criteria), n(%) | ||
| 37 (67.3) | 1 (20) |
| 8 (14.5) | 1 (20) |
| 10 (18.2) | 3 (60) |
| Location of infective endocarditis, n (%) | ||
| 25 (45.5) | 2 (40) |
| 10 (18.2) | 0 (0.0) |
| 7 (12.7) | 1 (20) |
| 6 (10.9) | 0 (0.0) |
| 1 (1.8) | 0 (0.0) |
| Cardiac surgery, n (%) | ||
| 9 (16.4) | 0 (0.0) |
| 21 (38.2) | 1 (20) |
| 11 (20.0) | 1 (20) |
| 5 (9.0) | 0 (0.0) |
| Sepsis or septic shock, n (%) | 21 (38.2) | 3 (60) |
| Microbiological isolation, n (%) | 46 (83.6) | 5 (100.0) |
| Staphylococcus aureus | 22 (40.0) | 1 (20.0) |
| 7 (12.7) | 1 (20.0) |
| 15 (27.3) | 0 (0.0) |
| CoNS | 22 (40.0) | 1 (20.0) |
| Enterococcus faecalis | 0 (0.0) | 3 (60.0) |
| Corynebacterium coyleae | 1 (1.8) | 0 (0.0) |
| Streptococcus pneumoniae | 1 (1.8) | 0 (0.0) |
| Antibiotic combination, n (%) | 55 (100) | 5 (100) |
| 6 (10.9) | 1 (20.0) |
| 3 (5.5) | 2 (40.0) |
| 42 (76.4) | 2 (40.0) |
| Antibiotic prescription, n (%) | ||
| 7 (12.7) | 1 (20) |
| 48 (87.3) | 4 (80) |
| Reason for switching, n (%) | ||
| 36 (75.0) | 2 (50.0) |
| 8 (16.7) | 2 (50.0) |
| 4 (8.3) | 0 (0.0) |
| Total dose of antibiotic (mg), median (IQR) | 16,200 (9200–39,600) | 54,400 (7000–60,000) |
| Duration of antibiotic therapy (days), median (IQR) | 13 (6–23) | 28 (4.50–40) |
| Length of hospital stay (days), median (IQR) | 38 (20–64) | 46 (35–100) |
| Total mortality, n (%) | 22 (40) | 1 (20) |
| Related mortality, n (%) | 13 (23.6) | 1 (20) |
| 8 (14.5) | 1 (20) |
| 3 (5.5) | 0 (0.0) |
| 2 (3.6) | 0 (0.0) |
| Readmission, n (%) | 1 (1.8) | 0 (0.0) |
| Ceftaroline (N = 227) | Ceftobiprole (N = 212) | p * | |
|---|---|---|---|
| Total adverse effects, n (%) | 9 (4.0) | 5 (2.4) | 0.338 |
| Severity of adverse effects, n (%) | |||
| 3 (1.3) | 3 (1.4) | |
| 6 (2.6) | 2 (0.9) | |
| 0 (0.0) | 0 (0.0) | |
| 0 (0.0) | 0 (0.0) | |
| Antibiotic withdrawal for adverse effects, n (%) | 5 (2.2) | 2 (0.9) | 1.000 |
| Adverse effects by symptoms, n (%) | |||
| 2 (0.9) | 1 (0.5) | 1.000 |
| 2 (0.9) | 3 (1.4) | 0.266 |
| 1 (0.4) | 1 (0.5) | 1.000 |
| 1 (0.4) | 1 (0.5) | 1.000 |
| 1 (0.4) | 0 (0.0) | 1.000 |
| 2 (0.9) | 0 (0.0) | 0.505 |
| 2 (0.9) | 0 (0.0) | 0.505 |
| Deaths in Ceftaroline Group (N = 43) | Deaths in Ceftobiprole Group (N = 25) | p * | OR (95%CI) | |
|---|---|---|---|---|
| Age, mean (years) (± SD) | 65.84 (±10.50) | 75.88 (±13.64) | 0.001 | 0.9 (0.81–1.2) |
| Charlson index, median (IQR) | 5 (4–7) | 4 (4–6.50) | 0.330 | |
| Men, n (%) | 29 (67.4) | 17 (68.0) | 0.962 | |
| Sepsis | 25 (58.1) | 14 (56.0) | 0.863 | |
| Septic shock | 19 (44.2) | 7 (28.0) | 0.185 | |
| Infection acquisition, n (%) | ||||
| Community acquired | 15 (34.9) | 11 (44.0) | 0.456 | |
| Nosocomial/Nosohusial | 28 (65.1) | 14 (56.0) | ||
| Inpatient department, n (%) | ||||
| Medical department | 40 (93.0) | 22 (88.0) | 0.481 | |
| Surgical department | 3 (7.0) | 2 (8.0) | 0.876 | |
| OPAT, n (%) | 0 (0.0) | 1 (4.0) | 0.186 | |
| Comorbidities, n (%) | ||||
| Cardiovascular risk factors | 36 (83.7) | 19 (76.0) | 0.435 | |
| Obesity | 8 (18.6) | 0 (0.0) | 0.022 | 0 (0–0) |
| Cardiovascular diseases | 23 (53.5) | 6 (24.0) | 0.018 | 3.1 (0.2–69) |
| Ischemic heart disease | 11 (25.6) | 1 (4.0) | 0.024 | 0.15 (0.001–27) |
| Moderate-severe valve disease | 12 (27.9) | 0 (0.0) | 0.004 | 0 (0–0) |
| Respiratory diseases | 13 (30.2) | 6 (24.0) | 0.581 | |
| Cirrhosis—chronic liver disease | 3 (7.0) | 2 (8.0) | 1.000 | |
| Chronic kidney disease | 10 (23.3) | 3 (12.0) | 0.255 | |
| Active solid malignancy | 4 (9.3) | 2 (8.0) | 1.000 | |
| Active hematologic disease | 3 (7.0) | 3 (12.0) | 0.481 | |
| Neurological disease | 7 (16.3) | 7 (28.0) | 0.249 | |
| Immunocompromised patients | 16 (37.2) | 10 (40.0) | 0.819 | |
| Infection pathways, n (%) | ||||
| Bloodstream infections | 24 (54.5) | 5 (17.2) | 0.001 | 0.09 (0.004–2.7) |
| Infective endocarditis | 13 (29.5) | 1 (3.4) | 0.006 | 8.6 (0.35–210) |
| Community-acquired pneumonia | 6 (13.6) | 12 (41.4) | 0.007 | 0 (0–0) |
| Nosocomial pneumonia | 5 (11.4) | 9 (31.0) | 0.037 | |
| Ventilator-associated pneumonia | 9 (20.5) | 3 (10.3) | 0.254 | |
| Coinfection with SARS-CoV-2 | 6 (13.6) | 8 (27.6) | 0.139 | |
| Fungal coinfection | 5 (11.4) | 0 (0.0) | 0.150 | |
| Soft tissue and skin infection | 2 (4.5) | 3 (10.3) | 0.380 | |
| Intraabdominal infection | 3 (6.8) | 1 (3.4) | 0.925 | |
| Central nervous system infection | 1 (2.3) | 0 (0.0) | 1.000 | |
| Bone and joint infection | 1 (2.3) | 1 (3.4) | 1.000 | |
| Spondylodiscitis | 3 (6.8) | 0 (0.0) | 0.272 | |
| Microbiological isolates, n (%) | ||||
| General microbial profile | ||||
| 20 (45.5) | 15 (51.7) | 0.600 | |
| 24 (54.5) | 14 (48.3) | ||
| Microbial profile of isolates | ||||
| 22 (91.7) | 7 (50.0) | 0.004 | 2.6 (0.01–644) |
| 2 (8.3) | 7 (50.0) | ||
| Gram-positive cocci | ||||
| 5 (20.8) | 5 (35.7) | 0.315 | |
| 9 (37.5) | 2 (14.3) | 0.128 | |
| 6 (25.0) | 0 (0.0) | 0.067 | |
| 2 (8.3) | 0 (0.0) | 0.522 | |
| 0 (0.0) | 2 (14.3) | 0.129 | |
| 2 (8.3) | (0.0) | 0.522 | |
| Gram-negative bacilli | 0 (0.0) | 5 (35.7) | 0.004 | |
| Pseudomonas aeruginosa | 0 (0.0) | 5 (35.7) | 0.002 | |
| Antibiotic therapy | ||||
| Total dose (mg), median (IQR) | 9600 (5400–14,400) | 9000 (5000–12,500) | 0.545 | |
| Duration of antibiotic therapy (days), median (IQR) | 7 (3–12) | 6 (3–8.50) | 0.527 | |
| Antibiotic prescription, n (%) | ||||
| 4 (9.3) | 6 (24.0) | 0.099 | 2.6 (0.02–391) |
| 39 (90.7) | 19 (76.0) | ||
| Treatment regimen, n (%) | ||||
| 3 (7.0) | 17 (56.0) | 0.0001 | 0 (0–0) |
| 40 (93.0) | 12 (44.0) | ||
| Empirical treatment, n (%) | 20 (46.5) | 21 (84.0) | 0.002 | 0.3 (0.01–6.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnés García, D.; Pitto-Robles, I.; Calderón Parra, J.; Calvo Salvador, M.; Herrero Rodríguez, C.; Gisbert, L.; Hidalgo-Tenorio, C. Ceft-to-Ceft Study: Real-Life Experience with Ceftaroline and Ceftobiprole in Treatment of the Principal Infectious Syndromes in a Spanish Multicenter Hospital Cohort. Antibiotics 2023, 12, 1692. https://doi.org/10.3390/antibiotics12121692
Arnés García D, Pitto-Robles I, Calderón Parra J, Calvo Salvador M, Herrero Rodríguez C, Gisbert L, Hidalgo-Tenorio C. Ceft-to-Ceft Study: Real-Life Experience with Ceftaroline and Ceftobiprole in Treatment of the Principal Infectious Syndromes in a Spanish Multicenter Hospital Cohort. Antibiotics. 2023; 12(12):1692. https://doi.org/10.3390/antibiotics12121692
Chicago/Turabian StyleArnés García, Daniel, Inés Pitto-Robles, Jorge Calderón Parra, Marina Calvo Salvador, Carmen Herrero Rodríguez, Laura Gisbert, and Carmen Hidalgo-Tenorio. 2023. "Ceft-to-Ceft Study: Real-Life Experience with Ceftaroline and Ceftobiprole in Treatment of the Principal Infectious Syndromes in a Spanish Multicenter Hospital Cohort" Antibiotics 12, no. 12: 1692. https://doi.org/10.3390/antibiotics12121692
APA StyleArnés García, D., Pitto-Robles, I., Calderón Parra, J., Calvo Salvador, M., Herrero Rodríguez, C., Gisbert, L., & Hidalgo-Tenorio, C. (2023). Ceft-to-Ceft Study: Real-Life Experience with Ceftaroline and Ceftobiprole in Treatment of the Principal Infectious Syndromes in a Spanish Multicenter Hospital Cohort. Antibiotics, 12(12), 1692. https://doi.org/10.3390/antibiotics12121692






