Focusing on Gordonia Infections: Distribution, Antimicrobial Susceptibilities and Phylogeny
Abstract
1. Introduction
2. Results and Discussion
2.1. Distribution of Gordonia Species in Human Infections
2.2. Gordonia Species Identification
2.3. Antimicrobial Susceptibilities of Gordonia Species
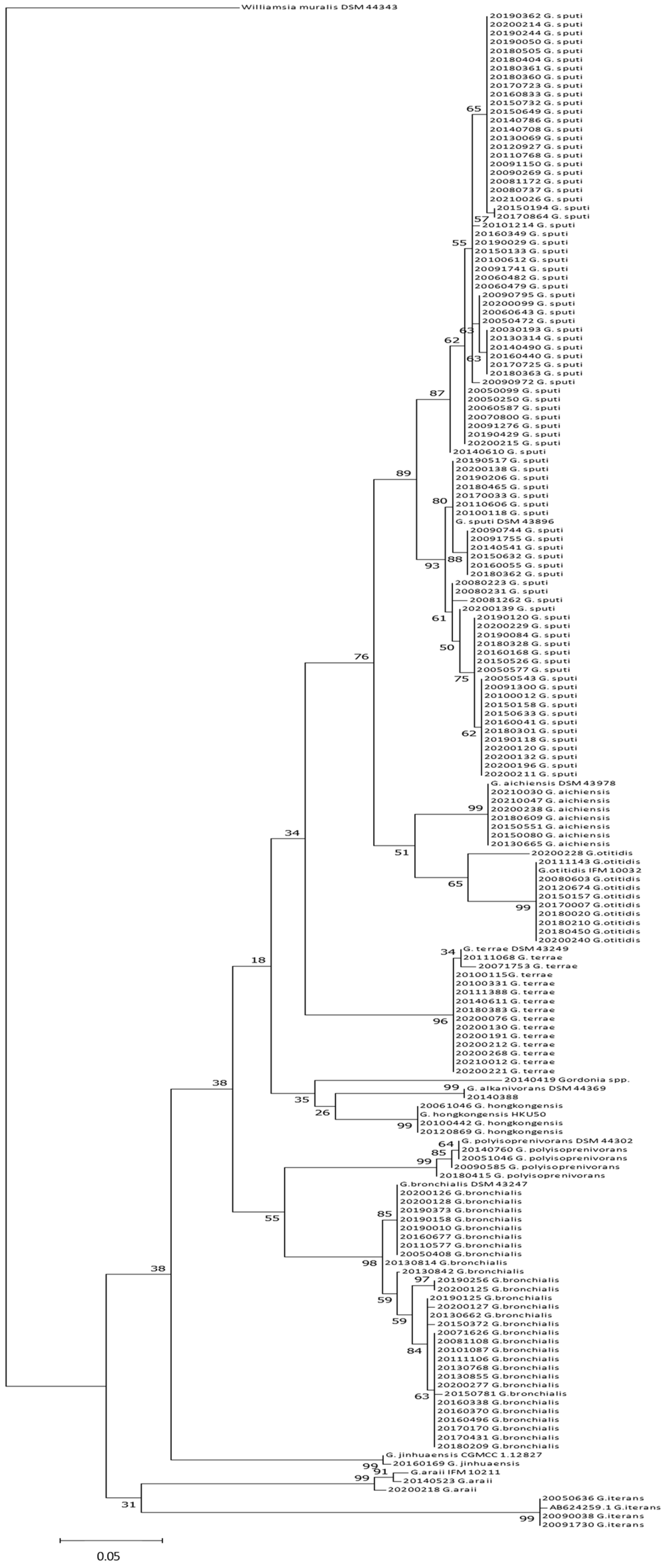
2.4. Phylogenetic Analysis of Gordonia spp.
2.4.1. 16S rDNA and secA1 phylogeny
2.4.2. Gordonia Species with High Prevalence: G. sputi
2.4.3. Gordonia Species with Medium Prevalence: G. bronchialis
2.4.4. Gordonia Species with Low Prevalence: G. terrae and G. otitidis
2.4.5. Other Gordonia species
3. Materials and Methods
3.1. Strains and Target Genes for Identification
3.2. Species Assignment
3.3. Antimicrobial Susceptibility Testing
3.4. 16S rDNA and secA1 Phylogeny
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsukamura, M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J. Gen. Microbiol. 1971, 68, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Andalibi, F.; Fatahi-Bafghi, M. Gordonia: Isolation and identification in clinical samples and role in biotechnology. Fol. Microbiol. 2017, 62, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sowani, H.; Kulkarni, M.; Zinjarde, S. An insight into the ecology, diversity and adaptations of Gordonia species. Critical Rev. Microbiol. 2018, 44, 393–413. [Google Scholar] [CrossRef]
- Kempf, V.A.; Schmalzing, M.; Yassin, A.F.; Schaal, K.P.; Baumeister, D.; Arenskötter, M.; Steinbüchel, A.; Autenrieth, I.B. Gordonia polyisoprenivorans septicemia in a bone marrow transplant patient. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 226–228. [Google Scholar] [CrossRef]
- Blaschke, A.J.; Bender, J.; Byington, C.L.; Korgenski, K.; Daly, J.; Petti, C.A.; Pavia, A.T.; Ampofo, K. Gordonia species: Emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin. Infect. Dis. 2007, 45, 483–486. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Liu, C.Y.; Tan, C.K.; Lin, S.H.; Liao, C.H.; Chou, C.H.; Huang, Y.T.; Lin, H.I.; Hsueh, P.R. Infections caused by Gordonia species at a medical centre in Taiwan, 1997 to 2008. Clin. Microbiol. Infect. 2010, 16, 1448–1453. [Google Scholar] [CrossRef]
- Siddiqui, N.; Toumeh, A.; Georgescu, C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J. Clin. Microbiol. 2012, 50, 3119–3121. [Google Scholar] [CrossRef]
- Ambesh, P.; Kapoor, A.; Kazmi, D.H.; Elsheshtawy, M.; Shetty, V.; Lin, Y.S.; Kamholz, S. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann. Card. Anaesth. 2019, 22, 221–224. [Google Scholar] [CrossRef]
- Verma, P.; Brown, J.M.; Nunez, V.H.; Morey, R.E.; Steigerwalt, A.G.; Pellegrini, G.J.; Kessler, H.A. Native valve endocarditis due to Gordonia polyisoprenivorans: Case report and review of literature of bloodstream infections caused by Gordonia species. J. Clin. Microbiol. 2006, 44, 1905–1908. [Google Scholar] [CrossRef]
- Johnson, J.A.; Onderdonk, A.B.; Cosimi, L.A.; Yawetz, S.; Lasker, B.A.; Bolcen, S.J.; Brown, J.M.; Marty, F. Gordonia bronchialis bacteremia and pleural infection: Case report and review of the literature. J. Clin. Microbiol. 2011, 49, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yu, Y.; Chen, M.; Wang, C.; Kang, Y.; Li, H.; Lou, J. Bacteremia due to Gordonia polyisoprenivorans: Case report and review of literature. BMC Infect. Dis. 2017, 17, 419. [Google Scholar] [CrossRef][Green Version]
- Guerrero, C.; Casañe, C.; Antequera, P.; Candel, C.; Blázquez, R. Catheter-related bloodstream infection caused by Gordonia terrae in a bone-marrow transplant patient: Case report and review of the literature. J. Med. Microbiol. Case Rep. 2014, 1, e001032. [Google Scholar] [CrossRef]
- Lai, C.C.; Hsieh, J.H.; Tsai, H.Y.; Liao, C.H.; Hsueh, P.R. Cutaneous Infection Caused by Gordonia amicalis after a Traumatic Injury. J. Clin. Microbiol. 2012, 50, 1821–1822. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Deziel, P.J.; Wengenack, N.L. Gordonia Bacteremia. J. Clin. Microbiol. 2012, 51, 3443–3447. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.C.; Xiong, L.; Poon, R.W.S.; Chen, J.H.K.; Leung, K.W.; Lam, J.Y.W.; Wu, A.K.L.; Chan, J.F.W.; Lau, S.K.P.; Woo, P.C.Y. Gordonia hongkongensis sp. nov., isolated from blood culture and peritoneal dialysis effluent of patients in Hong Kong. Int. J. Syst. Evol. Microbiol. 2016, 66, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Akrami, K.; Coletta, J.; Mehta, S.; Fierer, J. Gordonia sternal wound infection treated with ceftaroline: Case report and literature review. J. Med. Microbiol. Case Rep. 2017, 13, e005113. [Google Scholar] [CrossRef] [PubMed]
- Gueneau, R.; Blanchet, D.; Rodriguez-Nava, V.; Bergeron, E.; Soulier, M.; Bestandji, N.; Demar, M.; Couppie, P.; Blaizot, R. Actinomycetoma caused by Gordonia westfalica: First reported case of human infection. New Microbes New Infect. 2020, 14, 100658. [Google Scholar] [CrossRef]
- Margalit, I.; Lebeaux, D.; Tishler, O.; Goldberg, E.; Bishara, J.; Yahav, D.; Coussement, J. How do I manage nocardiosis? Clin. Microbiol. Infect. 2021, 27, 550–558. [Google Scholar] [CrossRef]
- Bartolomé-Álvarez, J.; Sáez-Nieto, J.A.; Escudero-Jiménez, A.; Barba-Rodríguez, N.; Galán-Ros, J.; Carrasco, G.; Muñoz-Izquierdo, M.P. Cutaneous abscess due to Gordonia bronchialis: Case report and literature review. Rev. Esp. Quimioter. 2016, 29, 170–173. Available online: https://seq.es/seq/0214-3429/29/3/bartolome26mar2016.pdf (accessed on 1 March 2023).
- Eribi, A.; Al-Amri, K.; Al-Jabri, A.; Osman, A.; Mohamed Elfadil, O. Gordonia sputi related multiple brain abscesses, an AIDS-presenting illness: Thinking outside the box. IDCases 2020, 21, e00906. [Google Scholar] [CrossRef]
- Jannat-Khah, D.P.; Halsey, E.S.; Lasker, B.A.; Steigerwalt, A.G.; Hinrikson, H.P.; Brown, J.M. Gordonia araii infection associated with an orthopedic device and review of the literature on medical device-associated Gordonia infections. J. Clin. Microbiol. 2009, 47, 499–502. [Google Scholar] [CrossRef]
- Kang, Y.; Takeda, K.; Yazawa, K.; Mikami, Y. Phylogenetic studies of Gordonia species based on gyrB and secA1 gene analyses. Mycopathologia 2009, 167, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.Y.; Wu, A.K.; Leung, W.S.; Cheung, I.; Tsang, C.C.; Chen, J.H.; Chan, J.F.; Tse, C.W.; Lee, R.A.; Lau, S.K.; et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rDNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J. Clin. Microbiol. 2015, 53, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Werno, A.M.; Anderson, T.P.; Chambers, S.T.; Laird, H.M.; Murdoch, D.R. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J. Clin. Microbiol. 2005, 43, 3009–3010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lesens, O.; Hansmann, Y.; Riegel, P.; Heller, R.; Benaissa-Djellouli, M.; Martinot, M.; Petit, H.; Christmann, D. Bacteremia and endocarditis caused by a Gordonia species in a patient with a central venous catheter. Emerg. Infect. Dis. 2000, 6, 382–385. [Google Scholar] [CrossRef]
- Mormeneo Bayo, S.; Palacián Ruíz, M.P.; Asin Samper, U.; Millán Lou, M.I.; Pascual Catalán, A.; Villuendas Usón, M.C. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2022, 40, 255–257, (In English and Spanish). [Google Scholar] [CrossRef]
- Choi, R.; Strnad, L.; Flaxel, C.J.; Lauer, A.K.; Suhler, E.B. Gordonia bronchialis—Associated Endophthalmitis, Oregon, USA. Emerg. Infect. Dis. 2019, 25, 1017–1019. [Google Scholar] [CrossRef]
- Martín, D.; Barrios, A.; Domingo, D.; Sánchez, P.; Sánchez, M.; Ruiz-Dassy, A.; Miqueleiz, A.; Sanz, J. Cerebrospinal fluid shunt-associated meningitis caused by Gordonia sputi: Case report and review of the literature. Infez. Med. 2017, 25, 174–178. Available online: https://www.infezmed.it/media/journal/Vol_25_2_2017_14.pdf (accessed on 1 March 2023). [PubMed]
- Hou, C.; Yang, Y.; Li, Z. A Chinese patient with peritoneal dialysis-related peritonitis caused by Gordonia terrae: A case report. BMC Infect. Dis. 2017, 17, 179. [Google Scholar] [CrossRef][Green Version]
- Chang, J.H.; Ji, M.; Hong, H.L.; Choi, S.H.; Kim, Y.S.; Chung, C.H.; Sung, H.; Kim, M.N. Sternal Osteomyelitis Caused by Gordonia bronchialis after Open-Heart Surgery. Infect. Chemother. 2014, 46, 110–114. [Google Scholar] [CrossRef]
- Drancourt, M.; Bollet, C.; Carlioz, A.; Martelin, R.; Gayral, J.P.; Raoult, D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J. Clin. Microbiol. 2000, 38, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sande, E.; Brun-Otero, M.; Campo-Cerecedo, F.; Esteban, E.; Aguilar, L.; García-de-Lomas, J. Etiological misidentification by routine biochemical tests of bacteremia caused by Gordonia terrae infection in the course of an episode of acute cholecystitis. J. Clin. Microbiol. 2006, 44, 2645–2647. [Google Scholar] [CrossRef]
- Zardawi, I.M.; Jones, F.; Clark, D.A.; Holland, J. Gordonia terrae-induced suppurative granulomatous mastitis following nipple piercing. Pathology 2004, 36, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Blanc, V.; Dalle, M.; Markarian, A.; Debunne, M.V.; Duplay, E.; Rodriguez-Nava, V.; Boiron, P. Gordonia terrae: A difficult-to-diagnose emerging pathogen? J. Clin. Microbiol. 2007, 45, 1076–1077. [Google Scholar] [CrossRef]
- Iida, S.; Taniguchi, H.; Kageyama, A.; Yazawa, K.; Chibana, H.; Murata, S.; Nomura, F.; Kroppenstedt, R.M.; Mikami, Y. Gordonia otitidis sp. nov., isolated from a patient with external otitis. Int. J. Syst. Evol. Microbiol. 2005, 55, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Lejeune, F.; Caillon, J.; Wiertlewski, S.; Crémet, L. Premier cas clinique de bactériémie à Gordonia aichiensis [First case report of Gordonia aichiensis bacteremia]. Med. Mal. Infect. 2017, 47, 508–509. (In French) [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Peña, C.; Ocaña-Cano, M.J.; Amores-Antequera, C.; Muñoz-Peña, C.; Ocaña-Cano, M.J.; Amores-Antequera, C.; Cantudo-Muñoz, P. Infección cutánea por Gordonia araii [Skin infection due to Gordonia araii]. Enferm. Infecc. Microbiol. Clin. 2016, 34, 685–686. (In Spanish) [Google Scholar] [CrossRef]
- Kang, Y.Q.; Ming, H.; Gonoi, T.; Chen, Y.; Cao, Y.; Wang, Y.Y.; Cheng, J.; Koga, T.; Mikami, Y.; Li, W.J. Gordonia iterans sp. nov., isolated from a patient with pneumonia. Int. J. Syst. Evol. Microbiol. 2014, 64, 3520–3525. [Google Scholar] [CrossRef]
- Ercibengoa Arana, M.; Alonso, M.; Idigoras, P.; Vicente, D.; Marimón, J.M. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) score algorithm for identification of Gordonia species. AMB Express 2018, 8, 121. [Google Scholar] [CrossRef]
- Moser, B.D.; Pellegrini, G.J.; Lasker, B.A.; Brown, J.M. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob. Agents Chemother. 2012, 56, 4991–4993. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 1 March 2023).
- Arenskötter, M.; Bröker, D.; Steinbüchel, A. Biology of the metabolically diverse genus Gordonia. Appl. Environ. Microbiol. 2014, 70, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Drzyzga, O. The strengths and weaknesses of Gordonia: A review of an emerging genus with increasing biotechnological potential. Crit. Rev. Microbiol. 2012, 38, 300–316. [Google Scholar] [CrossRef]
- Schlaberg, R.; Fisher, M.A.; Hanson, K.E. Susceptibility profiles of Nocardia isolates based on current taxonomy. Antimicrob. Agents Chemother. 2014, 58, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Valdezate, S.; Garrido, N.; Carrasco, G.; Medina-Pascual, M.J.; Villalón, P.; Navarro, A.M.; Saéz-Nieto, J.A. Epidemiology and susceptibility to antimicrobial agents of the main Nocardia species in Spain. J. Antimicrob. Chemother. 2017, 72, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 25, 1–43, Erratum in: Microbiol. Mol. Biol. Rev. 2016, 80, iii. [Google Scholar] [CrossRef]
- Conville, P.S.; Brown-Elliott, B.A.; Smith, T.; Zelazny, A.M. The Complexities of Nocardia Taxonomy and Identification. J. Clin. Microbiol. 2017, 56, e01419-17. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7, Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Richet, H.M.; Craven, P.C.; Brown, J.M.; Lasker, B.A.; Cox, C.D.; McNeil, M.M.; Tice, A.D.; Jarvis, W.R.; Tablan, O.C. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N. Engl. J. Med. 1991, 324, 104–109. [Google Scholar] [CrossRef]
- Wright, S.N.; Gerry, J.S.; Busowski, M.T.; Klochko, A.Y.; McNulty, S.G.; Brown, S.A.; Sieger, B.E.; Ken Michaels, P.; Wallace, M.R. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: Intraoperative transmission from a healthcare worker. Infect. Control Hosp. Epidemiol. 2012, 33, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Green, E.R.; Mecsas, J. Bacterial Secretion Systems: An Overview. Microbiol. Spectr. 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Ahdash, Z.; Pyle, E.; Allen, W.J.; Corey, R.A.; Collinson, I.; Politis, A. HDX-MS reveals nucleotide-dependent, anti-correlated opening and closure of SecA and SecY channels of the bacterial translocon. eLife 2019, 8, e47402. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Komives, E.A. Open, engage, bind, translocate: The multi-level dynamics of bacterial protein translocation. Structure 2021, 29, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Zelazny, A.M.; Calhoun, L.B.; Li, L.; Shea, Y.R. Identification of Mycobacterium species by secA1 sequences. J. Clin. Microbiol. 2005, 43, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Conville, P.S.; Zelazny, A.M.; Witebsky, F.G. Analysis of secA1 gene sequences for identification of Nocardia species. J. Clin. Microbiol. 2006, 44, 2760–2766. [Google Scholar] [CrossRef]
- Frantsuzova, E.; Bogun, A.; Vetrova, A.; Delegan, Y. Methods of Identifying Gordonia Strains in Clinical Samples. Pathogens 2022, 11, 1496. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Baker, G.C.; Smith, J.J.; Cowan, D.A. Review and re-analysis of domain-specific 16S primers. J. Microbiol. Meth. 2003, 55, 541–555. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Interpretive Criteria for Identification of Bacteria and Fungi by DNA Target Sequencing: Approved Guideline MM18-A; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardia spp. and other Aerobic Actinomycetes, 3rd ed.; CLSI Standard M24; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. Approved Standard-M24-A2, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999, 41, 95–98. [Google Scholar]
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-Del Barrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6, DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]


| No. of Strains (%) b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species (No. and %) a | Blood | CNS c | Respiratory Tract | Soft Tissue/Bone | Urine | Other | ||
| Sputum | Bronchial Fluid | Lung/Pleural Fluid | ||||||
| High prevalence | ||||||||
| G. sputi (87, 53.0) | 18 (20.7%) | 1 (1.1%) | 60 (69.0%) | 1(1.1%) | 1 (1.1%) | 4 (6.0%) | 2 (2.3%) | 0 |
| Medium prevalence | ||||||||
| G. bronchialis (30, 18.3) | 6 (20.0%) | 0 | 13 (43.3%) | 0 | 0 | 9 (30.0%) | 2 (6.6%) | 0 |
| Low prevalence | ||||||||
| G. terrae (14, 8.5) | 2 (14.3) | 0 | 6 (42.8) | 3 (21.4) | 1 (7.1) | 2 (14.3) | 0 | 0 |
| G. otitidis (10, 6.1%) | 2 (20.0) | 0 | 7 (70.0) | 0 | 0 | 0 | 1 (10.0) | 0 |
| Other species | ||||||||
| G. aichiensis (8, 4.8) | 3 | 0 | 4 | 0 | 0 | 0 | 0 | 1 |
| G. alkanivorans (1, 0.6) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| G. araii (2, 1.2) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| G. hongkongensis (3, 1.8%) | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G. iterans (3, 1.8) | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| G. jinhuaensis (1, 0.6) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| G. polyisoprenivorans (4, 2.4%) | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| Gordonia spp. (1, 0.6) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Total (164, 100) a | 36 (21.9) | 1 (0.61) | 94 (57.3) | 5 (3.0) | 2 (1.2) | 20 (12.2) | 5 (3.0) | 1 (0.6) |
| Species | Gordonia sputi | Gordonia bronchialis | Gordonia terrae | Gordonia otitidis | Gordonia aichiensis | Gordonia hongkongensis | Gordonia iterans | Gordonia polyisoprenivorans | Other Species b | All Species |
|---|---|---|---|---|---|---|---|---|---|---|
| (No. of Strains, % a) | (87, 53.0%) | (30, 18.3%) | (14, 8.5%) | (10, 6.1%) | (8, 4.8%) | (3, 1.8%) | (3, 1.8%) | (4, 2.4%) | (5, 3.0%) | (164, 100%) |
| Amoxycillin–clavulanate c,d | ||||||||||
| Range | ≤2 | ≤2 | ≤2–32 | ≤2 | ≤2 | ≤2–8 | ≤2 | ≤2 | ≤2 | ≤2–32 |
| MIC50/MIC90 e | ≤2/≤2 | ≤2/≤2 | 4/16 | ≤2/≤2 | ≤2/≤2 | 4/8 | ≤2/≤2 | ≤2/≤2 | ≤2/≤2 | 2/2 |
| Resistance f,g | 0 (0.0) | 0 (0.0) | 3 (21.4) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 3 (1.8) |
| Ceftriaxone | ||||||||||
| Range | ≤4 | ≤4 | ≤4–128 | ≤4 | ≤4 | ≤4 | ≤4 | ≤4 | ≤4 | ≤4–128 |
| MIC50/MIC90 | ≤4/≤4 | ≤4/≤4 | ≤4/≤4 | ≤4/≤4 | ≤4/≤4 | ≤4/≤4 | ≤4/≤4 | ≤4/≤4 | ≤4/≤4 | ≤4/≤4 |
| Resistance | 0 (0.0) | 0 (0.0) | 1 (0.78) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 1 (0.6) |
| Cefoxitin | ||||||||||
| Range | 8–32 | ≤4–32 | ≤4–32 | ≤4–16 | 16–16 | ≤4–32 | ≤4–8 | 8–16 | ≤4–32 | ≤4–32 |
| MIC50/MIC90 | 16/32 | 16/16 | 8/16 | 8/16 | 16/16 | 16/32 | 8/8 | 16/16 | 16/16 | 32/32 |
| Resistance h | 82 (94.2) | 20 (66.7) | 5 (35.7) | 5 (50.0) | 8 | 2 | 0 | 3 | 3 | 128 (76.6) |
| Cefepime | ||||||||||
| Range | ≤1 | ≤1 | ≤1–>32 | ≤1 | ≤1 | ≤1–32 | ≤1 | ≤1 | ≤1 | ≤1–>32 |
| MIC50/MIC90 | ≤1/≤1 | ≤1/≤1 | ≤1/>32 | ≤1/≤1 | ≤1/≤1 | ≤1/32 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 |
| Resistance | 0 (0.0) | 0 (0.0) | 6 (42.8) | 0 (0.0) | 0 | 1 | 0 | 0 | 0 | 7 (4.3) |
| Imipenem | ||||||||||
| Range | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 |
| MIC50/MIC90 | ≤2/≤2 | ≤2/≤2 | ≤2/≤2 | ≤2/≤2 | ≤2/≤2 | ≤2/≤2 | ≤2/≤2 | ≤2/≤2 | ≤2/≤2 | ≤2/≤2 |
| Resistance | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 0 (0.0) |
| Amikacin | ||||||||||
| Range | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
| MIC50/MIC90 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 |
| Resistance | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Tobramycin | ||||||||||
| Range | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1–4 | ≤1–4 |
| MIC50/MIC90 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/2 | ≤1/2 |
| Resistance | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 0 (0.0) |
| Clarithromycin | ||||||||||
| Range | ≤0.06–2 | ≤0.06–8 | 0.12–4 | ≤0.06–0.25 | 0.12–0.25 | ≤0.06–2 | 2 | ≤0.06 | ≤0.06–4 | ≤0.06–8 |
| MIC50/MIC90 | ≤0.06/0,12 | 2/4 | 2/2 | 0.12/0.25 | 0.12/0.25 | ≤0.06/2 | 2/2 | ≤0.06/≤0.06 | ≤0.5/1 | 1/2 |
| Resistance | 0 (0.0) | 9 (30) | 1 (7.1) | 0 (0.0) | 0 | 0 | 0 | 0 | 1 | 9 (5.5) |
| Doxycycline | ||||||||||
| Range | 0.25–4 | 0.25–1 | ≤0.12–0.5 | 0.25–0.5 | ≤0.12–1 | 0.25–0.5 | 0.25 | 0.25–0.5 | ≤0.12–0.5 | ≤0.12–4 |
| MIC50/MIC90 | 0.5/1 | 0.5/1 | 0.25/0.5 | 0.25/0.5 | 0.5/0.5 | 0.25/0.5 | 0.25/0.25 | 0.25/0.5 | 0.25/0.5 | 0.5/1 |
| Resistance | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 1 (0.6) |
| Minocycline | ||||||||||
| Range | ≤1–2 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
| MIC50/MIC90 | ≤1/2 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 |
| Resistance | 10 (11.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 10 (6.1) |
| Ciprofloxacin | ||||||||||
| Range | ≤0.12–2 | ≤0.12–1 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12 | ≤0.12–0.25 | ≤0.12–2 |
| MIC50/MIC90 | ≤0.12/≤0.12 | ≤0.12/≤0.12 | ≤0.12/≤0.12 | ≤0.12/≤0.12 | ≤0.12/≤0.12 | ≤0.12/≤0.12 | ≤0.12/≤0.12 | ≤0.12/≤0.12 | ≤0.12/≤0.12 | ≤0.12/≤0.12 |
| Resistance | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 0 (0.0) |
| Moxifloxacin | ||||||||||
| Range | ≤0.25 | ≤0.25–0.5 | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25–0.5 |
| MIC50/MIC90 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 |
| Resistance | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 0 (0.0) |
| Trimethoprim–sulphamethoxazole d | ||||||||||
| Range | ≤0.25–0.5 | ≤0.25 | ≤0.25–0.5 | ≤0.25–0.5 | ≤0.25–0.5 | ≤0.25–1 | ≤0.25 | ≤0.25 | ≤0.25–0.5 | ≤0.25–1 |
| MIC50/MIC90 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | 1/1 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 | ≤0.25/≤0.25 |
| Resistance | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 0 (0.0) |
| Tigecycline | ||||||||||
| Range | 0.25–2 | 0.12–1 | 0.25–1 | 0.25–0.5 | 0.12->4 | 0.25–0.5 | 0.25 | 0.25–1 | 0.25–1 | 0.12–4 |
| MIC50/MIC90 | 0.5/1 | 0.5/1 | 0.5/1 | 0.5/0.5 | 0.5/1 | 0.25/0.5 | 0.25/0.25 | 0.5/1 | 0.5/1 | 0.5/1 |
| Resistance i | 25 (28.7) | 12 (40.0) | 4 (28.5) | 0 (0.0) | 2 | 0 | 0 | 1 | 1 | 45 (43.0%) |
| Linezolid | ||||||||||
| Range | ≤1–2 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
| MIC50/MIC90 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 | ≤1/≤1 |
| Resistance | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 0 | 0 | 0 (0.0) |
| Species (No. of Strains) | Genes (bp) a | Haplotype Number (HGDI, S2, SD) b | SNP Number (Nucleotide Diversity) c | SNPs Per Strain (Average, Mode) d |
|---|---|---|---|---|
| Gordonia spp. (n = 174) e | 16S rRNA (954) | 20 (0.737, 0.00085, 0.029) | 81 (≤1.37) | -- |
| secA (337) | 44 (0.954, 0.00003, 0.006) | 165 (≤8.53) | -- | |
| High prevalence | ||||
| G. sputi (n = 87) | 16S rRNA (1088) | 6 (0.132, 0.0024, 0.049) | 7 (0.017) | 0–2 (0.18, 0) |
| secA (454) | 18 (0.890, 0.00033, 0.018) | 35 (2.55) | 0–20 (7, 20) | |
| Clonal lineage I (n = 36) f | secA (454) | 7 (0.803, 0.00107, 0.033) | 10 (0.864) | 0–7 (4.4, 7) |
| Clonal lineage II (n = 51) f | secA (454) | 11 (0.773, 0.00231, 0.048) | 15 (0.567) | 0–20 (2, 0) g |
| Medium prevalence | ||||
| G. bronchialis (n = 30) | 16S rRNA (1093) | 1 | 0 | 0–1 (0.0, 0) |
| secA (336) | 9 (0.751, 0.00342, 0.059) | 15 (1.5) | 0–11 (6.7, 10) | |
| Clonal lineage I (n = 10) h | secA (336) | 3 (0.345, 0.02967, 0.172) | 5 (0.358) | 0–4 (0.7, 0) |
| Clonal lineage II (n = 20) h | secA (336) | 6 (0.579, 0.0142, 0.124) | 10 (0.559) | 0–10 (3.15, 0) |
| Low prevalence | ||||
| G. terrae (n = 14) | 16S rRNA (1167) | 2 (0.0, 0.0, 0.0) | 1 (0.0) | 0 |
| secA (462) | 3 (0.362, 0.02098, 0.145) | 3 (0.132) | 0–2 (0.14, 1) | |
| G. otitidis (n = 10) | 16S rRNA (1293) | 2 (0.436, 0.0177, 0.133) | 1 (0.034) | 0–1 (0, 0) |
| secA (451) | 2 (0.182, 0.0261, 0.144) | 18 (5.2) | 0–18 (1.6, 0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino-Rosa, S.; Medina-Pascual, M.J.; Carrasco, G.; Garrido, N.; Villalón, P.; Valiente, M.; Valdezate, S. Focusing on Gordonia Infections: Distribution, Antimicrobial Susceptibilities and Phylogeny. Antibiotics 2023, 12, 1568. https://doi.org/10.3390/antibiotics12111568
Pino-Rosa S, Medina-Pascual MJ, Carrasco G, Garrido N, Villalón P, Valiente M, Valdezate S. Focusing on Gordonia Infections: Distribution, Antimicrobial Susceptibilities and Phylogeny. Antibiotics. 2023; 12(11):1568. https://doi.org/10.3390/antibiotics12111568
Chicago/Turabian StylePino-Rosa, Silvia, María J. Medina-Pascual, Gema Carrasco, Noelia Garrido, Pilar Villalón, Mónica Valiente, and Sylvia Valdezate. 2023. "Focusing on Gordonia Infections: Distribution, Antimicrobial Susceptibilities and Phylogeny" Antibiotics 12, no. 11: 1568. https://doi.org/10.3390/antibiotics12111568
APA StylePino-Rosa, S., Medina-Pascual, M. J., Carrasco, G., Garrido, N., Villalón, P., Valiente, M., & Valdezate, S. (2023). Focusing on Gordonia Infections: Distribution, Antimicrobial Susceptibilities and Phylogeny. Antibiotics, 12(11), 1568. https://doi.org/10.3390/antibiotics12111568






