Sensitization of Gram-Negative Bacteria to Aminoglycosides with 2-Aminoimidazole Adjuvants
Abstract
:1. Introduction
2. Results
2.1. Initial Screen
2.2. Follow-Up Screen of Additional Analogues
2.3. Activity of Lead Compounds with Additional Aminoglycosides
2.4. Activity of Lead Compounds against Other Gram-Negative Pathogens
2.5. Effect of Divalent Cations on Adjuvant Activity
2.6. Evolution of TOB Resistance in the Presence and Absence of Adjuvant and Identification of Mutated Genes in Evolved Strains
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Media, and Antimicrobial Agents
4.2. Broth Microdilution Method for Minimum Inhibitory Concentration Determination
4.3. Broth Microdilution Method for Measurement of Aminoglycoside Potentiation
4.4. Evolution of Tobramycin Resistance in A. baumannii
4.5. Sequencing Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. Antibiotic/Antimicrobial Resistance (AR/AMR); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Klug, D.M.; Idiris, F.I.M.; Blaskovich, M.A.T.; von Delft, F.; Dowson, C.G.; Kirchhelle, C.; Roberts, A.P.; Singer, A.C.; Todd, M.H. There is no market for new antibiotics: This allows an open approach to research and development. Wellcome Open Res. 2021, 6, 146. [Google Scholar] [CrossRef]
- Coates, A.R.; Halls, G.; Hu, Y. Novel classes of antibiotics or more of the same? Br. J. Pharmacol. 2011, 163, 184–194. [Google Scholar] [CrossRef]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, 5–8. [Google Scholar] [CrossRef]
- Ariano, R.E.; Zelenitsky, S.A.; Kassum, D.A. Aminoglycoside-induced vestibular injury: Maintaining a sense of balance. Ann. Pharmacother. 2008, 42, 1282–1289. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef]
- Labby, K.J.; Garneau-Tsodikova, S. Strategies to overcome the action of aminoglycoside-modifying enzymes for treating resistant bacterial infections. Future Med. Chem. 2013, 5, 1285–1309. [Google Scholar] [CrossRef]
- Kalan, L.; Wright, G.D. Antibiotic adjuvants: Multicomponent anti-infective strategies. Expert. Rev. Mol. Med. 2011, 13, e5. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Huang, X.; Ding, S.; Wang, Y.; Shen, J.; Zhu, K. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 2020, 5, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, C.R.; Fang, X.; Allison, K.R. Potentiating aminoglycoside antibiotics to reduce their toxic side effects. PLoS ONE 2020, 15, e0237948. [Google Scholar] [CrossRef] [PubMed]
- Maiden, M.M.; Hunt, A.M.A.; Zachos, M.P.; Gibson, J.A.; Hurwitz, M.E.; Mulks, M.H.; Waters, C.M. Triclosan Is an Aminoglycoside Adjuvant for Eradication of Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Minrovic, B.M.; Jung, D.; Melander, R.J.; Melander, C. New Class of Adjuvants Enables Lower Dosing of Colistin Against Acinetobacter baumannii. ACS Infect. Dis. 2018, 4, 1368–1376. [Google Scholar] [CrossRef]
- Harris, T.L.; Worthington, R.J.; Hittle, L.E.; Zurawski, D.V.; Ernst, R.K.; Melander, C. Small Molecule Downregulation of PmrAB Reverses Lipid A Modification and Breaks Colistin Resistance. ACS Chem. Biol. 2014, 9, 122–127. [Google Scholar] [CrossRef]
- Hubble, V.B.; Bartholomew, K.R.; Weig, A.W.; Brackett, S.M.; Barlock, S.L.; Mattingly, A.E.; Nemeth, A.M.; Melander, R.J.; Melander, C. Augmenting the Activity of Macrolide Adjuvants against. ACS Med. Chem. Lett. 2020, 11, 1723–1731. [Google Scholar] [CrossRef]
- Martin, S.E.; Melander, R.J.; Brackett, C.M.; Scott, A.J.; Chandler, C.E.; Nguyen, C.M.; Minrovic, B.M.; Harrill, S.E.; Ernst, R.K.; Manoil, C.; et al. Small Molecule Potentiation of Gram-Positive Selective Antibiotics against. ACS Infect. Dis. 2019, 5, 1223–1230. [Google Scholar] [CrossRef]
- Marrujo, S.A.; Hubble, V.B.; Yang, J.; Wang, M.; Nemeth, A.M.; Barlock, S.L.; Juarez, D.; Smith, R.D.; Melander, R.J.; Ernst, R.K.; et al. Dimeric 2-aminoimidazoles are highly active adjuvants for gram-positive selective antibiotics against Acinetobacter baumannii. Eur. J. Med. Chem. 2023, 253, 115329. [Google Scholar] [CrossRef]
- Wesseling, C.M.J.; Martin, N.I. Synergy by Perturbing the Gram-Negative Outer Membrane: Opening the Door for Gram-Positive Specific Antibiotics. ACS Infect. Dis. 2022, 8, 1731–1757. [Google Scholar] [CrossRef]
- Stokes, J.M.; MacNair, C.R.; Ilyas, B.; French, S.; Côté, J.P.; Bouwman, C.; Farha, M.A.; Sieron, A.O.; Whitfield, C.; Coombes, B.K.; et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat. Microbiol. 2017, 2, 17028. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, Y.; Yang, K.; Li, R.; Xiao, X.; Zhu, K.; Wang, Z. Metformin Restores Tetracyclines Susceptibility against Multidrug Resistant Bacteria. Adv. Sci. 2020, 7, 1902227. [Google Scholar] [CrossRef] [PubMed]
- Brackett, C.M.; Melander, R.J.; An, I.H.; Krishnamurthy, A.; Thompson, R.J.; Cavanagh, J.; Melander, C. Small-Molecule Suppression of Beta-Lactam Resistance in Multidrug-Resistant Gram-Negative Pathogens. J. Med. Chem. 2014, 57, 7450–7458. [Google Scholar] [CrossRef] [PubMed]
- Clinical & Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Milton, M.E.; Minrovic, B.M.; Harris, D.L.; Kang, B.; Jung, D.; Lewis, C.P.; Thompson, R.J.; Melander, R.J.; Zeng, D.; Melander, C.; et al. Re-sensitizing Multidrug Resistant Bacteria to Antibiotics by Targeting Bacterial Response Regulators: Characterization and Comparison of Interactions between 2-Aminoimidazoles and the Response Regulators BfmR from Acinetobacter baumannii and QseB from Francisella spp. Front. Mol. Biosci. 2018, 5, 15. [Google Scholar] [CrossRef]
- Bunders, C.A.; Richards, J.J.; Melander, C. Identification of aryl 2-aminoimidazoles as biofilm inhibitors in Gram-negative bacteria. Bioorg Med. Chem. Lett. 2010, 20, 3797–3800. [Google Scholar] [CrossRef]
- Douraghi, M.; Aris, P.; To, J.; Myers, G.S.A.; Hamidian, M. Two carbapenem-resistant ST1:ST231:KL1:OCL1. JAC Antimicrob. Resist. 2021, 3, dlab112. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, V.; Medina, R.; Wright, G.D. Venturicidin A, A Membrane-active Natural Product Inhibitor of ATP synthase Potentiates Aminoglycoside Antibiotics. Sci. Rep. 2020, 10, 8134. [Google Scholar] [CrossRef]
- Hui, Z.; Liu, S.; Cui, R.; Zhou, B.; Hu, C.; Zhang, M.; Deng, Q.; Cheng, S.; Luo, Y.; Chen, H.; et al. A small molecule interacts with pMAC-derived hydroperoxide reductase and enhances the activity of aminoglycosides. J. Antibiot. 2021, 74, 324–329. [Google Scholar] [CrossRef]
- Clifton, L.A.; Skoda, M.W.; Le Brun, A.P.; Ciesielski, F.; Kuzmenko, I.; Holt, S.A.; Lakey, J.H. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 2015, 31, 404–412. [Google Scholar] [CrossRef]
- Barrett, J.F.; Goldschmidt, R.M.; Lawrence, L.E.; Foleno, B.; Chen, R.; Demers, J.P.; Johnson, S.; Kanojia, R.; Fernandez, J.; Bernstein, J.; et al. Antibacterial agents that inhibit two-component signal transduction systems. Proc. Natl. Acad. Sci. USA 1998, 95, 5317–5322. [Google Scholar] [CrossRef]
- Walsh, B.J.C.; Wang, J.; Edmonds, K.A.; Palmer, L.D.; Zhang, Y.; Trinidad, J.C.; Skaar, E.P.; Giedroc, D.P. The Response of Acinetobacter baumannii to Hydrogen Sulfide Reveals Two Independent Persulfide-Sensing Systems and a Connection to Biofilm Regulation. mBio 2020, 11, e01254-20. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef]
- Lv, B.; Huang, X.; Lijia, C.; Ma, Y.; Bian, M.; Li, Z.; Duan, J.; Zhou, F.; Yang, B.; Qie, X.; et al. Heat shock potentiates aminoglycosides against gram-negative bacteria by enhancing antibiotic uptake, protein aggregation, and ROS. Proc. Natl. Acad. Sci. USA 2023, 120, e2217254120. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, R.Q.; Wang, L.; Yang, W.T.; Zou, Q.H.; Xiao, D. Proteomic Analyses of Acinetobacter baumannii Clinical Isolates to Identify Drug Resistant Mechanism. Front. Cell. Infect. Microbiol. 2021, 11, 625430. [Google Scholar] [CrossRef] [PubMed]
- Pineau, R.M.; Hanson, S.E.; Lyles, J.T.; Quave, C.L. Growth Inhibitory Activity of Callicarpa americana Leaf Extracts Against Cutibacterium acnes. Front. Pharmacol. 2019, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
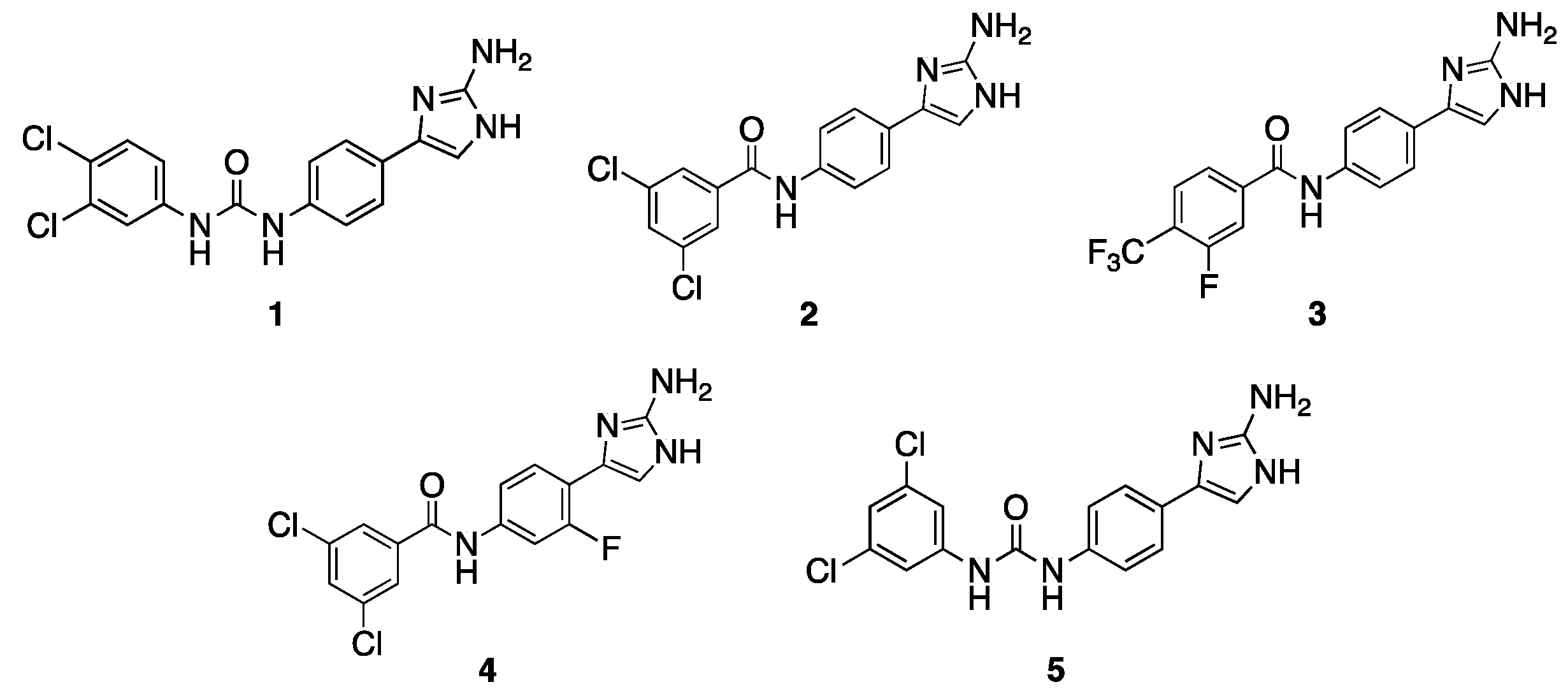
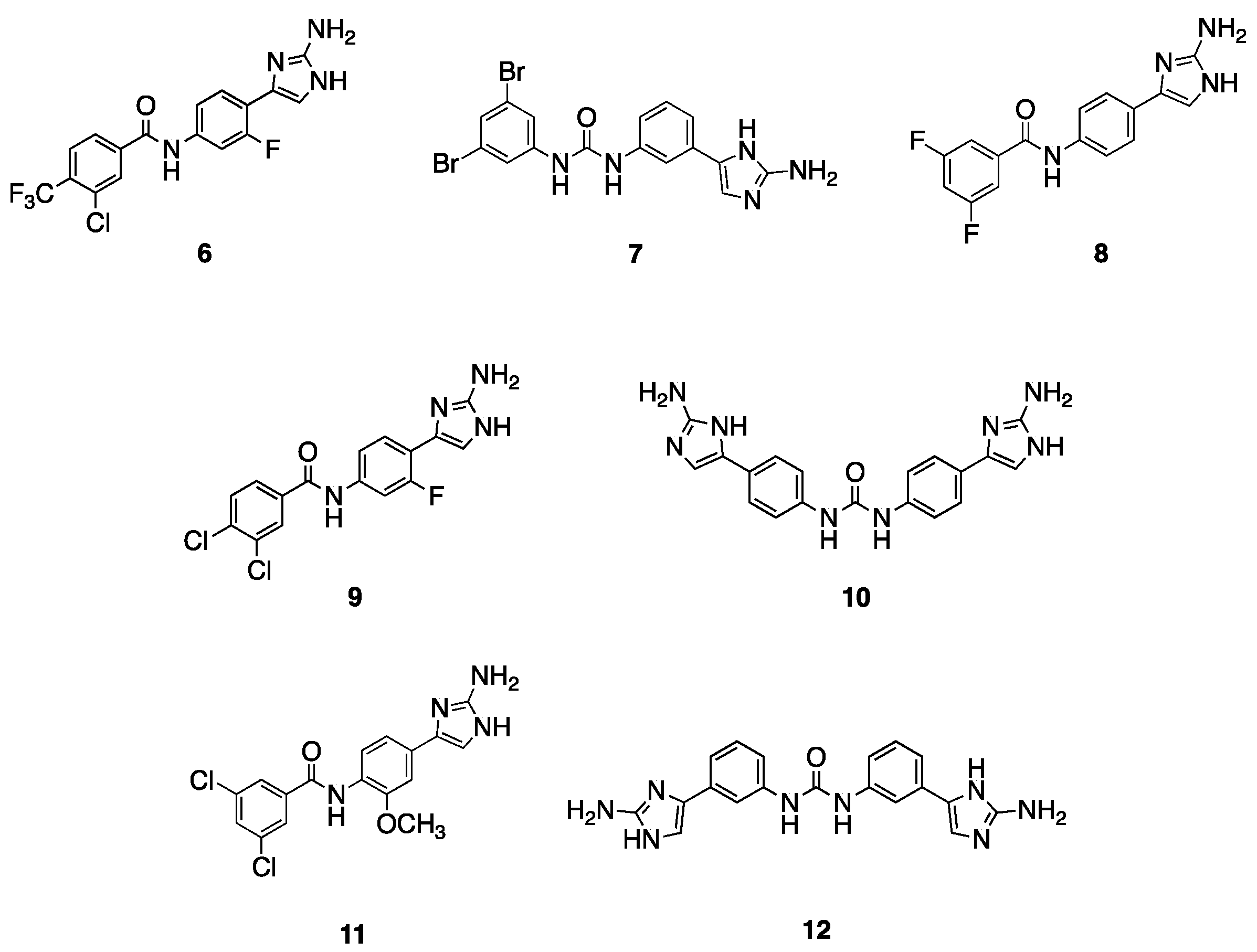
| AB5075 | AB19606 | |||
|---|---|---|---|---|
| Compound | Concentration (μM) | TOB MIC (μg/mL) (Fold-Reduction) | Concentration (μM) | TOB MIC (μg/mL) (Fold-Reduction) |
| 128 | 4 | |||
| 1 | 30 | 2 (64) | 30 | 1 (4) |
| 2 | 30 | 16 (8) | 30 | 1 (4) |
| 3 | 15 | 64 (2) | 30 | 0.5 (8) |
| 4 | 60 | 32 (4) | 60 | 2 (2) |
| 5 | 7.5 | 32 (4) | 15 | 2 (2) |
| AB5075 | AB19606 | |||
|---|---|---|---|---|
| Compound | Concentration (μM) | TOB MIC (μg/mL) (Fold-Reduction) | Concentration (μM) | TOB MIC (μg/mL) (Fold-Reduction) |
| 128 | 4 | |||
| 6 | 30 | 4 (32) | 30 | 0.5 (8) |
| 7 | 30 | 2 (64) | 15 | 0.5 (8) |
| 8 | 60 | 4 (32) | 60 | 2 (2) |
| 9 | 60 | 16 (8) | 60 | 0.0625 (64) |
| 10 | 30 | 32 (4) | 60 | 0.25 (16) |
| 11 | 60 | 16 (8) | 30 | 1 (4) |
| 12 | 60 | 8 (16) | 60 | 0.5 (8) |
| Compound | Concentration (μM) | STM MIC (μg/mL) (Fold-Reduction) | Concentration (μM) | KAN MIC (μg/mL) (Fold-Reduction) | Concentration (μM) | NEO MIC (μg/mL) (Fold-Reduction) | Concentration (μM) | GEN MIC (μg/mL) (Fold-Reduction) |
|---|---|---|---|---|---|---|---|---|
| 2048 | 2048 | 64 | 512 | |||||
| 1 | 30 | 128 (16) | 15 | 2048 (0) | 15 | 32 (2) | 15 | 32 (16) |
| 6 | 30 | 32 (64) | 30 | 1024 (2) | 30 | 16 (4) | 30 | >512 (0) |
| 7 | 15 | 128 (16) | 7.5 | 2048 (0) | 7.5 | >64 (0) | 7.5 | 256 (2) |
| 8 | 30 | 512 (4) | 60 | 2048 (0) | 60 | 4 (16) | 60 | 16 (32) |
| Compound | Concentration (μM) | STM MIC (μg/mL) (Fold-Reduction) | Concentration (μM) | KAN MIC (μg/mL) (Fold-Reduction) | Concentration (μM) | NEO MIC (μg/mL) (Fold-Reduction) | Concentration (μM) | GEN MIC (μg/mL) (Fold-Reduction) |
|---|---|---|---|---|---|---|---|---|
| 512 | 8 | 8 | 32 | |||||
| 1 | 30 | 128 (4) | 15 | >8 (0) | 15 | 4 (2) | 15 | 4 (8) |
| 3 | 30 | 64 (8) | 30 | 2 (4) | 30 | 1 (8) | 30 | 1 (32) |
| 5 | 15 | 64 (8) | 15 | 4 (2) | 15 | 2 (4) | 15 | 2 (16) |
| 6 | 30 | 16 (32) | 30 | 1 (8) | 30 | 1 (8) | 30 | 32 (0) |
| 7 | 7.5 | 512 (0) | 15 | 1 (8) | 15 | 2 (4) | 15 | 4 (8) |
| 10 | 60 | 16 (32) | 60 | 0.5 (16) | 60 | 0.5 (16) | 60 | 0.25 (128) |
| 30 | 2 (4) | 45 | 2 (16) |
| Strain | TOB MIC (μg/mL) | TOB MIC (μg/mL) + 1 (μM) | TOB MIC (μg/mL) + 3 (μM) | TOB MIC (μg/mL) + 5 (μM) | TOB MIC (μg/mL) + 10 (μM) | TOB MIC (μg/mL) + 11 (μM) | TOB MIC (μg/mL) + 12 (μM) |
|---|---|---|---|---|---|---|---|
| KP1705 | 32 | (15) 16 | (15) 32 | (15) 16 | (60) 16 | (60) 32 | (60) 8 |
| KP43816 | 1 | (15) 0.125 | (15) 0.5 | (15) 0.125 | (30) 0.25 | (60) 0.5 | (10) 0.5 |
| EC197 | 32 | (3.75) 32 | (7.5) 16 | (7.5) 16 | (15) 16 | (60) 16 | (15) 16 |
| EC199 | 0.5 | (7.5) 0.5 | (7.5) 0.5 | (7.5) 0.5 | (15) 0.5 | (60) 0.5 | (15) 0.5 |
| AB5075 Passaged in TOB Only | AB5075 Passaged in TOB + 1 (15 μM) | |
|---|---|---|
| Day 0 | 128 | 128 |
| Day 2 | 1024 | 256 |
| Day 4 | 1024 | 1024 |
| Day 6 | 4096 | 1024 |
| Day 8 | 4096 | 2048 |
| Gene Product (Gene) | Locus Tag | # of Mutations | Type of Mutation |
|---|---|---|---|
| Alkyl hydroperoxide reductase subunit F (ahpF) | A591_RS08360 | 11 | 8 snp; 3 complex |
| Aldehyde dehydrogenase | A591_RS16100 | 3 | 1 snp; 2 complex |
| Bacteriophage protein | A591_RS13885 | 3 | 2 snp; 1 complex |
| Stress-induced protein | A591_RS20205 | 1 | snp |
| Replication initiation factor domain-containing protein | A591_RS15850 | 1 | Insertion |
| Ig-like domain repeat protein | A591_RS19590 | 1 | snp |
| Hypothetical protein (elongation factor Tu (tuf)) | A591_RS01280 | 1 | snp |
| Hypothetical protein (replication protein) | A591_RS20250 | 1 | Insertion |
| Unknown hypothetical protein | A591_RS20245 | 1 | Insertion |
| Unknown hypothetical protein | A591_RS15845 | 1 | Insertion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crotteau, A.N.; Hubble, V.B.; Marrujo, S.A.; Mattingly, A.E.; Melander, R.J.; Melander, C. Sensitization of Gram-Negative Bacteria to Aminoglycosides with 2-Aminoimidazole Adjuvants. Antibiotics 2023, 12, 1563. https://doi.org/10.3390/antibiotics12111563
Crotteau AN, Hubble VB, Marrujo SA, Mattingly AE, Melander RJ, Melander C. Sensitization of Gram-Negative Bacteria to Aminoglycosides with 2-Aminoimidazole Adjuvants. Antibiotics. 2023; 12(11):1563. https://doi.org/10.3390/antibiotics12111563
Chicago/Turabian StyleCrotteau, Ashley N., Veronica B. Hubble, Santiana A. Marrujo, Anne E. Mattingly, Roberta J. Melander, and Christian Melander. 2023. "Sensitization of Gram-Negative Bacteria to Aminoglycosides with 2-Aminoimidazole Adjuvants" Antibiotics 12, no. 11: 1563. https://doi.org/10.3390/antibiotics12111563
APA StyleCrotteau, A. N., Hubble, V. B., Marrujo, S. A., Mattingly, A. E., Melander, R. J., & Melander, C. (2023). Sensitization of Gram-Negative Bacteria to Aminoglycosides with 2-Aminoimidazole Adjuvants. Antibiotics, 12(11), 1563. https://doi.org/10.3390/antibiotics12111563





