Prevalence of Multidrug-Resistant and ESBL-Producing Bacterial Pathogens in Patients with Chronic Wound Infections and Spinal Cord Injury Admitted to a Tertiary Care Rehabilitation Hospital
Abstract
:1. Introduction
- To evaluate the prevalence of different bacterial pathogens in patients diagnosed with spinal cord injuries and pressure ulcers.
- To determine the prevalence of MDR and extended-spectrum beta-lactamase (ESBL)-producing Gram-negative bacteria isolated from chronic wounds.
- To investigate how frequently antibiotics are prescribed in patients with chronic wound infections.
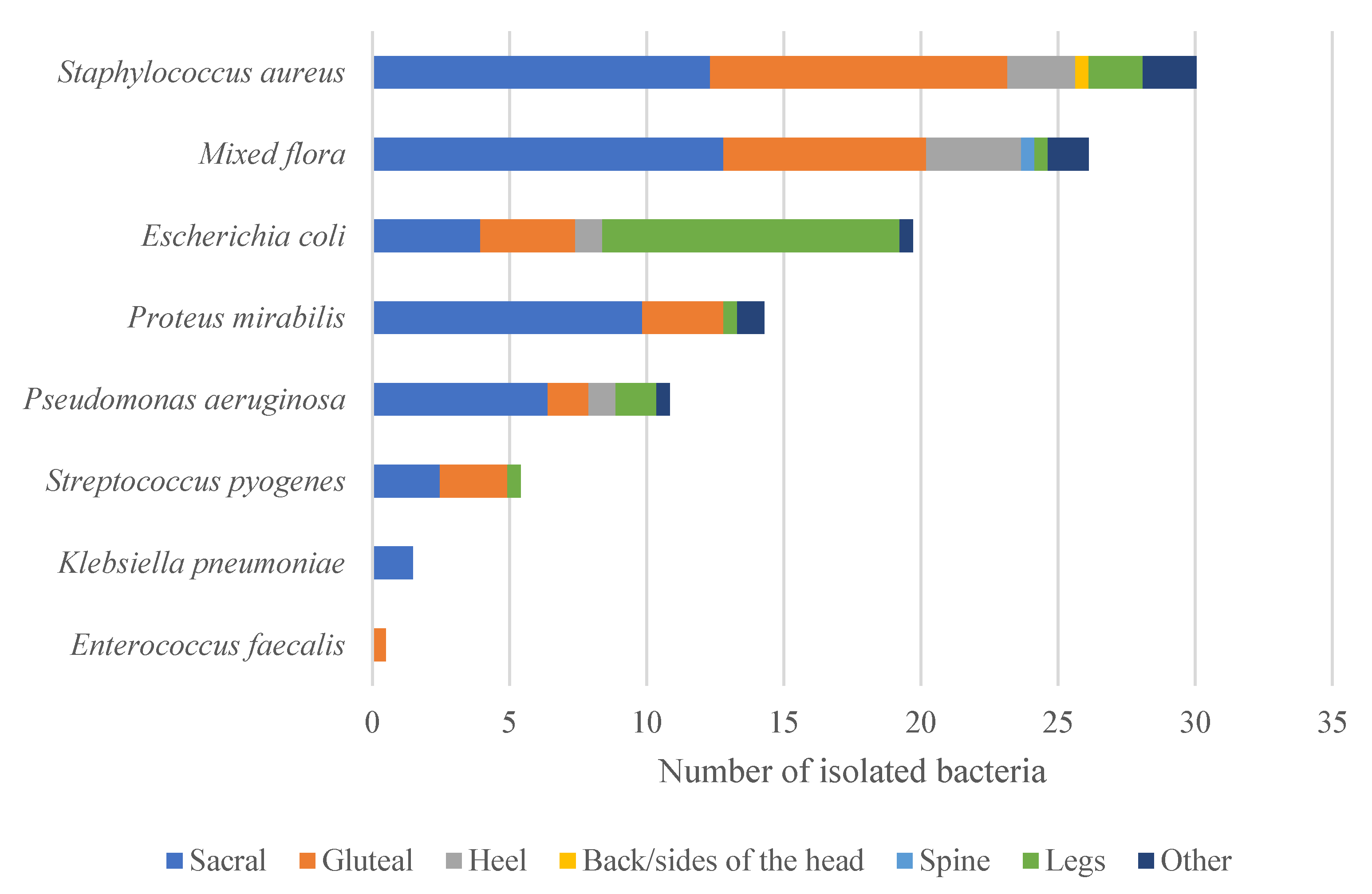
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Data Collection
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDR | Multidrug-resistant |
| IDSA | The Infectious Disease Society of America |
| SSTI | Skin and soft tissue infections |
| MRSA | Methicillin-resistant S. aureus |
| ESBL | Extended-spectrum beta-lactamase |
| PMN | Polymorphonuclear |
| PDR | Pan-drug-resistant |
| IRB | Institutional review board |
| SPSS | Statistical Package for Social Sciences |
References
- Dinh, A.; Bouchand, F.; Davido, B.; Duran, C.; Denys, P.; Lortat-Jacob, A.; Rottman, M.; Salomon, J.; Bernard, L. Management of established pressure ulcer infections in spinal cord injury patients. Med. Mal. Infect. 2019, 49, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Garber, S.L.; Rintala, D.H.; Hart, K.A.; Fuhrer, M.J. Pressure ulcer risk in spinal cord injury: Predictors of ulcer status over 3 years. Arch. Phys. Med. Rehabil. 2000, 81, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Dana, A.N.; Bauman, W.A. Bacteriology of pressure ulcers in individuals with spinal cord injury: What we know and what we should know. J. Spinal Cord Med. 2015, 38, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Scheel-Sailer, A.; Wyss, A.; Boldt, C.; Post, M.; Lay, V. Prevalence, location, grade of pressure ulcers and association with specific patient characteristics in adult spinal cord injury patients during the hospital stay: A prospective cohort study. Spinal Cord 2013, 51, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Filius, P.M.G.; Gyssens, I.C. Impact of increasing antimicrobial resistance on wound management. Am. J. Clin. Dermatol. 2002, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kruger, E.A.; Pires, M.; Ngann, Y.; Sterling, M.; Rubayi, S. Comprehensive management of pressure ulcers in spinal cord injury: Current concepts and future trends. J. Spinal Cord Med. 2013, 36, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.; Dumville, J.C.; Moore, Z.E.; Tanner, J.; Christie, J.; Goto, S. Antibiotics and antiseptics for pressure ulcers. Cochrane Database Syst. Rev. 2016. [CrossRef]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial contribution in chronicity of wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice Guidelines for the diagnosis and management of skin and soft tissue infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Heym, B.; Rimareix, F.; Lortat-Jacob, A.; Nicolas-Chanoine, M. Bacteriological investigation of infected pressure ulcers in spinal cord-injured patients and impact on antibiotic therapy. Spinal Cord 2004, 42, 230–234. [Google Scholar] [CrossRef]
- Evans, M.E.; Kralovic, S.M.; Simbartl, L.A.; Obrosky, D.S.; Hammond, M.C.; Goldstein, B.; Evans, C.T.; Roselle, G.A.; Jain, R. Prevention of methicillin-resistant Staphylococcus aureus infections in spinal cord injury units. Am. J. Infect. Control 2013, 41, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.; Duerden, B.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Summa, M.; Russo, D.; Penna, I.; Margaroli, N.; Bayer, I.S.; Bandiera, T.; Athanassiou, A.; Bertorelli, R. A biocompatible sodium alginate/povidone iodine film enhances wound healing. Eur. J. Pharm. Biopharm. 2018, 122, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ousey, K.; Blackburn, J. Understanding antimicrobial resistance and antimicrobial stewardship in wound management. Wounds UK 2020, 16, 36–39. [Google Scholar]
- Edsberg, L.E.; Black, J.M.; Goldberg, M.; McNichol, L.; Moore, L.; Sieggreen, M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. J. Wound Ostomy Cont. Nurs. Off. Publ. Wound Ostomy Cont. Nurses Soc. 2016, 43, 585–597. [Google Scholar] [CrossRef]
- Brienza, D.; Krishnan, S.; Karg, P.; Sowa, G.; Allegretti, A.L. Predictors of pressure ulcer incidence following traumatic spinal cord injury: A secondary analysis of a prospective longitudinal study. Spinal Cord 2018, 56, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Prang, P.; Schuld, C.; Rupp, R.; Hensel, C.; Weidner, N. Influence of patient isolation due to colonization with multidrug-resistant organisms on functional recovery after spinal cord injury. PLoS ONE 2021, 16, e0249295. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Islam, M.N.; Hawlader, M.D.H.; Ahmed, S.; Wahab, A.; Islam, M.; Uddin, K.R.; Hossain, A. Prevalence of multidrug resistance bacterial isolates from infected wound patients in Dhaka, Bangladesh: A cross-sectional study. Int. J. Surg. Open 2021, 28, 56–62. [Google Scholar] [CrossRef]
- Goldberg, S.R.; Diegelmann, R.F. What makes wounds chronic. Surg. Clin. 2020, 100, 681–693. [Google Scholar] [CrossRef]
- Puca, V.; Marulli, R.Z.; Grande, R.; Vitale, I.; Niro, A.; Molinaro, G.; Prezioso, S.; Muraro, R.; Di Giovanni, P. Microbial Species isolated from infected wounds and antimicrobial resistance analysis: Data emerging from a three-years retrospective study. Antibiotics 2021, 10, 1162. [Google Scholar] [CrossRef] [PubMed]
- Dakorah, M.P.; Agyare, E.; Acolatse, J.E.E.; Akafity, G.; Stelling, J.; Chalker, V.J.; Spiller, O.B.; Aidoo, N.B.; Kumi-Ansah, F.; Azumah, D.; et al. Utilising cumulative antibiogram data to enhance antibiotic stewardship capacity in the Cape Coast Teaching Hospital, Ghana. Antimicrob. Resist. Infect. Control 2022, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Truong, W.R.; Hidayat, L.; Bolaris, M.A.; Nguyen, L.; Yamaki, J. The antibiogram: Key considerations for its development and utilization. JAC-Antimicrob. Resist. 2021, 3, dlab060. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Balkhy, H.H.; Walsh, T.R.; Paterson, D.L. β-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin. Microbiol. Rev. 2013, 26, 361–380. [Google Scholar] [CrossRef] [PubMed]
| Character | Number (N) | Frequency (%) | |
|---|---|---|---|
| Total | 203 | 100.00 | |
| Gender | Male | 186 | 91.62 |
| Female | 17 | 8.38 | |
| Age | 18–39 | 160 | 78.88 |
| 40–60 | 28 | 13.79 | |
| >60 | 15 | 7.39 | |
| Hospitalization time (weeks) | ≤4 | 27 | 13.30 |
| 5–8 | 65 | 32.02 | |
| 9–12 | 72 | 35.47 | |
| ≥13 | 39 | 19.21 | |
| Trauma | Traumatic | 183 | 90.15 |
| Non-traumatic | 20 | 9.85 | |
| Mobility | Full mobility | 5 | 2.47 |
| Limited mobility | 131 | 64.53 | |
| Immobile | 67 | 33.00 | |
| Underlying condition | Hypertension | 18 | 8.86 |
| Diabetes | 25 | 12.31 | |
| Dyslipidemia | 5 | 2.46 | |
| Organ failure | 1 | 0.49 | |
| Asthma | 5 | 2.46 | |
| Obesity | 18 | 8.86 | |
| Number of ulcers | 1 | 124 | 61.08 |
| 2 | 63 | 31.04 | |
| 3 | 14 | 6.89 | |
| Multiple | 2 | 0.99 | |
| Number of bacterial isolates | 1 | 98 | 48.27 |
| 2 | 72 | 35.45 | |
| 3 | 14 | 6.89 | |
| Character | Number of Ulcers (N) | Frequency (%) | |
|---|---|---|---|
| Ulcer Location | Sacral | 101 | 49.76 |
| Gluteal | 59 | 29.08 | |
| Heel | 16 | 7.88 | |
| Back or side of the head | 1 | 0.49 | |
| Spine | 1 | 0.49 | |
| Legs | 13 | 6.40 | |
| Other | 12 | 5.90 | |
| Wound | Colonized | 42 | 20.68 |
| Infected | 161 | 79.31 | |
| Ulcer Stage | Level 2 | 11 | 5.41 |
| Level 3 | 50 | 24.63 | |
| Level 4 | 120 | 59.11 | |
| N/A | 22 | 10.83 | |
| Antibiotics | Prescription Frequency N (%) | Ulcer Stage | ||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | Not Known | |||
| Total | 203 (100) | 11 (5.41) | 50 (24.63) | 120 (59.11) | 22 (10.83) | |
| Topical | Fucidin topical cream | 1 (0.49) | - | 1 (2) | - | - |
| Mupirocin ointment | 8 (3.94) | - | 4 (8) | 4 (3.33) | - | |
| Silver sulphadiazine cream | 1 (0.49) | - | 1 (2) | - | - | |
| Triamcinolone, nystatin, neomycin, gramicidin | 4 (1.97) | - | 1 (2) | 1 (0.83) | 2 (9.09) | |
| Oral | Amoxicillin/clavulanic acid | 17 (8.37) | 1 (9.09) | 8 (16) | 7 (5.83) | 1 (4.54) |
| Cefuroxime | 2 (0.99) | - | - | 2 (1.67) | - | |
| Ciprofloxacin | 3 (1.48) | - | - | 2 (1.67) | - | |
| Clindamycin | 7 (3.45) | - | - | 6 (5) | 1 (4.54) | |
| Levofloxacin | 1 (0.49) | - | 1 (2) | - | - | |
| Rifampin | 1 (0.49) | - | - | 1 (0.83) | - | |
| Sulfamethoxazole, trimethoprim | 1 (0.49) | - | - | 1 (0.83) | - | |
| Systemic | Amoxicillin, clavulanic acid | 2 (0.99) | - | - | 2 (1.67) | - |
| Cefotaxime | 1 (0.49) | - | 1 (0.83) | - | ||
| Ceftriaxone | 5 (2.45) | - | 4 (8) | 1 (0.83) | - | |
| Ciprofloxacin | 2 (0.99) | - | - | 2 (1.67) | - | |
| Clindamycin | 1 (0.49) | - | - | 1 (0.83) | - | |
| Linezolid | 1 (0.49) | - | - | 1 (0.83) | - | |
| Meropenem | 9 (4.43) | - | 1 (2) | 7 (5.83) | 1 (4.54) | |
| Dressing | Antimicrobial dressing | 135 (66.50) | 6 (54.55) | 35 (70.0) | 83 (69.17) | 11 (50.00) |
| Non-antimicrobial dressing | 58 (28.57) | 4 (36.36) | 13 (26.0) | 32 (26.67) | 9 (40.90) | |
| Not treated | 10 (4.93) | 1 (9.09) | 2 (4.0) | 5 (4.17) | 2 (9.09) | |
| Bacteria (n) | S. aureus | P. aeruginosa | E. faecalis | Coagulase-Negative Staphylococci | E. coli | S. pyogenes | K. pneumoniae |
|---|---|---|---|---|---|---|---|
| Ampicillin | - | - | 1 (R) | - | 13 (R), 7 (S) | 7 (S) | 3 (R) |
| Amikacin | - | - | - | - | 6 (S) | - | 1 (S) |
| Ceftazidime | - | 1 (R), 17 (S), 1 (I) | - | - | 4 (R), 2 (S) | - | 2 (R) |
| Clindamycin | 4 (R), 58 (S) | - | - | 1 (S) | - | 6 (R), 5 (S) | - |
| Ciprofloxacin | 8 (R), 4 (S) | 2 (R), 16 (S), 1 (I) | - | - | 6 (R) | - | 2 (R), 1 (I) |
| Ceftriaxone | - | - | - | - | 4 (R), 2 (S) | 11 (S) | 2 (R) |
| Cefepime | - | 1 (R), 16 (S), 1 (I) | - | - | 4 (R), 2 (S) | - | 2 (S) |
| Gentamicin | 1 (R) | 1 (R), 18 (S) | - | - | 5 (R), I (S) | - | 1 (R), 2 (S) |
| Linezolid | 32 (S) | - | - | - | - | - | - |
| Meropenem | - | 1 (R), 16 (S), 2 (I) | - | - | 4 (S) | - | 1 (S) |
| Mupirocin | 28 (S) | - | - | - | - | - | - |
| Oxacillin | 35 (R), 23 (S) | - | - | 1 (S) | - | - | - |
| Rifampin | 4 (I), 33 (S) | - | - | - | - | - | - |
| Trimethoprim | 6 (R), 23 (S) | - | - | 1 (S) | 3 (R), 3 (S) | 2 (S) | 2 (R), 1 (S) |
| Tigecycline | 2 (S) | 7 (R) | - | - | 5 (R) | 3 (S) | 1 (S) |
| Pattern | N | % |
|---|---|---|
| Sensitive | 128 | 63.05 |
| MDR | 16 | 7.88 |
| PDR | 5 | 2.46 |
| MRSA | 52 | 25.61 |
| ESBL | 36 | 17.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binsuwaidan, R.; Khan, M.A.; Alzahrani, R.H.; Aldusaymani, A.M.; Almallouhi, N.M.; Alsabti, A.S.; Ali, S.; Khan, O.S.; Youssef, A.M.; Alnajjar, L.I. Prevalence of Multidrug-Resistant and ESBL-Producing Bacterial Pathogens in Patients with Chronic Wound Infections and Spinal Cord Injury Admitted to a Tertiary Care Rehabilitation Hospital. Antibiotics 2023, 12, 1587. https://doi.org/10.3390/antibiotics12111587
Binsuwaidan R, Khan MA, Alzahrani RH, Aldusaymani AM, Almallouhi NM, Alsabti AS, Ali S, Khan OS, Youssef AM, Alnajjar LI. Prevalence of Multidrug-Resistant and ESBL-Producing Bacterial Pathogens in Patients with Chronic Wound Infections and Spinal Cord Injury Admitted to a Tertiary Care Rehabilitation Hospital. Antibiotics. 2023; 12(11):1587. https://doi.org/10.3390/antibiotics12111587
Chicago/Turabian StyleBinsuwaidan, Reem, Mohammad Aatif Khan, Raghad H. Alzahrani, Aljoharah M. Aldusaymani, Noura M. Almallouhi, Alhanouf S. Alsabti, Sajjad Ali, Omar Sufyan Khan, Amira M. Youssef, and Lina I. Alnajjar. 2023. "Prevalence of Multidrug-Resistant and ESBL-Producing Bacterial Pathogens in Patients with Chronic Wound Infections and Spinal Cord Injury Admitted to a Tertiary Care Rehabilitation Hospital" Antibiotics 12, no. 11: 1587. https://doi.org/10.3390/antibiotics12111587
APA StyleBinsuwaidan, R., Khan, M. A., Alzahrani, R. H., Aldusaymani, A. M., Almallouhi, N. M., Alsabti, A. S., Ali, S., Khan, O. S., Youssef, A. M., & Alnajjar, L. I. (2023). Prevalence of Multidrug-Resistant and ESBL-Producing Bacterial Pathogens in Patients with Chronic Wound Infections and Spinal Cord Injury Admitted to a Tertiary Care Rehabilitation Hospital. Antibiotics, 12(11), 1587. https://doi.org/10.3390/antibiotics12111587






