Abstract
The objective of this review is to investigate the distribution and the characteristics of coagulase-negative Staphylococci (CoNS) implicated in ovine mastitis, and especially in subclinical cases, in order to provide a global perspective of the current research data and analyze specific critical aspects of the issue. PRISMA guidelines were implemented in the search of the last 20 years of the related literature in two databases. In total, 139 studies were included in this review. Relevant data were tracked down, assembled, and compared. Regarding the geographical distribution, most studies originated from Europe (68), followed by South America (33). Lacaune was the most examined breed, while S. epidermidis was the predominantly identified species, representing approximately 39% of the obtained isolates. Antibiotic resistance in the relevant bacteria was documented mostly for Penicillin (32.8%) and Amoxicillin (32.1%), while biofilm- and toxin-associated genes were encountered in variable rates because significant inequalities were observed between different articles. Significantly higher rates of antimicrobial resistance were detected in Asia and South America compared to Europe. Finally, the diagnostic procedures carried out in the respective studies were evaluated. Conventional culture and biochemical tests were mostly performed for simple strain identification; therefore, further molecular investigation of isolates should be pursued in future studies, as this will provide important data regarding specific aspects of the implication of CoNS in ovine mastitis.
1. Introduction
Mastitis, the inflammation of the mammary gland, is a major trouble in breeding ruminants, including sheep, as it causes significant economic losses, mostly due to a reduction in milk yield, the downfall of its quality, and rejection after antibiotic administration [1,2]. Furthermore, it constitutes a major welfare issue for the infected animals, and it is associated with increased animal replacement and veterinary expenses [3,4]. The subclinical type of the disease requires a more complex and challenging approach because it is commonly spread in flocks, affecting a considerable percentage of the lactating ewes, and it is, in some cases, misdiagnosed [5].
Coagulase-negative Staphylococci (CoNS) are opportunistic pathogens regularly associated with intramammary infection (IMI) in ruminants, and mostly in subclinical cases. During the last years, this group has become a main etiologic agent of ruminant mastitis [6]. Furthermore, recent studies indicate that these species are capable of causing more severe tissue damage in the mammary gland than was previously considered [7]. The bacterial species most frequently identified in cases of mastitis in sheep are S. epidermidis, S. chromogenes, S. simulans, S. xylosus, and S. haemolyticus [2,3,8].
Several studies have been performed worldwide investigating the prevalence, etiology, predisposing factors, and pathogenesis of ovine mastitis, as well as the implication of Staphylococci in the development of the disease.
The objective of this review is to collect and analyze data regarding the implication of CoNS in ovine mastitis, with a focus on the more challenging, subclinical type of the disease. A global perspective of the subject will be presented, and recently obtained information will be provided, by including studies published worldwide during the last two decades (2003–2023). Moreover, critical aspects of the subject will be investigated, such as pathogens’ prevalence, virulence, antibiotic resistance, and public health concerns.
2. Results
2.1. Geographical Distribution
The distribution of the included articles in relation to the continent of origin is:
- Europe: 67
- South America: 33
- Asia: 21
- Africa: 12
- North America: 5
- Europe and South America: 1
Assuming equal proportions, significantly more studies originate from Europe, while significantly fewer studies originate from Africa and North America (Chi-square test: 88.21; Degree of freedom: 4; p-value: <0.001).
In reference to the country/area of origin, the respective distribution is presented in Table 1 and visualized in the map presented in Figure 1.

Table 1.
Number of studies included in this review per country of origin.
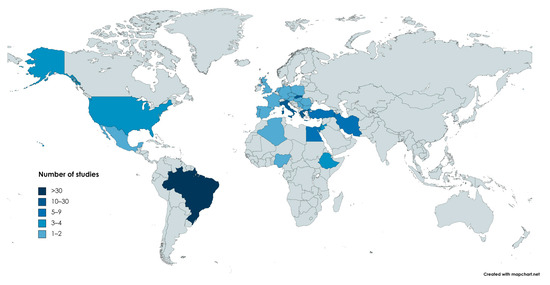
Figure 1.
Distribution of the studies (countries in shades of blue) included in this review throughout the world. Color indicates the arithmetical range to which the number of relevant articles from the corresponding country belongs. (https://www.mapchart.net/world.html, accessed οn 20 September 2023).
2.2. Relevant Findings of the Studies per Country
Due to the number of studies and the size of the data, only generic information is presented in this part of the results section. More detailed data about the findings of the selected articles are available in the following parts and the respective tables. The order of Table 2 is used to present the findings per country.

Table 2.
Breeds of sheep mostly included in the relevant studies.
2.2.1. Brazil
Thirty-three studies from Brazil were selected. They were published from 2009 to 2022. Over 12,000 milk samples were totally tested in all of them.
Intramammary infections in Santa Ines ewes were investigated in several studies [9,10,11,12,13,14,15,16,17,18,19,20,21]. Both Santa Ines and Morada Nova breed ewes were researched in four other articles [22,23,24,25], while Santa Ines and Bergamacia were researched in one [26]. Samples were received from farms located in various regions, such as Sao Paolo, Pernambuco, Bauru, Para, Montes Claros, Sergipe State, and Parana State.
Lacaune and their crossbreeds were also examined in seven studies [27,28,29,30,31,32,33], which were carried out in Chapeco-SC, Rio Grande do Sul, Santa Catarina, and Minas Gerais. Coagulase-negative Staphylococci were regularly obtained, especially from subclinical udder infections. Ovine milk samples of various breeds were tested in a few more articles, such as Coriedalle and Texel [34], Santa Ines, Ile de France, Dorper, and Texel [35,36,37]. Finally, ovine Staphylococci and their phenotypic and molecular characteristics were investigated in four more studies [38,39,40,41]. In some cases, concerning results regarding drug resistance were reported [39].
2.2.2. Greece
A total of 18 studies from Greece are included in this review. They were published from 2007 to 2022. The majority of the samples were received from central and northern parts of the country. Regarding breeds, Chios (5), Karagouniko (4), and Lacaune (4) were the most frequently encountered.
In particular, two studies that investigated aspects of milking and mammary glands’ health were accomplished for ewes of the Karagouniko breed [42,43]. Control measures for subclinical mastitis [44], the survival of CoNS species during the dry period [45], and the effect of the drying-off procedure [46], were investigated in Chios ewes in three research studies carried out in northern and central Greece. The effects of the drying-off procedure in the mammary health status were also examined in Lacaune-cross sheep in Central Greece [47]. In all of these studies, CoNS were regularly obtained, identified, and, in some cases, molecularly characterized [45,46]. Furthermore, the hypothesis that parasitic infections could predispose ewes to ovine subclinical mastitis was investigated in two articles [48,49]. The potential of specific enzymes for the diagnosis of subclinical mastitis in small ruminants was examined in another study [50]. The consequences of reduced vitamin A administration in the health of the mammary gland of Mytilene breed ewes were investigated [51], while mphC-positive Staphylococci obtained from cases of ovine subclinical mastitis were described in another article [52]. Extensive countrywide research on subclinical mastitis was accomplished, while various factors, such as breeds, susceptibility of the isolates, including numerous CoNS, and biofilm production, were investigated [53,54,55,56]. The efficacy of a vaccine against staphylococcal mastitis was evaluated in a study carried out in central Greece [57], while the association of subclinical mastitis with biotic and abiotic factors was examined for sheep of the Sfakia breed in Crete [58]. In these studies, a total of 272 and 652 CoNS strains were isolated, respectively. Finally, 33 S. epidermidis strains obtained in the two previous studies [53,57] were submitted for multi-locus sequence typing (MLST) and evaluation of their susceptibility profiles [59].
2.2.3. Italy
A total of 16 studies from Italy was selected for this review. They were published from 2005 to 2022. A large number of examined milk samples was included in most of them (total > 60,000 samples), while the isolated bacteria were, in various cases, submitted to molecular assays [60,61,62,63,64,65,66,67].
Several studies originated from Sardinia. The antibiotic residues in ovine milk samples were evaluated [68], a clinical investigation of mastitis cases in 2198 Sarda sheep was carried out [60], and 226 CoNS strains of ovine milk origin were identified through PCR-Restriction Fragment Length Polymorphism (RFLP) assay [61], while 131 S. epidermidis isolates from the previous study were submitted for susceptibility testing and genotyping [62]. Furthermore, the effects of infection in Somatic Cell Count (SCC) and milk yield of Sarda-breed ewes were examined [69], enterotoxigenic and methicillin-resistant CoNS isolates from ovine milk were identified and molecularly characterized [63], and the efficacy of an essential oil in teat disinfection was evaluated [70]. Finally, a total of 199 S. epidermidis strains isolated from sheep milk were investigated for biofilm production, quorum sensing system, and virulence factors [64]; pathogens associated with small ruminant mastitis were identified using a Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometer (MALDI-TOF MS) and PCR-RFLP [66], and a comparative profiling of 70 human and 125 ovine Staphylococci was accomplished [67].
Regarding other regions, an investigation of the associations among genetic parameters, somatic cell score, and mammary infection status was carried out in Valle del Belice ewes [71]. For the same breed, an assessment of the genetic background of pathogen-specific mastitis resistance was accomplished [72]. A survey of small ruminant mastitis during 2013–2014 included data from 23,040 ovine samples obtained in different areas of the country [73]. In Sicily, the efficacy of intramammary-infused Lactococcus lactis against staphylococcal mastitis was examined in 67 ewes [74], while in the Piedmont region, microbial agents from macroscopically healthy mammary glands of small ruminants were obtained and examined [75]. Furthermore, 73 CoNS isolates from ovine milk were submitted to a phenotypic and molecular investigation [65].
2.2.4. Slovakia
Twelve articles from Slovakia were included, which were published from 2009 to 2022. Local breeds were mostly studied, such as Tsigai and Valaska. Furthermore, the region of the sampling (when available) was mainly located in the eastern parts of the country.
Specifically, 240 CoNS isolates from ovine milk were identified and genotyped [76], whereas in another article, the production of enterotoxins in Staphylococci of the same origin was evaluated [77]. The prevalence and characteristics of mastitis pathogens in sheep farms located in marginal parts of the country were examined in another study. Among these pathogens, 76 CoNS isolates were identified [78]. The antibiotic resistance profiles of 288 and 158 CoNS isolates obtained from sheep milk during 2015–2017 and 2017–2019, respectively, were investigated in two other articles [79,80]. Susceptibility patterns of 131 common udder pathogens isolated in 2017 and 2018 were examined, and relatively high rates were documented for some agents [81], while the distribution of leukocytes and epithelial cells in Tsigai-breed ewes was researched in association with their udder health status, including cases of CoNS-caused mastitis [82]. In another study, fatty acid profiles of infected ovine milk samples were examined [83]. Moreover, bacterial pathogens and somatic cells in 303 ewes were investigated in 2019 [84], the presence of pathogens in association with SCC and subpopulations of leukocytes in 45 Lacaune ewes were researched [85], and the effect of udder infection on oxidative status was evaluated in 981 ovine milk samples of various breeds [86]. In all of these three studies, CoNS were the prevalent pathogens detected. Finally, in a study accomplished in eastern Slovakia, 44 CoNS strains were obtained from ovine subclinical mastitis cases and submitted to phenotypic and molecular investigation for antibiotic resistance, virulence factors, and biofilm formation [87].
2.2.5. Iran
Eight studies from Iran were selected. They were published from 2003 to 2018 and originated from various regions of the country. Native breeds were mostly researched.
Frequency, causative agents, and enzymatic activity of subclinical mastitis were investigated in 178 ewes in Urmia Province [88], while in the Shahrekord region, two studies were performed on susceptibility and phenotypic characteristics of isolates from 400 and 600 milk samples of native-breed sheep, respectively [89,90]. Additionally, the prevalence and aetiology of subclinical mastitis were researched in ewes from Tabriz [91], Nagadeh [92], and Semnan [93]. In 196 Sangsari-breed sheep from the latter region, the activity of specific enzymes was evaluated as a diagnostic tool for subclinical mastitis [94]. Coagulase-negative Staphylococci were regularly obtained in all of these studies, and they were the prevalent isolated bacteria in several cases [88,91,93,94]. Finally, in another study carried out in West Azerbaijan Province, 27 Staphylococci obtained from cases of ovine subclinical mastitis were phenotypically and molecularly investigated, and high resistance rates were detected for specific agents [95].
2.2.6. Egypt
Six of the included articles originated from Egypt. They were published from 2005 to 2019. Ovine subclinical mastitis was investigated in dairy ewes for two studies carried out in Fayoum [96] and Kafr-el-Sheikh [97] Governorates, with 38 and 45 CoNS isolations, respectively. In the latter article, concerning rates of resistance were documented for some antibiotics [97]. Nine CoNS isolates were also identified from sheep with subclinical mastitis both in the Assiut Governorate [98] and Sharkia Governorate [99]. The susceptibility of udder pathogens, including eight CoNS strains of ovine origin, to antibiotics, essential oils, honey, and plant extracts was evaluated [100]. Finally, the role of Staphylococci in subclinical mastitis was examined in 455 sheep milk samples, with 18 isolates being coagulase-negative [101].
2.2.7. Turkey
Six studies from Turkey were selected. They were published from 2009 to 2019. Three of them included Awassi-breed sheep from the Hatay region. Coagulase-negative Staphylococci were the predominant cause of subclinical mastitis in the first [102], while 70 of the obtained isolates were examined in a second study for phenotypic and molecular characteristics, and relatively high rates were documented regarding resistance to specific antibiotics and biofilm production ability [103]. In the third study, the E-test was evaluated for susceptibility determination of 50 ovine Staphylococci isolates to specific antibiotics [104]. In two other articles, CoNS from milk infections in the Kirikalle region were examined for the identification of susceptibility profiles and specific virulence factors [105,106]. Finally, 31 CoNS isolates from subclinical cases of udder infection in Pirlak sheep were molecularly investigated for specific resistance and toxin-encoding genes [107].
2.2.8. USA
The impact of dry treatment and teat sanitation on the health status of the mammary gland was examined in sheep in Wisconsin. During the research, 73 CoNS strains were obtained from milk samples [108]. The prevalence and aetiology of subclinical mastitis at weaning were investigated in ewes in Wyoming, and four CoNS were isolated [109], while in a study on extensively managed sheep of various breeds in Montana and Idaho, 25 more isolates were identified [110]. In another study, subclinical mastitis was investigated in 42 ewes in Wyoming, and CoNS were the aetiologic agent in 59% of the cases [111].
2.2.9. Ethiopia
Three studies from Ethiopia were included in this review. A total of 531 milk samples were tested, and CoNS were the prevalent isolated pathogens in all cases. Local breeds were examined in farms located in Kafta Humera [112], Haramaya [113], and Jimma [114].
2.2.10. Israel
Three studies with Assaf-breed sheep were included. In the first, subclinical mastitis and changes in milk composition were examined, and 36 CoNS were totally isolated [115]. In the second, dry-off treatment was evaluated in 159 ewes, and, in total, 134 CoNS were obtained [116]. In the third, the effects of udder infection caused by CoNS were investigated in 61 ovine milk samples [117].
2.2.11. Jordan
In all three selected studies from Jordan, Awassi-breed sheep were examined. In total, 1646 ovine milk samples were included, and CoNS along with S. aureus were the pathogens mostly detected. These studies were accomplished in southern Jordan [118], northern Jordan [119], and in the Al Balqa region [120].
2.2.12. Other Countries
Two studies were carried out in Algeria with Ouled-Djellal sheep. Antibiotic residues were examined in ovine milk samples [121], while the aetiology of subclinical mastitis was investigated [122]. Sixteen and thirty-four CoNS were isolated, respectively. In Austria, CoNS were the predominantly obtained pathogens in two studies. In particular, 267 [123] and 908 [124] ovine milk samples were examined. Regarding Bulgaria, two studies were caried out by Stoimenov et al. investigating udder pathogens in lactating sheep. In both cases, CoNS were regularly identified [125,126].
Staphylococci from the milk of ruminants were examined in a study in the Czech Republic [127], while methicillin-resistant Staphylococci, including coagulase-negative ones, were identified in another one from samples originating from both the Czech Republic and Slovakia [128]. In France, two studies were carried out, in which 4880 ovine milk samples were tested. Somatic cell count thresholds were investigated [129], while an SCC-based selection for mastitis resistance in sheep was evaluated [130]. Coagulase-negative Staphylococci were the most frequently isolated bacteria in both cases. In an article from Germany, the diagnostic value of CMT in dairy ewes was examined, and during the process, 24 CoNS were detected [131]. Staphylococci of ovine origin were identified through MALDI-TOF MS in Hungary [132], and the prevalence and aetiologic agents of subclinical mastitis were researched in ewes from the Pajacuaran Michoacan municipality, Mexico [133]. Ovine mastitis was also investigated in Bauchi State, Nigeria, and two CoNS strains were identified [134]. In a study of Awassi-breed ewes in Palestine, CoNS were the predominant udder pathogens [135].
In Poland, 108 Staphylococci were obtained from ovine milk samples of native breeds [136]. In a study from Romania, one S. epidermidis strain was obtained from a subclinical udder infection [137], and during research carried out in Portugal and Brazil, 20 CoNS isolates were detected [138]. Queiroga investigated sheep mastitis in Alentejo, Portugal, and 249 CoNS were totally isolated from subclinical cases [139], while Queiroga et al. submitted 109 ovine S. epidermidis isolates for phenotypic biofilm production and adhesion assays [140]. In Serbia, 25 cases of CoNS subclinical infection were detected [141], while in Spain, the effects of dry-therapy were investigated in lactating ewes [142], and the major udder pathogens were identified in small ruminants [143]. In all of these articles, numerous CoNS were isolated. Texel-breed sheep were examined in two studies accomplished in the Netherlands, in which CoNS were prevalent among the isolated pathogens [144,145]. Finally, in the UK, ovine milk samples were bacteriologically tested in two studies, and in both of them, several CoNS were associated with cases of infection [146,147].
2.3. Breeds of Sheep Included in the Studies
Sheep of various breeds are included in the selected articles. Those breeds with more than five references are listed in Table 2.
Studies including Lacaune sheep are distributed worldwide, whereas most other breeds are, unsurprisingly, strongly related to articles from specific countries or regions (Santa Ines—Brazil, Valachian and Tsigai—Slovakia, Chios—Greece, Sarda—Italy, etc.).
2.4. Prevalence of Pathogens
In 118 studies, the prevalent pathogen from all of the isolated bacteria is defined; the pathogen was identified in most of the samples investigated, even though, in all studies included in this review, a number of CoNS was isolated. Respective data are presented in Table 3.

Table 3.
Prevalent pathogens per number of studies.
In the majority of the studies (95/118, 80.5%) included in this review, cases of mastitis (mainly subclinical) were mostly associated with infections by CoNS, which are the prevalent and main cause of the problem.
2.5. Species of Identified CoNS
As presented in Table 4, there were references to several CoNS species from the cases of ovine mastitis. Data from 77 studies, in which the numbers of these species were available, have been included.

Table 4.
Species of CoNS in the selected studies.
As it is clearly noticed, S. epidermidis is the predominant species of CoNS in the included studies, as it was observed in approximately 40.0% of CoNS identification cases. This species was detected, at least once, in 61/77 (79.2%) studies, which provided the respective data, while in 34 of them (44.2%), it was prevalent among CoNS. Other species regularly obtained are S. chromogenes, S. simulans, and S. xylosus. These four species represent over 70% of total cases. Numerous other species are only occasionally obtained.
2.6. Antibiotic Resistance
In various studies, antibiotic susceptibility testing has been accomplished. Respective data have been accumulated and presented in Table 5.

Table 5.
Antibiotic resistance rates of the CoNS isolates in the included studies.
The isolates were usually susceptible to the majority of the antibiotics tested. However, relatively higher resistance rates were documented for Penicillin (32.8%), Amoxicillin (32.1%), Ampicillin (28%), Tetracycline (18%), Streptomycin (17.9%), Neomycin (14.8%), and Erythromycin (11.3%).
Because a sufficient number of studies was carried out in Europe, South America, and Asia, the resistance data included in Table 5 are separated according to their origin in Table 6. This task was accomplished in order to identify possible geographical variations in resistance rates.

Table 6.
Resistance rates in reference to the origin of the studies. In bold are the statistically significant results (when p< 0.005).
The highest resistance rates for Ampicillin and Penicillin were documented in Asia (41.05% and 50.85%) compared to South America (32.76% and 31.33%) and Europe (22.31% and 23.23%). Furthermore, oxacillin-resistant strains were detected significantly more frequently in South America than in Europe. Finally, rates against Tetracycline and Erythromycin were also relatively higher in South America (Brazil), while insignificant variations were observed for Cefoxitin, Enrofloxacin, and Gentamicin.
2.7. Biofilm Production and Biofilm- and Toxin-Associated Genes
An issue frequently investigated in the selected studies was the ability of the strains to produce biofilm and/or specific toxins, because these factors affect the severity and the persistence of the disease. Relevant available data are accumulated in Table 7.

Table 7.
Phenotypic assays for biofilm formation, detection of biofilm, and toxin-related genes.
The ability of CoNS isolates to produce biofilm was detected in higher rates when the plate adhesion technique was carried out (28.7%) compared to Congo Red Agar assay (8.4%). Biofilm-related genes were present in variable percentages of the isolates, with embp (78.3%) and bhp (31%) the most frequently encountered. A noteworthy fact is that in several cases, a significant inequality existed between the results of the phenotypic and molecular assays, indicating that the presence of the relevant genes is not always interrelated with a respective phenotype.
Toxin genes were rarely identified in the majority of the studies, and in only four articles were relatively higher percentages of positive isolates observed [21,25,26,106]. Three of them originated from Brazil.
2.8. Procedures of Microbiological Examination in the Selected Studies
Data regarding the diagnostic procedures followed by each study during the microbiological examination of the ovine milk samples were evaluated. Accumulated data are presented in Table 8.

Table 8.
Procedures of microbiological examination per number of respective studies.
In the majority of the studies, milk samples were inoculated in blood agar and identified through conventional phenotypic and biochemical tests. MacConkey agar and Mannitol salt agar were the most commonly used selective media during the initial inoculation process. From the available commercial identification kits, API Staph was the most widely used, while in a relatively small number of studies, identification was accomplished through MALDI-TOF MS or VITEK II. Antibiotic susceptibility was assessed mainly through the disc diffusion method.
Data regarding molecular investigation of the CoNS isolates are available in 37 articles. Techniques associated with molecular identification of the species level of the isolates were used in 20 cases. Various molecular assays were carried out occasionally, such as Pulse Field Gel Electrophoresis (PFGE) [45,46,60,62,76], PCR-RFLP [28,60,61,65,66,95], etc. PCR for the detection of ARGs and biofilm-related genes was accomplished in 19 [21,26,27,39,40,41,52,56,59,62,63,65,87,103,105,106,107,138,143] and 10 studies [25,26,27,39,55,64,65,67,103,138], respectively, while in 9 cases, genes associated with the production of enterotoxins were investigated [21,25,26,63,64,67,77,106,107]. The most commonly examined gene was mecA, with 16 relevant studies [21,26,27,39,40,41,59,62,63,65,87,103,105,106,107,138]. An interesting fact is that all of the aforementioned articles have been published since 2009, and the majority of them (22/36) were published during the last decade (2014–2023).
3. Discussion
Τhe results of this review indicate the importance of CoNS in ovine mastitis, and especially in subclinical cases. In a great number of the selected articles, these species were obtained repeatedly during the examination of ovine milk samples, regardless of breed, country, or culture media.
Countries traditionally associated with small ruminant breeding, such as Brazil, Greece, Italy, etc., accommodate numerous respective studies, including mainly local breeds. The majority of these breeds are dairy; however, research on wool and meat-producing sheep has been accomplished, too. This is anticipated because mastitis is a factor in deficient welfare, increased mortality, and reduced lamb growth [145].
Staphylococcus epidermidis was the predominant species identified (Table 4). This is in accordance with previous reports for ovine IMI [3], while S. chromogenes, S. xylosus, and S. simulans are also commonly detected. Moreover, because current knowledge suggests that S. epidermidis is a human-adapted species [8], the contribution of human sources to its distribution as well as its zoonotic potential are concerning facts that need further investigation.
Nevertheless, the diagnostic procedures that were carried out in the selected articles could affect the respective results. As it is clearly presented in Table 8, the identification of the pathogen causing udder infection is mostly based on aerobic culture after inoculation of a milk quantity in general purpose media. However, because some microorganisms are fastidious or require specific techniques to be detected, the prevalence of CoNS could be overestimated. When culture-independent methods for the detection of the etiologic agent were carried out, a greater diversity of identified pathogens was observed [8]. Therefore, the modification of the diagnostic procedures in the future could contribute to an alteration of the CoNS predominance in IMI cases.
In reference to antibiotic resistance, the highest rate was observed for Penicillin (unsurprisingly, similar rates were detected for Amoxicillin and Ampicillin) both worldwide and per continent (Table 5 and Table 6). These results correspond to previously published data [2,148]. Tetracycline and Erythromycin were also agents with noteworthy percentages of resistant isolates. Furthermore, comparable rates have been identified for CoNS originating from bovine mastitis [148], while the distribution of ARGs associated with respective phenotypes has been investigated and identified at various occasions in sheep [2,52,56,59,65,103,138]. On the other hand, antibiotics like Fluoroquinolones, Sulphamethoxazole–Trimethoprim, Gentamicin, Cephalosporins, Rifampicin, Vancomycin, and Chloramphenicol exhibited in vitro effectiveness against the grand majority of the relevant strains. It is important to note that the disc diffusion method is the one mainly used in most studies; MIC calculation is something that should be applied on a larger scale in future studies, as it will offer more information on antimicrobial resistance patterns.
Higher resistance rates are detected in Asia and South America compared to Europe (Table 6). This fact is anticipated, because both antibiotic administration and resistance rates are increasing in livestock animals in these areas [149]. Therefore, reduction in the usage of antibacterial agents and preventive measures for the distribution of resistant strains are required. Furthermore, surveillance measures should be established, because recent research suggests that CoNS could act as a reservoir for several transferrable ARGs [150]; therefore, the danger of horizontal or vertical gene transfer at the farm level is not negligible.
Coagulase-negative Staphylococci isolates with an ability to produce biofilm were occasionally encountered using the Congo Red Agar (CRA) method (8.4%), while more bacteria exhibited a positive phenotype when tested using the plate adhesion technique (Table 7). The latter assay is referenced as more sensitive [151,152]; thus, more biofilm-producing bacteria are usually detected. In addition, most associated genes were identified at relatively higher rates (from 9.8% to 78.3%), and this could indicate that a percentage of them could be misdiagnosed by phenotypic tests. However, due to the complexity of the biofilm formation process and the possible implication of more genes that are not comprehensively researched [55], this evaluation constitutes a challenging task.
Finally, toxin-related genes were regularly present in low percentages in the isolates, with some exceptions (Table 7). Staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin (TSST) could contribute to bacterial virulence [3,67]. They were generally correlated with S. aureus. Nevertheless, references to enterotoxin-producing CoNS are continuously increasing [153]. This could be concerning given their presence in milk and, consequently, food products, because cases of subclinical mastitis could be misdiagnosed. Therefore, future studies should include a more extensive investigation of the obtained bacteria, including tests for the detection of toxins or the respective genes and the determination of the factors affecting toxin production in cases of mastitis. Consequently, appropriate surveillance measures should be established in order to ascertain the safety of milk and dairy products.
Subclinical mastitis is undoubtedly a troublesome disease in sheep. It is a cause of significant animal welfare issues and financial losses at the farm level. Furthermore, concerns regarding public health arise. The distribution of bacteria or genetic elements associated with antibiotic resistance and specific virulence factors, such as biofilm and toxin production, is possible not only inside the farm but also in relation to its environment through animal transportation, milk, or bio-waste. The prevalence of CoNS in cases of subclinical mastitis is also an important aspect, because they are bacteria with a zoonotic potential [154]. Additionally, even though their implication in food poisoning has not been thoroughly investigated yet, enterotoxin production from relevant strains has been identified [153]. Therefore, surveillance and management measures are essential at the farm level in order to limit the number of cases, achieve an early diagnosis and treatment of infected animals, and prevent further distribution of strains with a pathogenic potential. Novel techniques that could allow for the early detection of mastitis cases, such as the detection of specific biomarkers [2] or infrared thermography [155], could constitute effective tools for veterinarians in this endeavour. Furthermore, the application of molecular techniques, like whole genome sequencing, next-generation sequencing, and MLST technologies, should be further increased in future studies, as they could contribute to a comprehensive investigation of the genetic basis of significant pathogens’ characteristics, like virulence and antibiotic resistance, as numerous respective studies have been accomplished for human isolates [156].
4. Materials and Methods
The Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines were implemented for this study [157]. Each individual step of the process is presented in Figure 2. Initially, a search for reviews on the subject of mastitis in sheep was accomplished using the following databases: Google Scholar and PubMed. Only studies published during the last 20 years (2003–2023) were included. In these databases, 5296 studies were found using the keywords coagulase negative, Staphylococcus, and ovine mastitis, as well as extra keywords, such as subclinical, milk, and sheep, in various combinations.
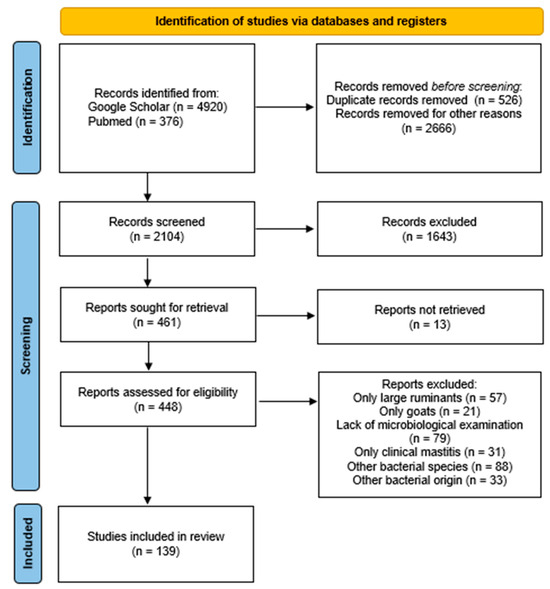
Figure 2.
Identification of studies regarding CoNS and ovine mastitis via databases using the PRISMA guidelines [157].
All of the selected studies were published in peer-reviewed journals, websites of organizations, books, and dissertations, and they were mostly written in the English language, with a limited number of them published in Portuguese. The initial step was a screening based on the titles of the articles. Articles not related were excluded, like duplicates, studies referring to human medicine, studies referring to other animal species, such as large ruminants and goats, and studies referring to other bacterial species or to CoNS of other origin, except mastitis. Subsequently, the second selection phase was carried out, where the abstracts of the reviewed studies were examined independently and in detail in order to identify their relevance. During this step, studies exclusively including cases of clinical mastitis were excluded due to the focus of this review on the subclinical type. Generic information was collected from each article, such as the author, year of publication, country where the study was conducted, study design, number of samples tested, number of isolates obtained, diagnostic procedures, antibiotic resistance, and virulence of the strains.
In particular, a total of 5296 manuscripts were detected, with 4920 from Google Scholar and 376 from PubMed. A total of 3192 publications were first excluded as their title was completely irrelevant or they were duplicates. Subsequently, the abstracts of the 2104 remaining articles were examined. During this phase, 1643 were rejected because their abstracts were not relevant to the scope of this review, according to the previously referenced criteria. Therefore, 461 studies were left to be examined, and 13 of them could not be retrieved. Among the remaining 448 articles, 57 were rejected as they only concerned large ruminants, 21 were rejected as they only concerned goats, 79 were rejected as they did not include a detailed microbiological examination of the milk samples, 31 were rejected as they only included cases of clinical mastitis, 88 were rejected as they only concerned other bacterial species, and 33 were rejected as the bacteria did not originate from mastitis (bulk milk, environmental samples, etc.). Finally, 139 manuscripts were included in this review.
The generic information extracted from each selected article is presented in Table 9. The country/area of isolation, the breed of sheep, the number of milk samples tested, the number of isolated CoNS, and the prevalent isolated pathogen in the study (the pathogen that was identified in most of the samples investigated, even though in all studies presented here a number of CoNS was isolated) are listed.

Table 9.
Generic information of the studies included in this review.
5. Conclusions
Coagulase-negative Staphylococci are predominant etiologic agents of subclinical mastitis in sheep causing significant economic losses and animal welfare issues. Their distribution in respective cases is worldwide and breed independent. Thus, reduction of their presence at the farm level should be considered of major importance. To achieve this, various actions must take place. Good milking hygiene practices (with proper operation of the milking machines), implementation of cleaning and disinfection programs while keeping the animal’s living environment dry and comfortable, segregation and killing of persistently infected animals, preventive use of antibiotics in the dry period, and, potentially, vaccination are major actions that can be applied. Staphylococcus epidermidis is the most frequently encountered species, and its zoonotic potential raises concerns regarding cross-contamination between humans and animals. This highlights the need for further investigation of mastitis in a broader context and with a One Health perspective. Approximately three out of ten strains isolated are Penicillin resistant, while noteworthy rates have also been observed for Tetracycline and Erythromycin, which are concerning facts for the efficacy of these broadly used antibiotics in therapeutic protocols. The dispersion of ARGs through epidemic strains or transferrable genes is possible, especially in regions where higher rates are detected, like Asia and South America; therefore, surveillance measures should be established. Biofilm- and toxin-associated genes have been identified in several articles, with considerable variations observed among the respective rates. The aforementioned results could be affected by the diagnostic approach in the selected studies, because aerobic culture and conventional identification tests are mainly carried out. Thus, the application of more advanced, novel techniques in the future, which could detect cases at an early phase, and further investigation of the isolated strains in a timely manner (especially molecular typing and sequencing technologies), could provide a comprehensive examination of significant, specific aspects of the disease, like strains’ virulence or antimicrobial resistance profiles. Relevant data could contribute to appropriate management and counteracting the consequences of mastitis for animal welfare, economics, and public health.
Author Contributions
Conceptualization, M.L. and G.V.; methodology, M.L. and G.V.; investigation, M.L.; writing—original draft preparation, M.L.; writing—review and editing, G.V., C.B., and V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data collected in this study is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Selvaggi, M.; D’Alessandro, A.G.; Dario, C. Environmental and Genetic Factors Affecting Milk Yield and Quality in Three Italian Sheep Breeds. J. Dairy Res. 2017, 84, 27–31. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Sarrou, S.; Fragkou, I.A.; Katsafadou, A.I.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Role of Staphylococci in Mastitis in Sheep. J. Dairy Res. 2019, 86, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Gelasakis, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.C.; Fthenakis, G.C. Mastitis in Sheep—The Last 10 Years and the Future of Research. Vet. Microbiol. 2015, 181, 136–146. [Google Scholar] [CrossRef]
- Libera, K.; Konieczny, K.; Grabska, J.; Smulski, S.; Szczerbal, I.; Szumacher-Strabel, M.; Pomorska-Mól, M. Potential Novel Biomarkers for Mastitis Diagnosis in Sheep. Animals 2021, 11, 2783. [Google Scholar] [CrossRef]
- Fragkou, I.A.; Boscos, C.M.; Fthenakis, G.C. Diagnosis of Clinical or Subclinical Mastitis in Ewes. Small Rumin. Res. 2014, 118, 86–92. [Google Scholar] [CrossRef]
- Ruiz-Romero, R.A.; Vargas-Bello-Pérez, E. Non-Aureus Staphylococci and Mammaliicocci as a Cause of Mastitis in Domestic Ruminants: Current Knowledge, Advances, Biomedical Applications, and Future Perspectives—A Systematic Review. Vet. Res. Commun. 2023, 47, 1067–1084. [Google Scholar] [CrossRef]
- Jesse, F.; Bitrus, A.; Peter, I.; Chung, E.; Tukiran, N. Clinical and Subclinical Mastitis in Ruminants: A Review of Etiological Agents, Diagnosis, Clinical Management and Risk Factors. J. Res. Vet. Sci. 2023, 1, 51–65. [Google Scholar] [CrossRef]
- Vanderhaeghen, W.; Piepers, S.; Leroy, F.; Van Coillie, E.; Haesebrouck, F.; De Vliegher, S. Identification, Typing, Ecology and Epidemiology of Coagulase Negative Staphylococci Associated with Ruminants. Vet. J. 2015, 203, 44–51. [Google Scholar] [CrossRef]
- Almeida, M.Z.P.R.B.; Oliveira, L.G.L.; Afonso, J.A.B.; Lázaro, N.S.; Mendonça, C.L. Influence of Intrammary Infections on the Physical-Chemical Characteristics of Milk from Santa-Ines Ewes. Cienc. Anim. Bras. 2009, 10 (Suppl. S1), 760–765. [Google Scholar]
- Guaraná, E.D.S.; dos Santos, R.A.; Silva, N.D.; Campos, A.G.S.S.; Afonso, J.A.B.; de Mendonça, C.L. Influence of Subclinical Mastitis on the Physical-Chemical Characteristics of Milk from Santa Inês Ewes in Different Lactation Stages: A Preliminary Study. Ciênc. Anim. Bras. 2009, 10 (Suppl. S1), 754–759. [Google Scholar]
- Blagitz, M.G.; Batista, C.F.; Nunes, G.R.; Souza, F.N.D.; Gomes, V.; Azedo, M.R.; Sucupira, M.C.A.; Libera, A.M.M.P.D. Características físico-químicas, celulares e microbiológicas da secreção mamária de ovelhas Santa Inês no período lactante e pós-desmame. Rev. Ciênc. Agrar. 2010, 53, 137–142. [Google Scholar] [CrossRef]
- Lucheis, S.B.; Hernandes, G.S.; Troncarelli, M.Z. Monitoramento Microbiológico da Mastite Ovina na Região de Bauru, SP. Arq. Inst. Biol. 2010, 77, 395–403. [Google Scholar] [CrossRef]
- Silva, N.D.S.E.; Silveira, J.A.S.D.; Pinheiro, C.P.; Sousa, M.G.S.D.; Oliveira, C.M.C.; Mendonça, C.L.D.; Duarte, M.D.; Barbosa, B.J.D. Etiologia e Perfil de Sensibilidade de Bactérias Isoladas de Ovelhas com Mastite na Região Nordeste do Estado do Pará. Pesqui. Vet. Bras. 2010, 30, 1043–1048. [Google Scholar] [CrossRef]
- Veríssimo, C.J.; Zafalon, L.F.; Otsuk, I.P.; Nassar, A.F.C. Prejuízos Causados Pela Mastite em Ovelhas Santa Inês. Arq. Inst. Biol. 2010, 77, 583–591. [Google Scholar] [CrossRef]
- Guaraná, E.L.D.S.; Santos, R.A.D.; Campos, A.G.S.S.; Silva, N.D.S.E.; Afonso, J.A.B.; Mendonça, C.L.D. Dinâmica celular e microbiológica do leite de ovelhas Santa Inês acompanhadas durante a lactação. Pesqui. Vet. Bras. 2011, 31, 851–858. [Google Scholar] [CrossRef]
- Morais, G.D.; Almeida, A.C.; Teixeira, L.M.; Xavier, T.R.; Souza, R.M.D.; Duarte, E.R. Caracterização da Mastite Ovina no Norte de Minas Gerais: Ocorrência, Etiologia e Epidemiologia. Rev. Caatinga 2011, 24, 164–171. [Google Scholar]
- Zafalon, L.F.; Verissimo, C.J.; Mamizuka, E.M.; Martins, K.B.; Almeida, L.M.; Veschi, J.L.A. Estafilococos resistentes à oxacilina isolados em casos de mastite subclínica em ovinos. Arq. Inst. Biol. 2012, 79, 1–7. [Google Scholar] [CrossRef]
- Oliveira, A.A.; Melo, C.B.; Seixas, L.; Azevedo, H.C.; Teixeira, K.M.; Melo, P.O. Mastitis and Milk Composition in First Partum Santa Ines Ewes. J. Vet. Adv. 2013, 3, 220. [Google Scholar] [CrossRef]
- Zafalon, L.F.; Veschi, J.L.A.; Martins, K.B.; Santana, R.C.M. Occurrence of Ovine Subclinical Mastitis During Two Consecutive Lactations in a Santa Ines Breed Herd. Ciênc. Anim. Bras. 2015, 16, 116–124. [Google Scholar] [CrossRef]
- Pereira, P.F.V.; Reway, A.P.; Félix, A.; Beutemmüller, E.A.; Pretto-Giordano, L.G.; Alfieri, A.A.; Lisbôa, J.A.N.; Müller, E.E. Mammary Gland Health of Santa Inês Ewes at the Drying and Puerperium and Evaluation of a Dry-off Terapy with Gentamicin. Pesqui. Vet. Bras. 2018, 38, 2194–2200. [Google Scholar] [CrossRef]
- de Moura, G.S. Dinâmica da Infecção e Caracterização Molecular das Mastites Causadas por Staphylococcus spp. em Ovelhas da Raça Santa Inês. Ph.D. Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2020. [Google Scholar]
- Santana, R.C.M.; Zafalon, L.F.; Esteves, S.N.; Tanaka, E.V.; Pilon, L.E.; Massa, R. Occurrence of Etiologic Agents Causing Subclinical Mastitis in Morada Nova and Santa Ines Ewes. Ars Vet. 2013, 29, 148–152. [Google Scholar] [CrossRef]
- Santana, R.C.M.; Zafalon, L.F.; Brandão, H.D.M.; Junior, G.A.F.; Pilon, L.E.; Junior, W.B.; Giglioti, R.; Mosqueira, V.C.F. Uso de Antimicrobiano Nanoparticulado para o Tratamento da Mastite Subclínica de Ovelhas de Corte no Período Seco. Pesqui. Vet. Bras. 2016, 36, 826–830. [Google Scholar] [CrossRef][Green Version]
- Zafalon, L.F.; Santana, R.C.M.; Pilon, L.E.; Júnior, G.A.F. Diagnosis of Subclinical Mastitis in Santa Inês and Morada Nova Sheep in Southeastern Brazil. Trop. Anim. Health Prod. 2016, 48, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Zafalon, L.F.; Cunha, M.L.R.S.; Brandão, H.M.; Mosqueira, V.C.F.; Santana, R.C.M.; Barioni Júnior, W.; Martins, K.B.; Pilon, L.E. Relationship between Virulence Factor Genes in Coagulase-Negative Staphylococcus spp. and Failure of Antimicrobial Treatment of Subclinical Mastitis in Sheep. Pesqui. Vet. Bras. 2018, 38, 579–585. [Google Scholar] [CrossRef]
- Martins, K.B.; Faccioli, P.Y.; Bonesso, M.F.; Fernandes, S.; Oliveira, A.A.; Dantas, A.; Zafalon, L.F.; Cunha, M.D.L.R.S. Characteristics of Resistance and Virulence Factors in Different Species of Coagulase-Negative Staphylococci Isolated from Milk of Healthy Sheep and Animals with Subclinical Mastitis. J. Dairy Sci. 2017, 100, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Agnol, A.M.D.; Cavalcante, M.B.; Franca, C.A.D.; Krewer, C.D.C.; Queiros, A.A.D.; Costa, M.M.D.; Braganca, J.F.M.; Girardini, L.K. Caracterização Fenotípica e Molecular de Isolados de Staphylococcus spp. Obtidos de Leite de Ovelhas do Município de Chapecó-SC. Semin. Ciênc. Agrár. 2013, 34, 311–322. [Google Scholar] [CrossRef][Green Version]
- Moraes, C.D.R. Contagem de Células Somáticas e Isolamento Bacteriano em Leite de Ovelhas no Estado do Rio Grande do Sul-Brasil. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Rio Grande, Brazil, 2015. [Google Scholar]
- Silveira, R.F.; Costa, P.T.; Fernandes, T.A.; Moreira, S.M.; Silveira, I.D.B.; De Moraes, R.E.; de Lima Gonzalez, H. Características Produtivas e Comportamentais de Ovelhas Lacaune em Diferentes Estádios de Lactação. REDVET Rev. Electrón. Vet. 2017, 18, 1–11. [Google Scholar]
- Escopelli, K.S.; Lopes, E.J.C.; Pinto, A.T.; Schmidt, V. Diagnóstico de Mastite e Determinação da Composição do Leite de Ovelhas Laucane. Hig. Aliment. 2017, 31, 268–269. [Google Scholar]
- Takano, P.V.; Scapini, V.A.D.C.; Valentini, T.; Girardini, L.K.; De Souza, F.N.; Della Libera, A.M.M.P.; Heinemann, M.B.; Chande, C.G.; Cortez, A.; Collet, S.G.; et al. Milk Cellularity and Intramammary Infections in Primiparous and Multiparous Lacaune Ewes during Early Lactation. Small Rumin. Res. 2018, 167, 117–122. [Google Scholar] [CrossRef]
- Alba, D.F.; Da Rosa, G.; Hanauer, D.; Saldanha, T.F.; Souza, C.F.; Baldissera, M.D.; Da Silva Dos Santos, D.; Piovezan, A.P.; Girardini, L.K.; Schafer Da Silva, A. Subclinical Mastitis in Lacaune Sheep: Causative Agents, Impacts on Milk Production, Milk Quality, Oxidative Profiles and Treatment Efficacy of Ceftiofur. Microb. Pathog. 2019, 137, 103732. [Google Scholar] [CrossRef]
- Dias, B.V.; Da Costa, G.M.; Leite, R.F.; Lucas, F.A.; Custódio, D.A.D.C.; Lima, R.R.D.; Brighenti, C.R.G.; Alves, N.G. Relationship between Subclinical Mastitis and Reproduction in Lacaune Sheep. Small Rumin. Res. 2022, 216, 106809. [Google Scholar] [CrossRef]
- Pradieé, J.; da Rosa Moraes, C.; Gonçalves, M.; Vilanova, M.S.; Corrêa, G.F.; Lauz, O.G.; Osório, M.T.M.; Schmidt, V. Somatic cell count and California Mastitis Test as a diagnostic tool for subclinical mastitis in ewes. Acta Sci. Vet. 2012, 40, 1–7. [Google Scholar]
- Fim Junior, G.A.; Manieri, F.Z.; Lopes, N.S.D.S.; Pilon, L.E.; Zafalon, L.F. Occurrence of Subclinical Mastitis in Ewes of Different Breeds in the Same Production System. Rev. Bras. Hig. Sanid. Anim. 2014, 8, 478–489. [Google Scholar] [CrossRef]
- Fim Júnior, G.A. Contagem de Células Somáticas para o Diagnóstico da Mastite Subclínica Ovina em Diferentes Raças em Dois Períodos de Lactação. Master’s Thesis, Universidade Estadual Paulista, Sao Paolo, Brazil, 2015. [Google Scholar]
- Zafalon, L.F.; Santana, R.C.M.; Esteves, S.N.; Fim Júnior, G.A. Somatic Cell Count in the Diagnosis of Subclinical Mastitis in Sheep of Different Breeds. Semin. Ciênc. Agrár. 2018, 39, 1555. [Google Scholar] [CrossRef]
- Drescher, G.; Mattiello, S.P.; Peixoto, R.M.; Vargas, A.C.; Maciel, M.N.; Costa, M.M. Caracterização Bioquímica e Perfil de Sensibilidade aos Antimicrobianos de Agentes Bacterianos Isolados de Mastite Subclínica Ovina na Região Oeste de Santa Catarina. Ciênc. Anim. Bras. 2010, 11, 188–193. [Google Scholar] [CrossRef][Green Version]
- França, C.A.; Peixoto, R.M.; Cavalcante, M.B.; Melo, N.F.; Oliveira, C.J.B.; Veschi, J.L.A.; Mota, R.A.; Costa, M.M. Antimicrobial Resistance of Staphylococcus spp. from Small Ruminant Mastitis in Brazil. Pesqui. Vet. Bras. 2012, 32, 747–753. [Google Scholar] [CrossRef]
- Silva, G.V.D. Avaliação das Espécies e Perfil de Suscetibilidade aos Antimicrobianos de Staphylococcus Isolados de Leite de Ovelha. Master’s Thesis, Universidade Federal Rural do Rio De Janeiro, Sepetiba, Brazil, 2012. [Google Scholar]
- Santos, A.D.S.; Lima, D.C.V.D.; Abad, A.C.A.; Oliveira, P.R.F.D.; Silva, J.G.D.; Moura, G.S.D.; Silva, A.T.F.; Amorim, V.D.S.; Costa, M.M.D.; Mota, R.A. Antimicrobial Resistance Profile of Non-Aureus Staphylococci Isolates from Buffalo, Goat and Sheep Mastitis in the Northeast Region of Brazil. J. Dairy Res. 2020, 87, 290–294. [Google Scholar] [CrossRef]
- Gougoulis, D.A.; Kyriazakis, I.; Mavrogianni, V.S.; Fragkou, I.A.; Skoufos, J.; Tzora, A.; Taitzoglou, I.A.; Kokoli, A.N.; Fthenakis, G.C. Patterns of Maternal-Offspring Behaviour of Dairy Sheep and Potential Association with Mammary Health. Can. J. Anim. Sci. 2007, 87, 469–478. [Google Scholar] [CrossRef]
- Gougoulis, D.; Kyriazakis, I.; Tzora, A.; Taitzoglou, I.; Skoufos, J.; Fthenakis, G. Effects of Lamb Sucking on the Bacterial Flora of Teat Duct and Mammary Gland of Ewes. Reprod. Domest. Anim. 2007, 43, 22–26. [Google Scholar] [CrossRef]
- Kiossis, E.; Brozos, C.N.; Petridou, E.; Boscos, C. Program for the Control of Subclinical Mastitis in Dairy Chios Breed Ewes during Lactation. Small Rumin. Res. 2007, 73, 194–199. [Google Scholar] [CrossRef]
- Kiossis, E.; Brozos, C.N.; Petridou, E.; Zdragas, A.; Papadopoulos, T.; Boscos, C. Study on the Possible Survival of Staphylococcus Chromogenes through the Dry Period in Dairy Ewes. Small Rumin. Res. 2013, 115, 124–129. [Google Scholar] [CrossRef]
- Sougaris, S.; Brozos, C.; Petridou, E.; Papadopoulos, T.; Kiossis, E. Abrupt and Gradual Drying-off Procedure and Intramammary Dry Treatment: Impact on Udder Health Status of Chios Breed Dairy Sheep. J. Hell. Vet. Med. Soc. 2022, 73, 4031–4040. [Google Scholar] [CrossRef]
- Petridis, I.G.; Mavrogianni, V.S.; Fragkou, I.A.; Gougoulis, D.A.; Tzora, A.; Fotou, K.; Skoufos, I.; Amiridis, G.S.; Brozos, C.; Fthenakis, G.C. Effects of Drying-off Procedure of Ewes’ Udder in Subsequent Mammary Infection and Development of Mastitis. Small Rumin. Res. 2013, 110, 128–132. [Google Scholar] [CrossRef]
- Mavrogianni, V.S.; Papadopoulos, E.; Spanos, S.A.; Mitsoura, A.; Ptochos, S.; Gougoulis, D.A.; Barbagianni, M.S.; Kyriazakis, I.; Fthenakis, G.C. Trematode Infections in Pregnant Ewes Can Predispose to Mastitis during the Subsequent Lactation Period. Res. Vet. Sci. 2014, 96, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kordalis, N.G.; Arsenopoulos, K.; Vasileiou, N.G.C.; Mavrogianni, V.S.; Lianou, D.T.; Papadopoulos, E.; Fthenakis, G.C. Field Evidence for Association between Increased Gastrointestinal Nematode Burden and Subclinical Mastitis in Dairy Sheep. Vet. Parasitol. 2019, 265, 56–62. [Google Scholar] [CrossRef]
- Katsoulos, P.D.; Christodoulopoulos, G.; Minas, A.; Karatzia, M.A.; Pourliotis, K.; Kritas, S.K. The Role of Lactate Dehydrogenase, Alkaline Phosphatase and Aspartate Aminotransferase in the Diagnosis of Subclinical Intramammary Infections in Dairy Sheep and Goats. J. Dairy Res. 2010, 77, 107–111. [Google Scholar] [CrossRef]
- Koutsoumpas, A.T.; Giadinis, N.D.; Petridou, E.J.; Konstantinou, E.; Brozos, C.; Lafi, S.Q.; Fthenakis, G.C.; Karatzias, H. Consequences of Reduced Vitamin A Administration on Mammary Health of Dairy Ewes. Small Rumin. Res. 2013, 110, 120–123. [Google Scholar] [CrossRef]
- Papagiannitsis, C.C.; Malli, E.; Tsilipounidaki, K.; Sarrou, S.; Medvecky, M.; Hrabak, J.; Fthenakis, G.C.; Petinaki, E. First Description in Greece of mphC -Positive Staphylococci Causing Subclinical Mastitis in Ewes. Microb. Drug Resist. 2018, 24, 1050–1053. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Orfanou, D.C.; Politis, A.P.; Gonzalez-Valerio, T.C.; Argyros, S.; et al. Extensive Countrywide Field Investigation of Subclinical Mastitis in Sheep in Greece. J. Dairy Sci. 2018, 101, 7297–7310. [Google Scholar] [CrossRef]
- Vasileiou, N.G.; Gougoulis, D.A.; Riggio, V.; Ioannidi, K.S.; Chatzopoulos, D.C.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Association of Subclinical Mastitis Prevalence with Sheep Breeds in Greece. J. Dairy Res. 2018, 85, 317–320. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Katsafadou, A.I.; Spyrou, V.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Slime-Producing Staphylococci as Causal Agents of Subclinical Mastitis in Sheep. Vet. Microbiol. 2018, 224, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, N.G.C.; Sarrou, S.; Papagiannitsis, C.; Chatzopoulos, D.C.; Malli, E.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Antimicrobial Agent Susceptibility and Typing of Staphylococcal Isolates from Subclinical Mastitis in Ewes. Microb. Drug Resist. 2019, 25, 1099–1110. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Cripps, P.J.; Ioannidi, K.S.; Gougoulis, D.A.; Chouzouris, T.M.; Lianou, D.T.; Gonzalez-Valerio, T.C.; Vallverdu, R.G.; Argyros, S.; et al. Evaluation of Efficacy of a Biofilm-Embedded Bacteria-Based Vaccine against Staphylococcal Mastitis in Sheep—A Randomized, Placebo-Controlled Field Study. J. Dairy Sci. 2019, 102, 9328–9344. [Google Scholar] [CrossRef] [PubMed]
- Tzanidakis, N.; Brozos, C.N.; Voutzourakis, N.; Stefanakis, A.; Malama, E.; Zoller, D.; Zdragkas, A.; Hickford, J.; Sotiraki, S.; Kiossis, E. Effect of Abiotic and Biotic Factors on Subclinical Mastitis Occurrence in Low-Input Dairy Sheep Production Systems. Small Rumin. Res. 2021, 198, 106341. [Google Scholar] [CrossRef]
- Katsarou, E.I.; Chatzopoulos, D.C.; Giannoulis, T.; Ioannidi, K.S.; Katsafadou, A.I.; Kontou, P.I.; Lianou, D.T.; Mamuris, Z.; Mavrogianni, V.S.; Michael, C.K.; et al. MLST-Based Analysis and Antimicrobial Resistance of Staphylococcus Epidermidis from Cases of Sheep Mastitis in Greece. Biology 2021, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Marogna, G.; Rolesu, S.; Lollai, S.; Tola, S.; Leori, G. Clinical Findings in Sheep Farms Affected by Recurrent Bacterial Mastitis. Small Rumin. Res. 2010, 88, 119–125. [Google Scholar] [CrossRef]
- Onni, T.; Sanna, G.; Cubeddu, G.P.; Marogna, G.; Lollai, S.; Leori, G.; Tola, S. Identification of Coagulase-Negative Staphylococci Isolated from Ovine Milk Samples by PCR–RFLP of 16S rRNA and Gap Genes. Vet. Microbiol. 2010, 144, 347–352. [Google Scholar] [CrossRef]
- Onni, T.; Sanna, G.; Larsen, J.; Tola, S. Antimicrobial Susceptibilities and Population Structure of Staphylococcus Epidermidis Associated with Ovine Mastitis. Vet. Microbiol. 2011, 148, 45–50. [Google Scholar] [CrossRef]
- Perillo, J.; Ceccarelli, D.; Spagnoletti, M.; Lollai, S.; Cappuccinelli, P.; Colombo, M.M. Molecular Characterization of Enterotoxigenic and Borderline Oxacillin Resistant Staphylococcus Strains from Ovine Milk. Food Microbiol. 2012, 32, 265–273. [Google Scholar] [CrossRef]
- Abbondio, M.; Fois, I.; Longheu, C.; Azara, E.; Tola, S. Biofilm Production, Quorum Sensing System and Analysis of Virulence Factors of Staphylococcus Epidermidis Collected from Sheep Milk Samples. Small Rumin. Res. 2019, 174, 83–87. [Google Scholar] [CrossRef]
- Turchi, B.; Bertelloni, F.; Marzoli, F.; Cerri, D.; Tola, S.; Azara, E.; Longheu, C.M.; Tassi, R.; Schiavo, M.; Cilia, G.; et al. Coagulase Negative Staphylococci from Ovine Milk: Genotypic and Phenotypic Characterization of Susceptibility to Antibiotics, Disinfectants and Biofilm Production. Small Rumin. Res. 2020, 183, 106030. [Google Scholar] [CrossRef]
- Rosa, N.M.; Penati, M.; Fusar-Poli, S.; Addis, M.F.; Tola, S. Species Identification by MALDI-TOF MS and Gap PCR–RFLP of Non-Aureus Staphylococcus, Mammaliicoccus, and Streptococcus Spp. Associated with Sheep and Goat Mastitis. Vet. Res. 2022, 53, 84. [Google Scholar] [CrossRef] [PubMed]
- Azara, E.; Longheu, C.M.; Attene, S.; Sanna, S.; Sale, M.; Addis, M.F.; Tola, S. Comparative Profiling of Agr Locus, Virulence, and Biofilm-Production Genes of Human and Ovine Non-Aureus Staphylococci. BMC Vet. Res. 2022, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Calarescu, G.; Leori, G.; Testa, C.; Marogna, G.; Secchi, L. Is the Doubling of Withdrawal Time a Sufficient Measure? Evaluations of Oxytetracyline Residue Persistence in Sheep Milk. In Proceedings of the 4th SAFO Workshop, Frick, Switzerland, 17–19 March 2005; pp. 149–156. [Google Scholar]
- Cuccuru, C.; Meloni, M.; Sala, E.; Scaccabarozzi, L.; Locatelli, C.; Moroni, P.; Bronzo, V. Effects of Intramammary Infections on Somatic Cell Score and Milk Yield in Sarda Sheep. N. Z. Vet. J. 2011, 59, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Dore, S.; Ferrini, A.M.; Appicciafuoco, B.; Massaro, M.R.; Sotgiu, G.; Liciardi, M.; Cannas, E.A. Efficacy of a Terpinen-4-Ol Based Dipping for Post-Milking Teat Disinfection in the Prevention of Mastitis in Dairy Sheep. J. Essent. Oil Res. 2019, 31, 19–26. [Google Scholar] [CrossRef]
- Riggio, V.; Portolano, B.; Bovenhuis, H.; Bishop, S.C. Genetic Parameters for Somatic Cell Score According to Udder Infection Status in Valle Del Belice Dairy Sheep and Impact of Imperfect Diagnosis of Infection. Genet. Sel. Evol. 2010, 42, 30. [Google Scholar] [CrossRef]
- Tolone, M.; Larrondo, C.; Yáñez, J.M.; Newman, S.; Sardina, M.T.; Portolano, B. Assessment of Genetic Variation for Pathogen-Specific Mastitis Resistance in Valle Del Belice Dairy Sheep. BMC Vet. Res. 2016, 12, 158. [Google Scholar] [CrossRef]
- Dore, S.; Liciardi, M.; Amatiste, S.; Bergagna, S.; Bolzoni, G.; Caligiuri, V.; Cerrone, A.; Farina, G.; Montagna, C.O.; Saletti, M.A.; et al. Survey on Small Ruminant Bacterial Mastitis in Italy, 2013–2014. Small Rumin. Res. 2016, 141, 91–93. [Google Scholar] [CrossRef][Green Version]
- Mignacca, S.A.; Dore, S.; Spuria, L.; Zanghì, P.; Amato, B.; Duprè, I.; Armas, F.; Biasibetti, E.; Camperio, C.; Lollai, S.A.; et al. Intramammary Infusion of a Live Culture of Lactococcus Lactis in Ewes to Treat Staphylococcal Mastitis. J. Med. Microbiol. 2017, 66, 1798–1810. [Google Scholar] [CrossRef]
- Spuria, L.; Biasibetti, E.; Bisanzio, D.; Biasato, I.; De Meneghi, D.; Nebbia, P.; Robino, P.; Bianco, P.; Lamberti, M.; Caruso, C.; et al. Microbial Agents in Macroscopically Healthy Mammary Gland Tissues of Small Ruminants. PeerJ 2017, 5, e3994. [Google Scholar] [CrossRef]
- Pilipčincová, I.; Bhide, M.; Dudriková, E.; Trávniček, M. Genotypic Characterization of Coagulase-Negative Staphylococci Isolated from Sheep Milk in Slovakia. Acta Vet. Brno 2010, 79, 269–275. [Google Scholar] [CrossRef]
- Zigo, F.; Vasiľ, M.; Elečko, J.; Lapin, M.; Farkašova, Z. Production of Enterotoxins of Staphylococcus spp. Isolated from Samples of Sheep Milk. Potravin. Slovak J. Food Sci. 2014, 8, 92–96. [Google Scholar] [CrossRef]
- Zigo, F.; Vasiľ, M.; Takáč, L.; Zigová, M.; Elečko, J. Mastitis Pathogens Isolated from Raw Milk Samples on Sheep Farms Situated in Marginal Parts of Slovakia. Folia Vet. 2018, 62, 56–61. [Google Scholar] [CrossRef]
- Vasiľ, M.; Elečko, J.; Farkašová, Z.; Zigo, F. Development of Resistance to Antibiotics in Bacteria Staphylococcus spp. Isolated from Milk Samples in the Sheep Breedings on East of Slovakia. Potravin. Slovak J. Food Sci. 2018, 12, 273–278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vasiľ, M.; Farkašová, Z.; Elečko, J.; Zigo, F. Occurrence of Resistance to Antibiotics Therapy in Coagulase-Positive and Coagulase-Negative Staphylococci Isolated from Sheep´s Milk in Holding in Slovakia. Potravin. Slovak J. Food Sci. 2020, 14, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Holko, I.; Tančin, V.; Tvarožková, K.; Supuka, P.; Supuková, A.; Lucia, M. Occurence and Antimicrobial Resistance of Common Udder Pathogens Isolated from Sheep Milk in Slovakia. Potravin. Slovak J. Food Sci. 2019, 13, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Vašíček, J.; Tvarožková, K.; Uhrinčať, M.; Mačuhová, L.; Hleba, L.; Tančin, V. Distribution of Leucocytes and Epithelial Cells in Sheep Milk in Relation to the Somatic Cell Count and Bacterial Occurrence: A Preliminary Study. Slovak J. Anim. Sci. 2019, 52, 160–165. [Google Scholar]
- Pikhtirova, A.; Bujok, J.; Pecka-Kiełb, E.; Zachwieja, A.; Vasil, M.; Elečko, J.; Zigo, F. Fatty Acid Profile of Ewe’s Milk Infected with Staphylococcus spp. Iran. J. Vet. Res. 2020, 21, 216–220. [Google Scholar]
- Tvarožková, K.; Tančin, V.; Uhrinčať, M.; Hleba, L.; Mačuhová, L. Mastitis Pathogens and Somatic Cell Count in Ewes Milk. Potravin. Slovak J. Food Sci. 2020, 14, 164–169. [Google Scholar] [CrossRef]
- Tvarožková, K.; Vašíček, J.; Uhrinčať, M.; Mačuhová, L.; Hleba, L.; Tančin, V. The Presence of Pathogens in Milk of Ewes in Relation to the Somatic Cell Count and Subpopulations of Leukocytes. Czech J. Anim. Sci. 2021, 66, 315–322. [Google Scholar] [CrossRef]
- Zigo, F. Sheep Mastitis Caused by Staphylococci and Streptococci and Their Influence on Oxidative Status. Acta Fytotech. Zootech. 2021, 24, 53–57. [Google Scholar] [CrossRef]
- Zigo, F.; Farkašová, Z.; Farag Mohammed Rehan, I.; Sayed-Ahmed, A. Occurrence of Mastitis in Dairy Herds and the Detection of Virulence Factors in Staphylococci. In Infectious Diseases; Bustos-Martínez, J., José Valdez-Alarcón, J., Eds.; IntechOpen: Rijeka, Croatia, 2023; Volume 17, ISBN 978-1-83769-985-8. [Google Scholar]
- Batavani, R.A.; Mortaz, E.; Falahian, K.; Dawoodi, M.A. Study on Frequency, Etiology and Some Enzymatic Activities of Subclinical Ovine Mastitis in Urmia, Iran. Small Rumin. Res. 2003, 50, 45–50. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Lotfalian, S.; Karimi, S. Drug Resistance in Isolated Bacteria from Milk of Sheep and Goats with Subclinical Mastitis in Shahrekord District. Iran. J. Vet. Res. 2007, 8, 76–79. [Google Scholar]
- Ebrahimi, A.; Soleimani, F.; Moatamedi, A.; Shams, N.; Lotfalian, S. Study on Some Characteristics of Staphylococci Isolated from Sheep Subclinical Mastitis Milk in Shahrekord, Iran. Biol. J. Microorg. 2014, 2, 57–62. [Google Scholar]
- Beheshti, R.; Shaieghi, J.; Eshratkhah, B.; Ghalehkandi, J.G.; Maheri-Sis, N. Prevalence and Etiology of Subclinical Mastitis in Ewes of the Tabriz Region, Iran. Glob. Vet. 2010, 4, 299–302. [Google Scholar]
- Arighi Sareh, A.; Hadavi, E.; Abdi, K. Prevalence and Etiology of Subclinical Mastitis in Ghezel Ewes in Nagadeh District, Iran. J. Vet. Clin. Res. 2012, 3, 249–258. [Google Scholar]
- Narenji Sani, R.; Mahdavi, A.; Moezifar, M. Prevalence and Etiology of Subclinical Mastitis in Dairy Ewes in Two Seasons in Semnan Province, Iran. Trop. Anim. Health Prod. 2015, 47, 1249–1254. [Google Scholar] [CrossRef]
- Narenji Sani, R.; Hajigolikhani, B.; Ahmadi-Hamedani, M.; Kafshdouzan, K. Diagnostic Evaluation of Milk Lactate Dehydrogenase and Alkaline Phosphatase Activities by Receiver Operating Characteristic Analysis Curve in Early Lactation of Ewes with Subclinical Mastitis. Vet. Res. Forum 2018, 9, 343. [Google Scholar] [CrossRef]
- Rahman, B.; Ownagh, A.; Mardani, K.; Ardebili, F.F. Prevalence and Molecular Characterization of Staphylococci Isolated from Sheep with Subclinical Mastitis in West-Azerbaijan Province, Iran. Vet. Res. Forum 2016, 7, 155–162. [Google Scholar]
- Moawad, A.A.; Osman, S.A. Prevalence and Etiology of Subclinical Mastitis in Dairy Ewes at Fayoum Governorate, Egypt. Assiut Vet. Med. J. 2005, 51, 1–15. [Google Scholar] [CrossRef]
- Azab, Y.R. Subclinical Mastitis in Dairy Ewes at Kafr El-Sheikh Governorate, Egypt and Observation on Bacteria Associated with it. Kafrelsheikh Vet. Med. J. 2007, 5, 140–152. [Google Scholar] [CrossRef]
- El-Bassiony, T.; El-Prince, E.; Abdel-hameed, K.; Abdel-haleem, A.; Sadek, O. Prevalence and Public Health Hazard of Subclinical Mastitis in Goats and Sheep in Assiut Governorate. Assiut Vet. Med. J. 2008, 54, 1–10. [Google Scholar] [CrossRef]
- Abdallah, E.-S.; Eissa, M.; Menaze, A. The Prevalence and Etiology of Subclinical Mastitis in Sheep and Goats. Zagazig Vet. J. 2018, 46, 96–104. [Google Scholar] [CrossRef][Green Version]
- Abdalhamed, A.M.; Zeedan, G.S.G.; Abou Zeina, H.A.A. Isolation and Identification of Bacteria Causing Mastitis in Small Ruminants and Their Susceptibility to Antibiotics, Honey, Essential Oils, and Plant Extracts. Vet. World 2018, 11, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.; Nossair, M.; Habib, H.; Naggar, A.; Abdallah, M.; Farag, H. Prevalence of Subclinical Mastitis in Small Ruminants and Role of Staphylococcus Species in Such Infection. Alex. J. Vet. Sci. 2019, 62, 64. [Google Scholar] [CrossRef]
- Ergün, Y.; Aslantaş, Ö.; Doğruer, G.; Ki̇Reçci̇, E.; Saribay, M.K.; Ateş, C.T.; Ülkü, A.; Demi̇r, C. Prevalence and Etiology of Subclinical Mastitis in Awassi Dairy Ewes in Southern Turkey. Turk. J. Vet. Anim. Sci. 2009, 33, 477–483. [Google Scholar] [CrossRef]
- Ergün, Y.; Aslantaş, Ö.; Ki̇Reçci̇, E.; Öztürk, F.; Ceylan, A.; Boyar, Y. Antimicrobial Susceptibility, Presence of Resistance Genes and Biofilm Formation in Coagulase Negative Staphlococci Isolated from Subclinical Sheep Mastitis. Kafkas Univ. Vet. Fak. Derg. 2012, 18, 449–456. [Google Scholar] [CrossRef]
- Kirecci, E.; Ergun, Y.; Dogruer, G.; Saribay, M.K. Usefulness of the E-test for the Determination of the Susceptibility of Staphylococcus sp. Isolated from Milk of Sheep and Goats with Subclinical Mastitis to Amikacin and Amoxicillin-Clavulanic Acid. Bull. Vet. Inst. Pulawy 2009, 53, 401–405. [Google Scholar]
- Ünal, N.; Askar, Ş.; Macun, H.C.; Sakarya, F.; Altun, B.; Yıldırım, M. Panton–Valentine Leukocidin and Some Exotoxins of Staphylococcus Aureus and Antimicrobial Susceptibility Profiles of Staphylococci Isolated from Milks of Small Ruminants. Trop. Anim. Health Prod. 2012, 44, 573–579. [Google Scholar] [CrossRef]
- Ünal, N.; Çinar, O.D. Detection of Stapylococcal Enterotoxin, Methicillin-Resistant and Panton–Valentine Leukocidin Genes in Coagulase-Negative Staphylococci Isolated from Cows and Ewes with Subclinical Mastitis. Trop. Anim. Health Prod. 2012, 44, 369–375. [Google Scholar] [CrossRef]
- Şeker, E.; Özenç, E.; Baki Acar, D.; Yilmaz, M. Prevalence of Methicillin Resistance and Panton-Valentine Leukocidin Genes in Staphylococci Isolated from Pirlak Sheep with Subclinical Mastitis in Turkey. Kocatepe Vet. J. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Spanu, C.; Berger, Y.M.; Thomas, D.L.; Ruegg, P.L. Impact of Intramammary Antimicrobial Dry Treatment and Teat Sanitation on Somatic Cell Count and Intramammary Infection in Dairy Ewes. Small Rumin. Res. 2011, 97, 139–145. [Google Scholar] [CrossRef]
- Knuth, R.M.; Woodruff, K.L.; Hummel, G.L.; Williams, J.D.; Stewart, W.C.; Cunningham-Hollinger, H.C.; Bisha, B. Post-Weaning Management Strategies and Impacts on Ewe Subclinical Mastitis and Antimicrobial Susceptibility. Transl. Anim. Sci. 2021, 5, S80–S85. [Google Scholar] [CrossRef]
- Knuth, R.M.; Stewart, W.C.; Taylor, J.B.; Bisha, B.; Yeoman, C.J.; Van Emon, M.L.; Murphy, T.W. Relationships among Intramammary Health, Udder and Teat Characteristics, and Productivity of Extensively Managed Ewes. J. Anim. Sci. 2021, 99, skab059. [Google Scholar] [CrossRef]
- Knuth, R.M.; Woodruff, K.L.; Hummel, G.L.; Williams, J.D.; Austin, K.J.; Stewart, W.C.; Cunningham-Hollinger, H.C.; Bisha, B. Effects of Management Strategies during Early Lactation and Weaning on Etiological Agents of Ovine Subclinical Mastitis and Antimicrobial Susceptibility of Milk-Derived Bacterial Isolates. J. Anim. Sci. 2022, 100, skac171. [Google Scholar] [CrossRef]
- Gebrewahid, T.; Abera, B.; Menghistu, H. Prevalence and Etiology of Subclinical Mastitis in Small Ruminants of Tigray Regional State, North Ethiopia. Vet. World 2012, 5, 103–109. [Google Scholar] [CrossRef]
- Alemu, S.; Abraha, A. Prevalence of Bacteria Associated with Subclinical Mastitis in Haramaya University Dairy Cattle, Goat and Sheep Farms. East Afr. J. Vet. Anim. Sci. 2017, 1, 61–66. [Google Scholar]
- Hayle, W.A.; Ahmed, R.; Uddin, M.E. Prevalence of Subclinical Mastitis among Small Ruminants and Isolation of Some Etiological Bacterial Pathogens in Jimma Town, Ethiopia. Eur. J. Med. Health Sci. 2020, 2, 107–124. [Google Scholar] [CrossRef]
- Leitner, G.; Chaffer, M.; Shamay, A.; Shapiro, F.; Merin, U.; Ezra, E.; Saran, A.; Silanikove, N. Changes in Milk Composition as Affected by Subclinical Mastitis in Sheep. J. Dairy Sci. 2004, 87, 46–52. [Google Scholar] [CrossRef]
- Shwimmer, A.; Kenigswald, G.; Van Straten, M.; Lavi, Y.; Merin, U.; Weisblit, L.; Leitner, G. Dry-off Treatment of Assaf Sheep: Efficacy as a Management Tool for Improving Milk Quantity and Quality. Small Rumin. Res. 2008, 74, 45–51. [Google Scholar] [CrossRef]
- Leitner, G.; Merin, U.; Krifucks, O.; Blum, S.; Rivas, A.L.; Silanikove, N. Effects of Intra-Mammary Bacterial Infection with Coagulase Negative Staphylococci and Stage of Lactation on Shedding of Epithelial Cells and Infiltration of Leukocytes into Milk: Comparison among Cows, Goats and Sheep. Vet. Immunol. Immunopathol. 2012, 147, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Al-Majali, A.M.; Jawabreh, S. Period Prevalence and Etiology of Subclinical Mastitis in Awassi Sheep in Southern Jordan. Small Rumin. Res. 2003, 47, 243–248. [Google Scholar] [CrossRef]
- Alekish, M.O.; Alshehabat, M.A.; Abutarbush, S.M. The Prevalence and Etiology of Subclinical Mastitis in Awassi Sheep; Emphasis on the Relationship Between the Isolated Organisms and the Somatic Cell Count. Eur. J. Vet. Med. 2014, 8, 1–13. [Google Scholar]
- Hawari, A.D.; Obeidat, M.; Awaisheh, S.S.; Al-Daghistani, H.I.; Al-Abbadi, A.A.; Omar, S.S.; Qrunfleh, I.M.; Al-Dmoor, H.M.; El-Qudah, J. Prevalence of Mastitis Pathogens and their Resistance Against Antimicrobial Agents in Awassi Sheep in Al-Balqa Province of Jordan. Am. J. Anim. Vet. Sci. 2014, 9, 116–121. [Google Scholar] [CrossRef]
- Fatima, B.A.A.; Kheira, B.; Bettache, G.; Habib, A.; Mebrouk, K. Evaluation of Microbiological and Sanitary Quality of Ewe’s Raw Milk in Western of Algeria and Detection of Antibiotic Residue by Delvotest. Adv. Environ. Biol. 2013, 7, 1027–1033. [Google Scholar]
- Smaali, S. Etude de l’Έtiologie Βactérienne des Mammites Subcliniques des Ovins à l’Est de l’Algérie. Afr. Sci. Rev. Int. Sci. Technol. 2014, 10, 225–231. [Google Scholar]
- Winter, P.; Miny, M.; Fuchs, K.; Baumgartner, W. The Potential of Measuring Serum Amyloid A in Individual Ewe Milk and in Farm Bulk Milk for Monitoring Udder Health on Sheep Dairy Farms. Res. Vet. Sci. 2006, 81, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Dourakas, M.; Wurm, A.; Hess, C.; Urbantke, V.; Wittek, T.; Baumgartner, M. Untersuchungen zu Speziesverteilung, Pathogenität und Resistenzprofil von Staphylokokken aus aseptisch entnommenen Schaf- und Ziegenmilch-proben. Wien Tierärztl. Monatsschr. 2021, 108, 214–225. [Google Scholar]
- Stoimenov, A.; Popova, T.; Stoimenov, G.; Hristov, K. Microbiological Status of the Mammary Gland in Lactating Sheeps. Tradit. Mod. Vet. Med. 2022, 7, 10–17. [Google Scholar]
- Stoimenov, A.; Popova, T.; Stoimenov, G.; Hristov, K. Antibiotic Sensitivity of the Causative Microorganisms of Subclinical Mastitis in Lactating Sheep. Zhivotnov. Nauki 2022, 59, 73–79. [Google Scholar]
- Vyletělová, M.; Hanuš, O.; Karpíšková, R.; Šťástková, Z. Occurrence and Antimicrobial Sensitivity in Staphylococci Isolated from Goat, Sheep and Cow’s Milk. Acta Univ. Agric. Silvic. Mendel. Brun. 2014, 59, 209–214. [Google Scholar] [CrossRef]
- Vyletělová, M.; Vlková, H.; Manga, I. Occurrence and Characteristics of Methicillin Resistant Staphylococcus Aureus and Methicillin Resistant Coagulase-Negative Staphylococci in Raw Milk Manufacturing. Czech J. Food Sci. 2011, 29, S11–S16. [Google Scholar] [CrossRef]
- Berthelot, X.; Lagriffoul, G.; Concordet, D.; Barillet, F.; Bergonier, D. Physiological and Pathological Thresholds of Somatic Cell Counts in Ewe Milk. Small Rumin. Res. 2006, 62, 27–31. [Google Scholar] [CrossRef]
- Rupp, R.; Bergonier, D.; Dion, S.; Hygonenq, M.C.; Aurel, M.R.; Robert-Granié, C.; Foucras, G. Response to Somatic Cell Count-Based Selection for Mastitis Resistance in a Divergent Selection Experiment in Sheep. J. Dairy Sci. 2009, 92, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Barth, K.; Burow, E.; Knappstein, K. EC and CMT Detect Subclinical Mastitis in Dairy Sheep but Less Sensitive than in Dairy Cows. Landbauforsch. Volkenrode 2008, 58, 65–69. [Google Scholar]
- Tonamo, A.; Komlósi, I.; Varga, L.; Kačániová, M.; Peles, F. Identification of Ovine-Associated Staphylococci by MALDI-TOF Mass Spectrometry. Acta Aliment. 2021, 50, 210–218. [Google Scholar] [CrossRef]
- Cantero, R.A.P.; Jiménez, I.B.; Ramírez, J.M.T. Frecuencia de Mastitis Subclínicas en Ovejas en Ordeña y Sensibilidad Antibiótica de los Agentes Etiológicos Responsables. In Proceedings of the 5th Congreso Nacional de Ciencia y Tecnología Agropecuaria, Roque, Celaya, Mexico, 21–23 March 2018; pp. 169–174. [Google Scholar]
- Danmallam, F.A.; Pimenov, N.V.; Ngulukun, S.S.; Mwankon, S.E. Prevalence and Bacterial Etiology of Mastitis in Small Ruminants in Toro Local Government Area, Bauchi State of Nigeria. Russ. J. Agric. Soc.-Econ. Sci. 2018, 79, 341–345. [Google Scholar] [CrossRef]
- Adwan, G.; Abusafieh, D.; Aref, R.; Omar, J.A. Prevalence Of Microorganisms Associated with Intramammary Infection in Cows and Small Ruminants in the North of Palestine. IUG J. Nat. Stud. 2005, 13, 165–173. [Google Scholar]
- Świderek, W.P.; Charon, K.M.; Winnicka, A.; Gruszczyńska, J.; Pierzchała, M. Physiological Threshold of Somatic Cell Count in Milk of Polish Heath Sheep and Polish Lowland Sheep. Ann. Anim. Sci. 2016, 16, 155–170. [Google Scholar] [CrossRef]
- Mot, D.; Mot, T. Clinical, Microbiological and Hematological Findings in Ovine Subclinical Mastitis. Sci. Pap. Anim. Sci. Biotechnol. 2016, 49, 226–230. [Google Scholar]
- Andrade, N.C.; Laranjo, M.; Costa, M.M.; Queiroga, M.C. Virulence Factors in Staphylococcus Associated with Small Ruminant Mastitis: Biofilm Production and Antimicrobial Resistance Genes. Antibiotics 2021, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, M.C. Prevalence and Aetiology of Sheep Mastitis in Alentejo Region of Portugal. Small Rumin. Res. 2017, 153, 123–130. [Google Scholar] [CrossRef]
- Queiroga, M.C.; Duarte, E.L.; Laranjo, M. Sheep Mastitis Staphylococcus Epidermidis Biofilm Effects on Cell Adhesion and Inflammatory Changes. Small Rumin. Res. 2018, 168, 6–11. [Google Scholar] [CrossRef]
- Vakanjac, S.; Todorovic, I. Subclinical mastitis in sheep: Causes and their sensitivity to antibiotics. Vet. Glas. 2010, 64, 231–241. [Google Scholar] [CrossRef]
- Gonzalo, C.; Tardáguila, J.A.; De La Fuente, L.F.; San Primitivo, F. Effects of Selective and Complete Dry Therapy on Prevalence of Intramammary Infection and on Milk Yield in the Subsequent Lactation in Dairy Ewes. J. Dairy Res. 2004, 71, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Rovai, M.; Caja, G.; Salama, A.A.K.; Jubert, A.; Lázaro, B.; Lázaro, M.; Leitner, G. Identifying the Major Bacteria Causing Intramammary Infections in Individual Milk Samples of Sheep and Goats Using Traditional Bacteria Culturing and Real-Time Polymerase Chain Reaction. J. Dairy Sci. 2014, 97, 5393–5400. [Google Scholar] [CrossRef] [PubMed]
- Van den Crommenacker, L.W.J.H. Mastitis in Sheep. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2015. [Google Scholar]
- Van Den Crommenacker-Konings, L.W.J.H.; Van Dam, P.; Everts, R.; Shittu, A.; Nielen, M.; Lam, T.J.G.M.; Koop, G. Dynamics of Intramammary Infections in Suckler Ewes during Early Lactation. J. Dairy Sci. 2021, 104, 5979–5987. [Google Scholar] [CrossRef]
- Hariharan, H.; Donachie, W.; Macaldowie, C.; Keefe, G. Bacteriology and Somatic Cell Counts in Milk Samples from Ewes on a Scottish Farm. Can. J. Vet. Res. 2004, 68, 188–192. [Google Scholar]
- Zadoks, R.N.; Tassi, R.; Martin, E.; Holopainen, J.; McCallum, S.; Gibbons, J.; Ballingall, K.T. Comparison of Bacteriological Culture and PCR for Detection of Bacteria in Ovine Milk—Sheep Are Not Small Cows. J. Dairy Sci. 2014, 97, 6326–6333. [Google Scholar] [CrossRef]
- El Garch, F.; Youala, M.; Simjee, S.; Moyaert, H.; Klee, R.; Truszkowska, B.; Rose, M.; Hocquet, D.; Valot, B.; Morrissey, I.; et al. Antimicrobial Susceptibility of Nine Udder Pathogens Recovered from Bovine Clinical Mastitis Milk in Europe 2015–2016: VetPath Results. Vet. Microbiol. 2020, 245, 108644. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global Trends in Antimicrobial Resistance in Animals in Low- and Middle-Income Countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Andam, C.P. Extensive Horizontal Gene Transfer within and between Species of Coagulase-Negative Staphylococcus. Genome Biol. Evol. 2021, 13, evab206. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.D.C.; Ferreira, L.M.; Nader Filho, A.; Zafalon, L.F.; Vicente, H.I.G.; Souza, V.D. Comparison of Methods for the Detection of Biofilm Formation by Staphylococcus Aureus Isolated from Bovine Subclinical Mastitis. Braz. J. Microbiol. 2013, 44, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Deka, N. Comparison of Tissue Culture Plate Method, Tube Method and Congo Red Agar Method for the Detection of Biofilm Formation by Coagulase Negative Staphylococcus Isolated from Non-Clinical Isolates. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 810–815. [Google Scholar]
- Banaszkiewicz, S.; Wałecka-Zacharska, E.; Schubert, J.; Tabiś, A.; Król, J.; Stefaniak, T.; Węsierska, E.; Bania, J. Staphylococcal Enterotoxin Genes in Coagulase-Negative Staphylococci—Stability, Expression, and Genomic Context. Int. J. Mol. Sci. 2022, 23, 2560. [Google Scholar] [CrossRef]
- Piette, A.; Verschraegen, G. Role of Coagulase-Negative Staphylococci in Human Disease. Vet. Microbiol. 2009, 134, 45–54. [Google Scholar] [CrossRef]
- Martins, R.F.S.; Do Prado Paim, T.; De Abreu Cardoso, C.; Stéfano Lima Dallago, B.; De Melo, C.B.; Louvandini, H.; McManus, C. Mastitis Detection in Sheep by Infrared Thermography. Res. Vet. Sci. 2013, 94, 722–724. [Google Scholar] [CrossRef]
- Argemi, X.; Hansmann, Y.; Prola, K.; Prévost, G. Coagulase-Negative Staphylococci Pathogenomics. Int. J. Mol. Sci. 2019, 20, 1215. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).