Natural Compounds with Antifungal Properties against Candida albicans and Identification of Hinokitiol as a Promising Antifungal Drug
Abstract
:1. Introduction
2. Results
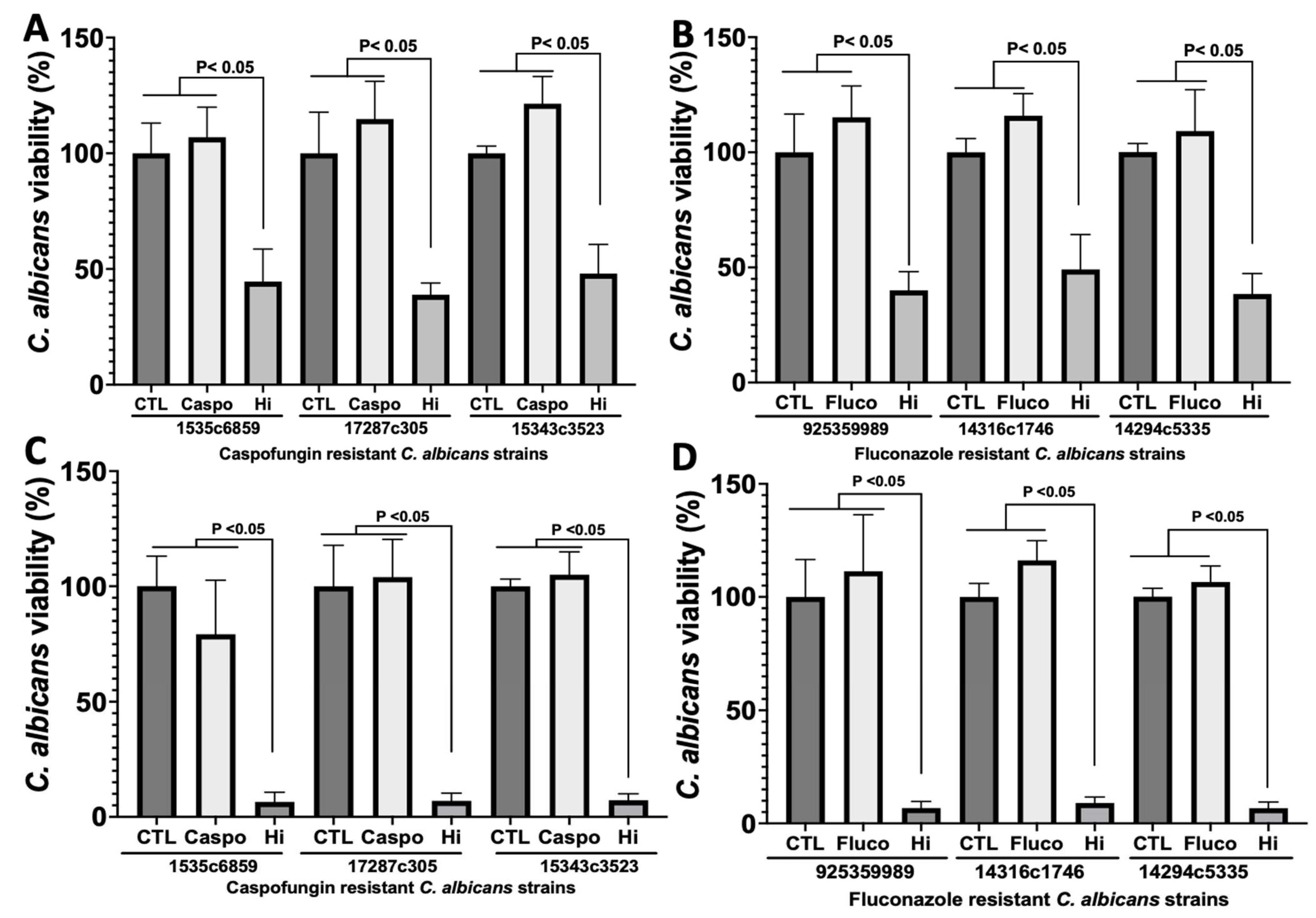
2.1. Analysis of the Antifungal Properties of Hi against Clinical Strains of C. albicans
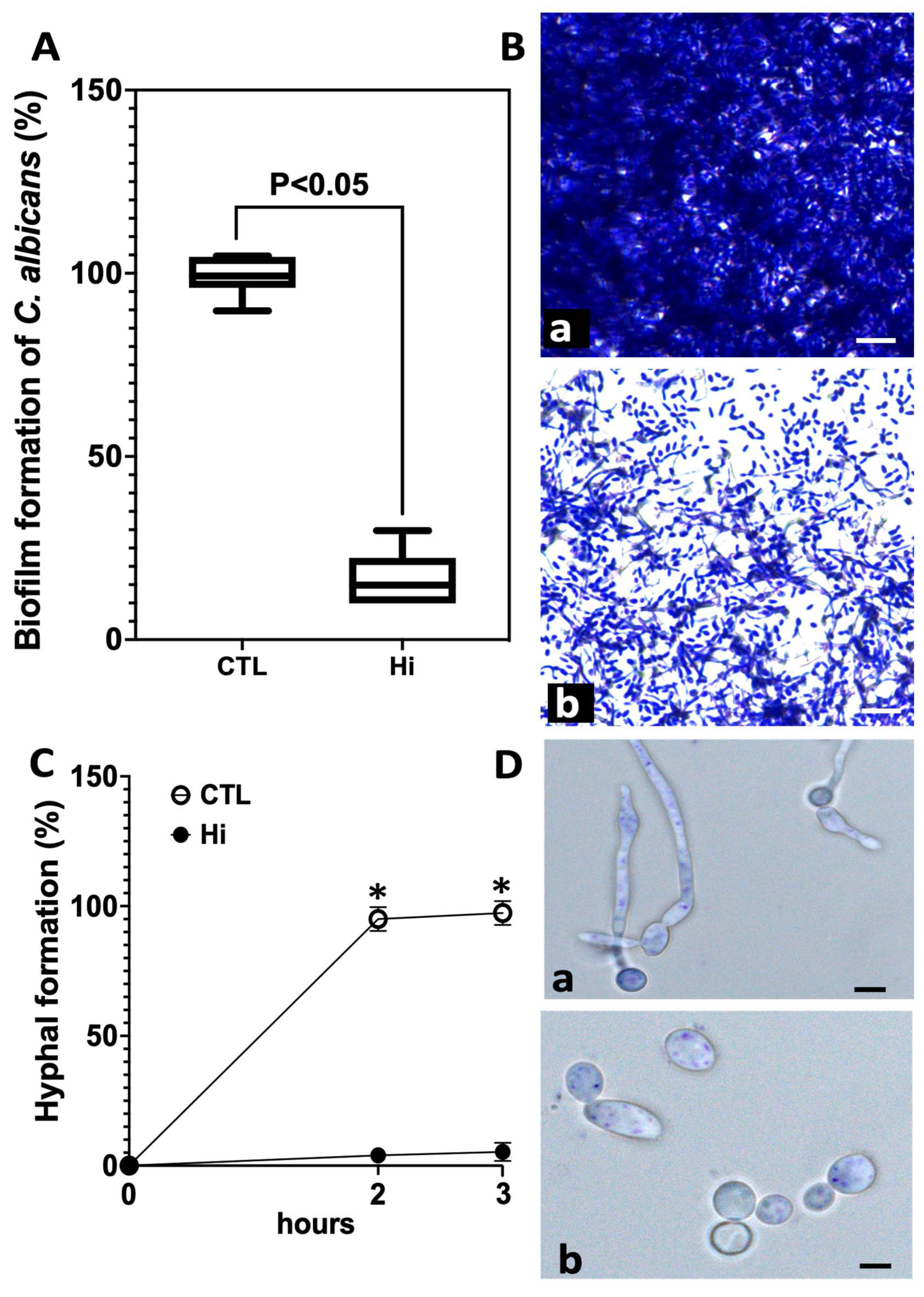
2.2. Effect of Hi on C. albicans Biofilm and Hyphal Formation
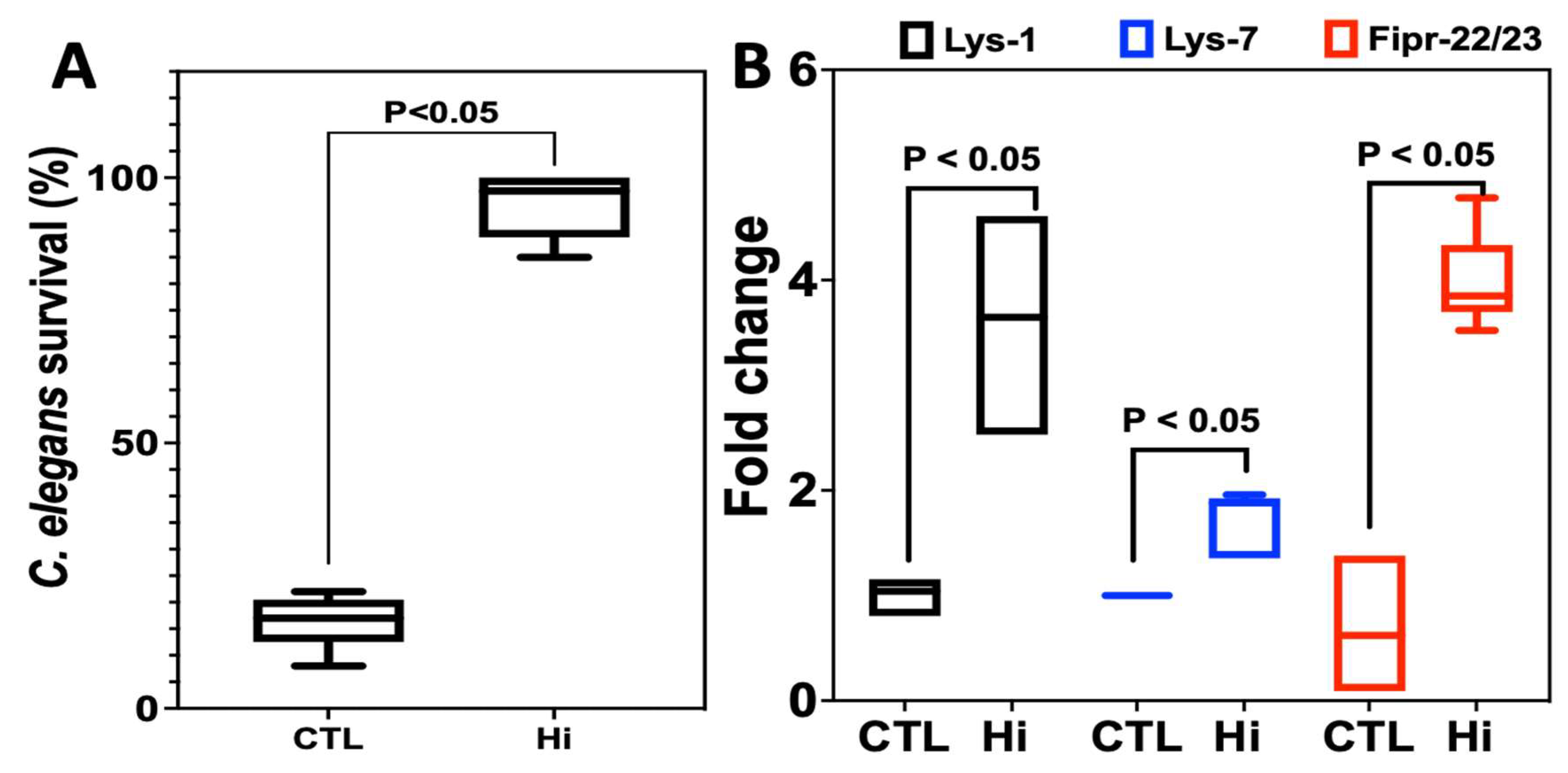
2.3. Effect of Hi Treatment on the Survival of C. elegans Infected with C. albicans
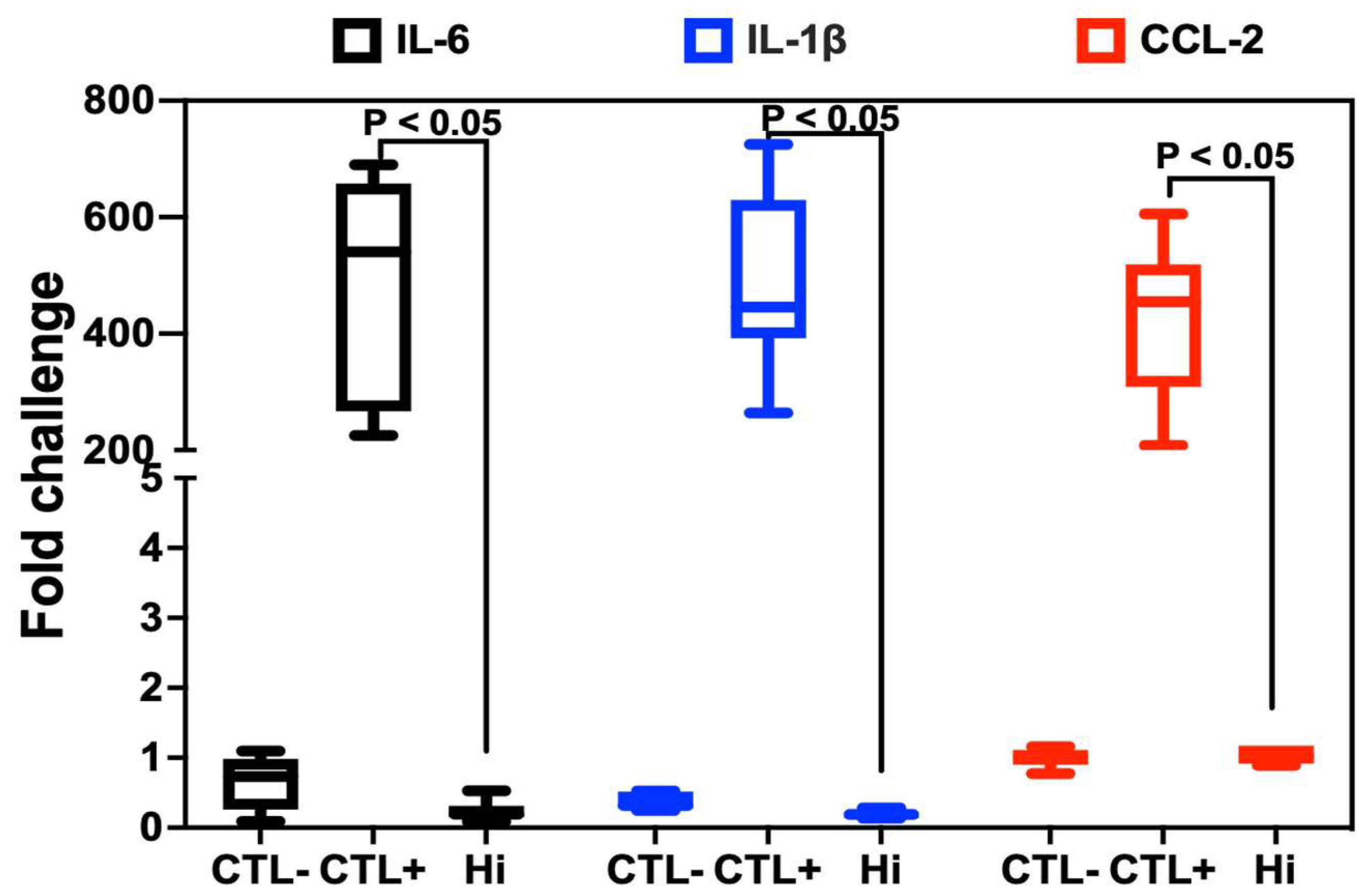
2.4. Anti-Inflammatory Properties of Hi
3. Discussion
4. Materials and Methods
4.1. Fungal and Human Cell Line Culture
4.2. Fungal Viability Assays
4.3. C. albicans Biofilm Formation
4.4. C. elegans Survival Assay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulain, D.; Sendid, B.; Standaert-Vitse, A.; Fradin, C.; Jouault, T.; Jawhara, S.; Colombel, J.F. Yeasts: Neglected pathogens. Dig. Dis. 2009, 27 (Suppl. 1), 104–110. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6 (Suppl. 1), S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. How Gut Bacterial Dysbiosis Can Promote Candida albicans Overgrowth during Colonic Inflammation. Microorganisms 2022, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics 2020, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R.; Ausubel, F.M. Models of Caenorhabditis elegans infection by bacterial and fungal pathogens. Methods Mol. Biol. 2008, 415, 403–427. [Google Scholar]
- Shu, C.; Sun, L.; Zhang, W. Thymol has antifungal activity against Candida albicans during infection and maintains the innate immune response required for function of the p38 MAPK signaling pathway in Caenorhabditis elegans. Immunol. Res. 2016, 64, 1013–1024. [Google Scholar] [CrossRef]
- Pukkila-Worley, R.; Peleg, A.Y.; Tampakakis, E.; Mylonakis, E. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot. Cell 2009, 8, 1750–1758. [Google Scholar] [CrossRef]
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.F.; Abid, M. Mechanistic Understanding of Candida albicans Biofilm Formation and Approaches for Its Inhibition. Front. Microbiol. 2021, 12, 638609. [Google Scholar] [CrossRef]
- D’Agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef]
- Pinto, E.; Pina-Vaz, C.; Salgueiro, L.; Goncalves, M.J.; Costa-de-Oliveira, S.; Cavaleiro, C.; Palmeira, A.; Rodrigues, A.; Martinez-de-Oliveira, J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2006, 55 Pt 10 Pt 10, 1367–1373. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis Cinerea, Fusarium sp. And Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Segvic Klaric, M.; Kosalec, I.; Mastelic, J.; Pieckova, E.; Pepeljnak, S. Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Lett. Appl. Microbiol. 2007, 44, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Han, F.; Ma, G.Q.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.C.; Zhao, Z.D.; Xu, H.L. Chemical composition and antioxidant activities of essential oils from different parts of the oregano. J. Zhejiang Univ. Sci. B 2017, 18, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha spp. Essential Oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef]
- Tampieri, M.P.; Galuppi, R.; Macchioni, F.; Carelle, M.S.; Falcioni, L.; Cioni, P.L.; Morelli, I. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia 2005, 159, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Bialon, M.; Krzysko-Lupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef]
- Behmanesh, F.; Pasha, H.; Sefidgar, A.A.; Taghizadeh, M.; Moghadamnia, A.A.; Adib Rad, H.; Shirkhani, L. Antifungal Effect of Lavender Essential Oil (Lavandula angustifolia) and Clotrimazole on Candida albicans: An In Vitro Study. Scientifica 2015, 2015, 261397. [Google Scholar] [CrossRef]
- D’Auria, F.D.; Tecca, M.; Strippoli, V.; Salvatore, G.; Battinelli, L.; Mazzanti, G. Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med. Mycol. 2005, 43, 391–396. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, Y.G.; Kim, J.C.; Han, J.G.; Lee, H.Y.; Cho, J.Y. Hinokitiol, a natural tropolone derivative, inhibits TNF-alpha production in LPS-activated macrophages via suppression of NF-kappaB. Planta Med. 2008, 74, 828–833. [Google Scholar] [CrossRef]
- Saeki, Y.; Ito, Y.; Shibata, M.; Sato, Y.; Okuda, K.; Takazoe, I. Antimicrobial action of natural substances on oral bacteria. Bull. Tokyo Dent. Coll. 1989, 30, 129–135. [Google Scholar]
- Le, C.Y.; Ye, Y.J.; Xu, J.; Li, L.; Feng, X.Q.; Chen, N.P.; Zhu, B.Q.; Ding, Z.S.; Qian, C.D. Hinokitiol Selectively Enhances the Antibacterial Activity of Tetracyclines against Staphylococcus aureus. Microbiol. Spectr. 2023, 11, e0320522. [Google Scholar] [CrossRef] [PubMed]
- Koufaki, M.; Theodorou, E.; Alexi, X.; Nikoloudaki, F.; Alexis, M.N. Synthesis of tropolone derivatives and evaluation of their in vitro neuroprotective activity. Eur. J. Med. Chem. 2010, 45, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Lin, S.T.; Chang, W.W.; Liu, L.W.; Li, T.Y.; Kuo, C.Y.; Hsieh, J.L.; Lee, C.H. Hinokitiol induces autophagy in murine breast and colorectal cancer cells. Environ. Toxicol. 2016, 31, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic Acid: A Promising Therapeutic Phytochemical and Recent Patents Advances. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Merlani, M.; Barbakadze, V.; Amiranashvili, L.; Gogilashvili, L.; Poroikov, V.; Petrou, A.; Geronikaki, A.; Ciric, A.; Glamoclija, J.; Sokovic, M. New Caffeic Acid Derivatives as Antimicrobial Agents: Design, Synthesis, Evaluation and Docking. Curr. Top Med. Chem. 2019, 19, 292–304. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M. Pharmacological and therapeutic applications of Sinapic acid-an updated review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Fatima, Z.; Hameed, S. Sesamol: A natural phenolic compound with promising anticandidal potential. J. Pathog. 2014, 2014, 895193. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Jirovetz, L.; Wlcek, K.; Buchbauer, G.; Gochev, V.; Girova, T.; Stoyanova, A.; Geissler, M. Antifungal Activity of Eugenol and Various Eugenol-Containing Essential Oils against 38 Clinical Isolates of Candida albicans. J. Essent. Oil Bear. Plants 2007, 10, 421–429. [Google Scholar] [CrossRef]
- Damiens, A.; Alebrahim, M.T.; Leonard, E.; Fayeulle, A.; Furman, C.; Hilbert, J.L.; Siah, A.; Billamboz, M. Sesamol-based terpenoids as promising bio-sourced crop protection compounds against the wheat pathogen Zymoseptoria tritici. Pest. Manag. Sci. 2021, 77, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- de Castro, R.D.; de Souza, T.M.; Bezerra, L.M.; Ferreira, G.L.; Costa, E.M.; Cavalcanti, A.L. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: An in vitro study. BMC Complement. Altern. Med. 2015, 15, 417. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Ben Miri, Y.; Nouasri, A.; Herrera, M.; Djenane, D.; Arino, A. Antifungal Activity of Menthol, Eugenol and Their Combination against Aspergillus ochraceus and Aspergillus niger In Vitro and in Stored Cereals. Foods 2023, 12, 2108. [Google Scholar] [CrossRef]
- Silva, D.; Diniz-Neto, H.; Cordeiro, L.; Silva-Neta, M.; Silva, S.; Andrade-Junior, F.; Leite, M.; Nobrega, J.; Morais, M.; Souza, J.; et al. (R)-(+)-beta-Citronellol and (S)-(−)-beta-Citronellol in Combination with Amphotericin B against Candida Spp. Int. J. Mol. Sci. 2020, 21, 1785. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2021, 11, 20. [Google Scholar] [CrossRef]
- Pereira Fde, O.; Mendes, J.M.; Lima, I.O.; Mota, K.S.; Oliveira, W.A.; Lima Ede, O. Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm. Biol. 2015, 53, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.I.S.; Sousa, M.N.A.; Filho, G.G.A.; Freitas, F.O.R.; Uchoa, D.P.L.; Nobre, M.S.C.; Bezerra, A.L.D.; Rolim, L.; Morais, A.M.B.; Nogueira, T.; et al. Antifungal activity of linalool against fluconazole-resistant clinical strains of vulvovaginal Candida albicans and its predictive mechanism of action. Braz. J. Med. Biol. Res. 2022, 55, e11831. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Li, H.; Wang, X.; Zhang, R.; Yang, X.; Jiang, Q.; Shi, Q. Revealing the Mechanisms for Linalool Antifungal Activity against Fusarium oxysporum and Its Efficient Control of Fusarium Wilt in Tomato Plants. Int. J. Mol. Sci. 2022, 24, 458. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.U.; Kwon, S.S.; Kong, T.Y.; Kim, J.H.; Lee, H.S. Inhibitory effects of cedrol, beta-cedrene, and thujopsene on cytochrome P450 enzyme activities in human liver microsomes. J. Toxicol. Environ. Health A 2014, 77, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, A.; Gamirov, R.; Panina, Y.; Sokolova, E.; Leonteva, Y.; Tarasova, E.; Potekhina, R.; Fitsev, I.; Shurpik, D.; Stoikov, I. Towards potential antifungal agents: Synthesis, supramolecular self-assembly and in vitro activity of azole mono-, sesqui- and diterpenoids. Org. Biomol. Chem. 2023, 21, 4863–4873. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Shi, X.C.; Wang, S.Y.; Wang, B.; Laborda, P. Antifungal Mechanism and Efficacy of Kojic Acid for the Control of Sclerotinia sclerotiorum in Soybean. Front. Plant Sci. 2022, 13, 845698. [Google Scholar] [CrossRef]
- Brtko, J. Biological functions of kojic acid and its derivatives in medicine, cosmetics, and food industry: Insights into health aspects. Arch. Pharm. 2022, 355, e2200215. [Google Scholar] [CrossRef]
- Balaz, S.; Uher, M.; Brtko, J.; Veverka, M.; Bransova, J.; Dobias, J.; Podova, M.; Buchvald, J. Relationship between antifungal activity and hydrophobicity of kojic acid derivatives. Folia Microbiol. 1993, 38, 387–391. [Google Scholar] [CrossRef]
- Komaki, N.; Watanabe, T.; Ogasawara, A.; Sato, N.; Mikami, T.; Matsumoto, T. Antifungal mechanism of hinokitiol against Candida albicans. Biol. Pharm. Bull. 2008, 31, 735–737. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojkovic, D.S.; Glamoclija, J.; Calhelha, R.C.; Ferreira, I.; Sanglard, D.; Sokovic, M. Camphor and Eucalyptol-Anticandidal Spectrum, Antivirulence Effect, Efflux Pumps Interference and Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef]
- Mishra, P.; Gupta, P.; Srivastava, A.K.; Poluri, K.M.; Prasad, R. Eucalyptol/ beta-cyclodextrin inclusion complex loaded gellan/PVA nanofibers as antifungal drug delivery system. Int. J. Pharm. 2021, 609, 121163. [Google Scholar] [CrossRef] [PubMed]
- Gillum, A.M.; Tsay, E.Y.; Kirsch, D.R. Isolation of the Candida albicans gene for orotidine-5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef]
- Bortolus, C.; Billamboz, M.; Charlet, R.; Lecointe, K.; Sendid, B.; Ghinet, A.; Jawhara, S. A Small Aromatic Compound Has Antifungal Properties and Potential Anti-Inflammatory Effects against Intestinal Inflammation. Int. J. Mol. Sci. 2019, 20, 321. [Google Scholar] [CrossRef] [PubMed]
- Isono, T.; Domon, H.; Nagai, K.; Maekawa, T.; Tamura, H.; Hiyoshi, T.; Yanagihara, K.; Kunitomo, E.; Takenaka, S.; Noiri, Y.; et al. Treatment of severe pneumonia by hinokitiol in a murine antimicrobial-resistant pneumococcal pneumonia model. PLoS ONE 2020, 15, e0240329. [Google Scholar] [CrossRef] [PubMed]
- Domon, H.; Hiyoshi, T.; Maekawa, T.; Yonezawa, D.; Tamura, H.; Kawabata, S.; Yanagihara, K.; Kimura, O.; Kunitomo, E.; Terao, Y. Antibacterial activity of hinokitiol against both antibiotic-resistant and -susceptible pathogenic bacteria that predominate in the oral cavity and upper airways. Microbiol. Immunol. 2019, 63, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Chang, K.W.; Hsia, S.M.; Yu, C.C.; Fuh, L.J.; Chi, T.Y.; Shieh, T.M. In vitro antimicrobial and anticancer potential of hinokitiol against oral pathogens and oral cancer cell lines. Microbiol. Res. 2013, 168, 254–262. [Google Scholar] [CrossRef]
- Hoang, B.X.; Han, B. A possible application of hinokitiol as a natural zinc ionophore and anti-infective agent for the prevention and treatment of COVID-19 and viral infections. Med. Hypotheses 2020, 145, 110333. [Google Scholar] [CrossRef]
- Chen, H.Y.; Cheng, W.P.; Chiang, Y.F.; Hong, Y.H.; Ali, M.; Huang, T.C.; Wang, K.L.; Shieh, T.M.; Chang, H.Y.; Hsia, S.M. Hinokitiol Exhibits Antitumor Properties through Induction of ROS-Mediated Apoptosis and p53-Driven Cell-Cycle Arrest in Endometrial Cancer Cell Lines (Ishikawa, HEC-1A, KLE). Int. J. Mol. Sci. 2021, 22, 8268. [Google Scholar] [CrossRef]
- Inamori, Y.; Muro, C.; Sajima, E.; Katagiri, M.; Okamoto, Y.; Tanaka, H.; Sakagami, Y.; Tsujibo, H. Biological activity of purpurogallin. Biosci. Biotechnol. Biochem. 1997, 61, 890–892. [Google Scholar] [CrossRef]
- Meng, F.; Liu, X.; Li, C.; Peng, X.; Wang, Q.; Xu, Q.; Sui, J.; Zhao, G.; Lin, J. Hinokitiol inhibits Aspergillus fumigatus by interfering with the cell membrane and cell wall. Front. Microbiol. 2023, 14, 1132042. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, M.W.; Choi, J.S.; Lee, S.G.; Park, J.Y.; Kim, S.W. Inhibitory activity of hinokitiol against biofilm formation in fluconazole-resistant Candida species. PLoS ONE 2017, 12, e0171244. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, T.; Domon, H.; Maekawa, T.; Yonezawa, D.; Kunitomo, E.; Tabeta, K.; Terao, Y. Protective effect of hinokitiol against periodontal bone loss in ligature-induced experimental periodontitis in mice. Arch. Oral. Biol. 2020, 112, 104679. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Thuru, X.; Standaert-Vitse, A.; Jouault, T.; Mordon, S.; Sendid, B.; Desreumaux, P.; Poulain, D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 2008, 197, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Procop, G.W.; Rinaldi, M.G. Multicenter comparison of the VITEK 2 antifungal susceptibility test with the CLSI broth microdilution reference method for testing amphotericin B, flucytosine, and voriconazole against Candida spp. J. Clin. Microbiol. 2007, 45, 3522–3528. [Google Scholar] [CrossRef]
- Camaioni, L.; Lambert, D.; Sendid, B.; Billamboz, M.; Jawhara, S. Antifungal Properties of Hydrazine-Based Compounds against Candida albicans. Antibiotics 2023, 12, 1043. [Google Scholar] [CrossRef]
- Charlet, R.; Le Danvic, C.; Sendid, B.; Nagnan-Le Meillour, P.; Jawhara, S. Oleic Acid and Palmitic Acid from Bacteroides thetaiotaomicron and Lactobacillus johnsonii Exhibit Anti-Inflammatory and Antifungal Properties. Microorganisms 2022, 10, 1803. [Google Scholar] [CrossRef]
- Dumortier, C.; Charlet, R.; Bettaieb, A.; Jawhara, S. H89 Treatment Reduces Intestinal Inflammation and Candida albicans Overgrowth in Mice. Microorganisms 2020, 8, 2039. [Google Scholar] [CrossRef]
- Jawhara, S.; Poulain, D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med. Mycol. 2007, 45, 691–700. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Mena, L.; Billamboz, M.; Charlet, R.; Despres, B.; Sendid, B.; Ghinet, A.; Jawhara, S. Two New Compounds Containing Pyridinone or Triazine Heterocycles Have Antifungal Properties against Candida albicans. Antibiotics 2022, 11, 72. [Google Scholar] [CrossRef]
- Zwirchmayr, J.; Kirchweger, B.; Lehner, T.; Tahir, A.; Pretsch, D.; Rollinger, J.M. A robust and miniaturized screening platform to study natural products affecting metabolism and survival in Caenorhabditis elegans. Sci. Rep. 2020, 10, 12323. [Google Scholar] [CrossRef] [PubMed]
| N° | Compound | Structure | M (g/mol) | MIC (µg/mL) | logP a | logKp cm/s b | TPSA (Å2) c | BBBP d | Ref e | |
|---|---|---|---|---|---|---|---|---|---|---|
| CINNAMIC DERIVATIVES | 1 | Ferulic acid | 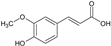 | 194.18 | 970.9 | 1.36 | −6.41 | 66.76 | Yes | [26,27] |
| 2 | Caffeic acid | 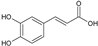 | 180.16 | 900.8 | 0.93 | −6.58 | 77.76 | No | [28,29] | |
| 3 | Sinapic acid |  | 224.21 | 1121.05 | 1.31 | −6.63 | 75.99 | No | [30,31] | |
| AROMATIC PHENOLS | 4 | Syringic acid | 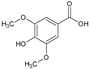 | 198.17 | 990.85 | 0.99 | −6.77 | 75.99 | No | [20] |
| 5 | Eugenol | 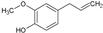 | 164.20 | 821 | 2.25 | −5.69 | 29.46 | Yes | [32] | |
| 6 | Sesamol | 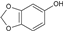 | 132.11 | 660.55 | 1.19 | −6.27 | 38.69 | Yes | [33,34] | |
| 7 | Thymol | 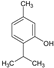 | 150.22 | 751.1 | 2.80 | −4.87 | 20.23 | Yes | [35,36] | |
| MONO & SESQUITERPENOLS | 8 | L-Menthol | 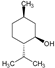 | 156.27 | 781.35 | 2.59 | −4.84 | 20.23 | Yes | [37,38] |
| 9 | Citronellol | 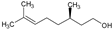 | 156.27 | 781.35 | 2.92 | −4.48 | 20.23 | Yes | [38,39] | |
| 10 | Geraniol | 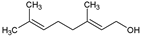 | 154.25 | 781.35 | 2.74 | −4.71 | 20.23 | Yes | [40,41] | |
| 11 | Linalool | 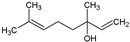 | 154.25 | 781.35 | 2.66 | −5.13 | 20.23 | Yes | [42,43] | |
| 12 | Cedrol | 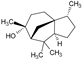 | 222.37 | 111.185 | 3.54 | −4.90 | 20.23 | Yes | [44,45] | |
| OTHERS | 13 | Kojic acid | 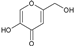 | 142.11 | 710.55 | −0.16 | −7.62 | 70.67 | No | [46,47,48] |
| 14 | Hinokitiol | 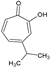 | 164.20 | 8.21 | 1.98 | −5.79 | 37.30 | Yes | [49] | |
| 15 | Eucalyptol |  | 154.25 | 781.35 | 2.77 | −5.13 | 9.23 | Yes | [50,51] |
| Strain | Description | Caspofungin MIC (µg/mL) | Fluconazole MIC (µg/mL) | Ref. |
|---|---|---|---|---|
| C. albicans SC5314 | Wild-type | 0.03 | 0.5 | [52] |
| C. albicans 15343c3523 | Blood, caspofungin-resistant | 2 | 0.5 | [53] |
| C. albicans 15351c6859 | Venous catheter, caspofungin-resistant | 4 | 1 | [53] |
| C. albicans 92535989 | Tracheal secretion, fluconazole-resistant | 0.06 | 64 | [53] |
| C. albicans 17287c305 | Blood, caspofungin-resistant | 8 | 0.5 | [53] |
| C. albicans 14316c1746 | Bronchoalveolar lavage, fluconazole-resistant | 0.03 | 128 | [53] |
| C. albicans 14294c5335 | Stools, fluconazole-resistant | 0.06 | 5 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camaioni, L.; Ustyanowski, B.; Buisine, M.; Lambert, D.; Sendid, B.; Billamboz, M.; Jawhara, S. Natural Compounds with Antifungal Properties against Candida albicans and Identification of Hinokitiol as a Promising Antifungal Drug. Antibiotics 2023, 12, 1603. https://doi.org/10.3390/antibiotics12111603
Camaioni L, Ustyanowski B, Buisine M, Lambert D, Sendid B, Billamboz M, Jawhara S. Natural Compounds with Antifungal Properties against Candida albicans and Identification of Hinokitiol as a Promising Antifungal Drug. Antibiotics. 2023; 12(11):1603. https://doi.org/10.3390/antibiotics12111603
Chicago/Turabian StyleCamaioni, Louis, Bastien Ustyanowski, Mathys Buisine, Dylan Lambert, Boualem Sendid, Muriel Billamboz, and Samir Jawhara. 2023. "Natural Compounds with Antifungal Properties against Candida albicans and Identification of Hinokitiol as a Promising Antifungal Drug" Antibiotics 12, no. 11: 1603. https://doi.org/10.3390/antibiotics12111603
APA StyleCamaioni, L., Ustyanowski, B., Buisine, M., Lambert, D., Sendid, B., Billamboz, M., & Jawhara, S. (2023). Natural Compounds with Antifungal Properties against Candida albicans and Identification of Hinokitiol as a Promising Antifungal Drug. Antibiotics, 12(11), 1603. https://doi.org/10.3390/antibiotics12111603








