Determination of Sulphonamides and Tetracycline Residues in Liver Tissues of Broiler Chicken Sold in Kinondoni and Ilala Municipalities, Dar es Salaam, Tanzania
Abstract
:1. Introduction
2. Results
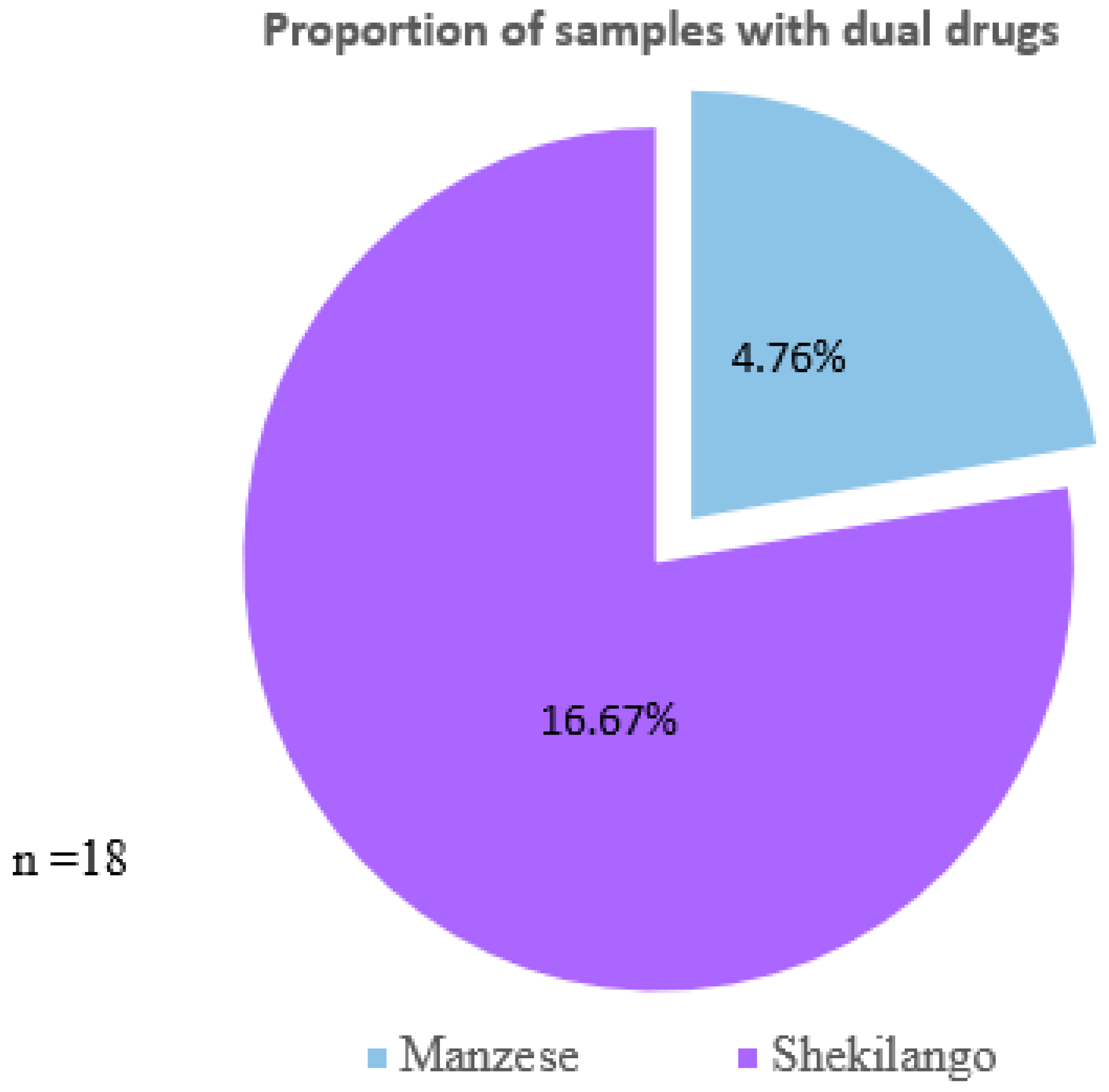
2.1. Percentage of Broiler Chicken Liver Tissue with Tetracycline and Sulphonamide Residues
| Sample Site | Drugs | * Positive (%) | * Negative (%) | * Not Detected (%) |
|---|---|---|---|---|
| Shekilango | Tetracycline | 42 (100) | 0 (0) | 0 (0) |
| Sulphonamides | 14 (33.33) | 22 (52.38) | 6 (14.29) | |
| Manzese | Tetracycline | 42 (100) | 0 (0) | 0 (0) |
| Sulphonamides | 4 (9.52) | 33 (78.57) | 5 (11.91) |
2.2. Concentration of Sulphonamides and Tetracycline Residues in Broiler Chicken Liver Tissues
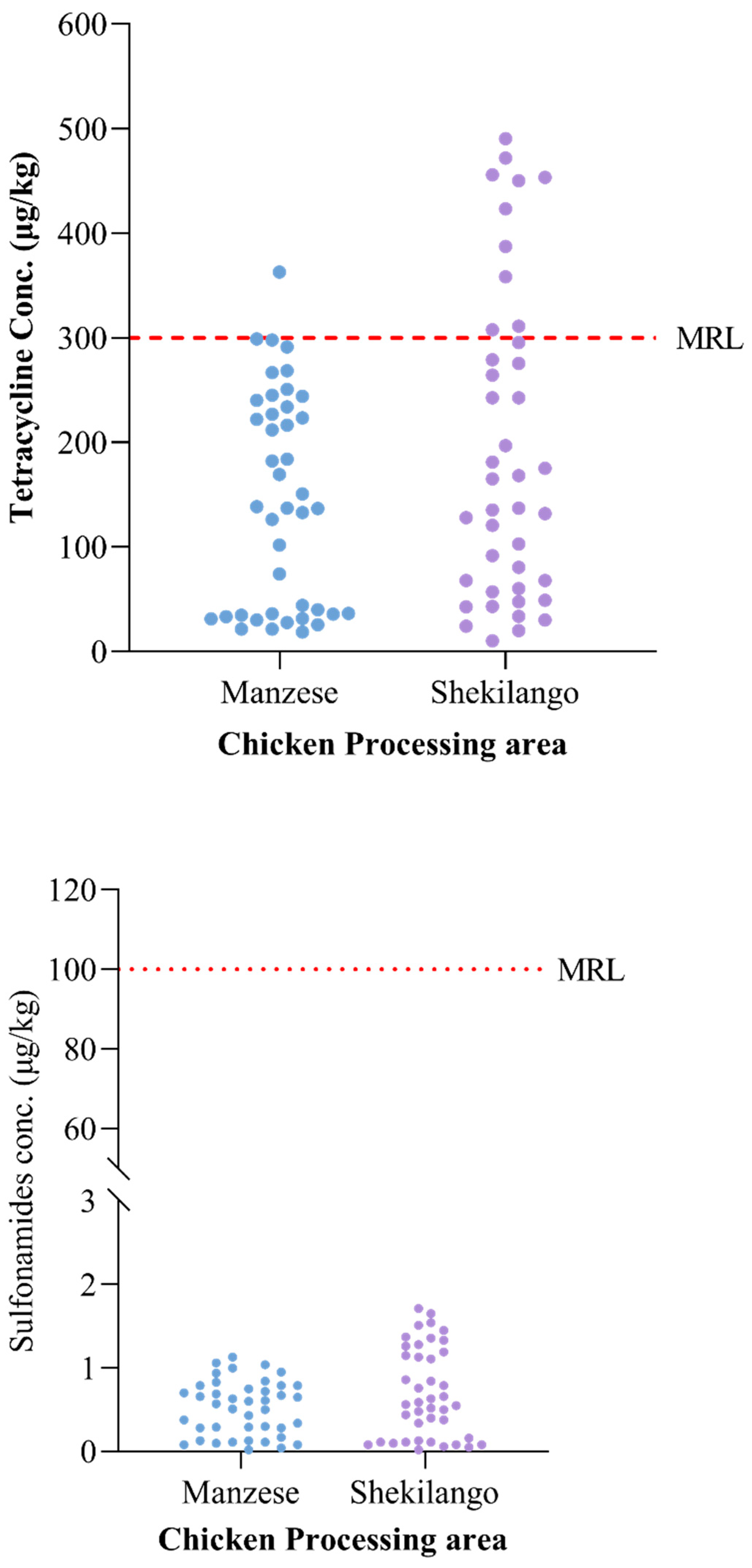
2.3. Concentrations of Tetracycline and Sulphonamides versus the Recommended Maximum Residue Limit (MRL)
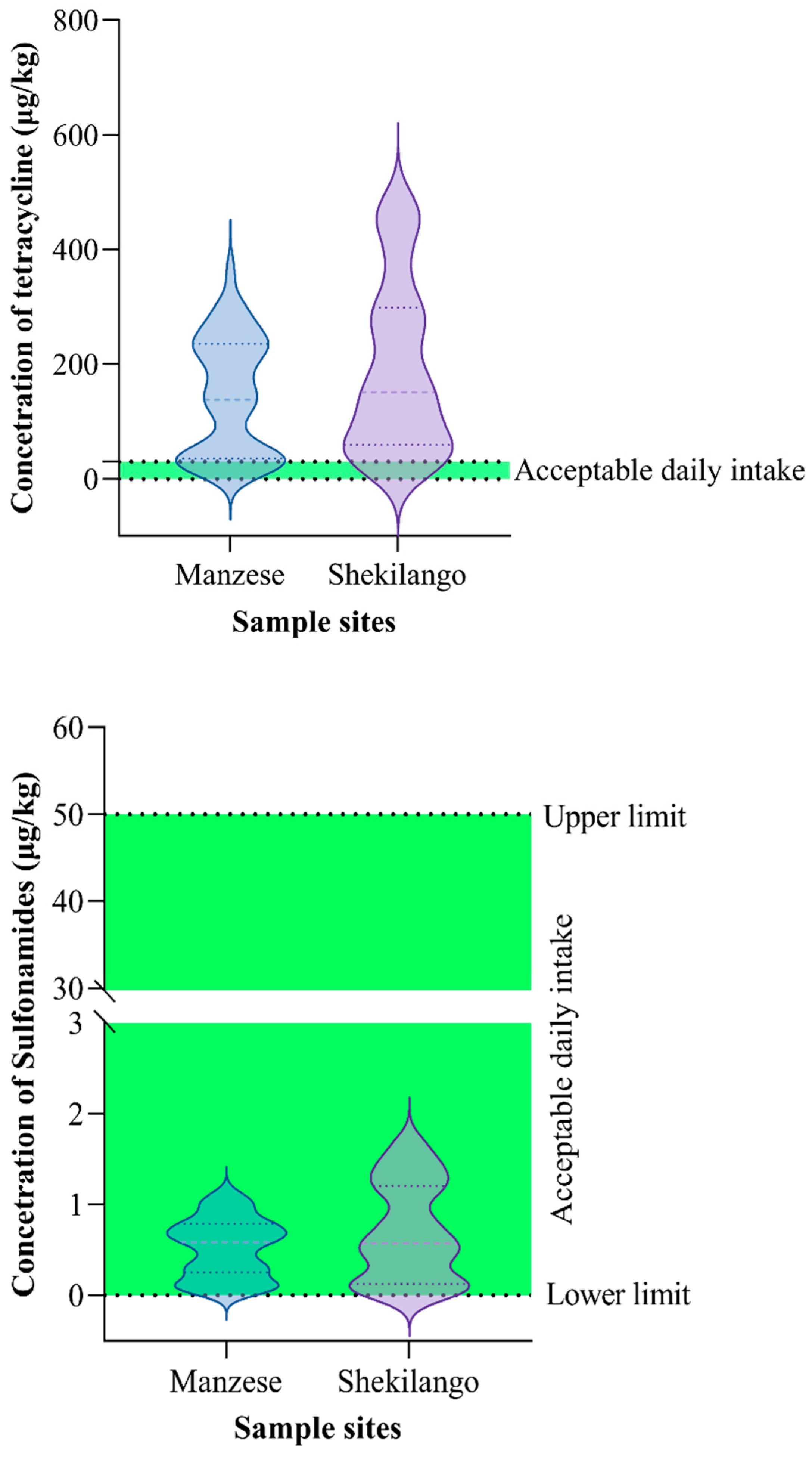
2.4. Concentrations of Sulphonamides and Tetracycline versus Acceptable Daily Intake (ADI)
3. Discussion
4. Material and Methods
4.1. Study Area
4.2. Study Design
4.3. Sampling Technique
4.4. Specimen Collection and Transportation
4.5. Chemicals and Reagents
4.6. Extraction Procedure
4.7. Competitive Enzyme Linked Immunosorbent Assay
4.8. Statistical Analysis
4.9. Ethical Consideration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, J.; Jasper, W.; Francis, E.; Margaret, M. Use of sulfonamides in layers in Kampala district, Uganda and sulfonamide residues in commercial eggs. Afr. Health Sci. 2005, 5, 33–39. [Google Scholar]
- Caudell, M.; Quinlan, M.; Subbiah, M.; Call, D.; Roulette, C.; Roulette, J. Antimicrobial use and veterinary care among agro-pastoralists in Northern Tanzania. PLoS ONE 2017, 12, e0170328. [Google Scholar] [CrossRef] [PubMed]
- Karimuribo, E.; Mdegela, R.; Kusiluka, L.; Kambarage, D. Assessment of drug usage and antimicrobial residues in milk on smallholder farms in Morogoro, Tanzania. Res. Dev. 2005, 53, 234–241. [Google Scholar]
- Kimera, Z.I.; Mshana, S.E.; Rweyemamu, M.M.; Mboera, L.E.G.; Matee, M.I.N. Antimicrobial use and resistance in food-producing animals and the environment: An African perspective. Antimicrob. Resist. Infect. Control 2020, 9, 37. [Google Scholar] [CrossRef]
- Mohameda, H.S.A.; Anders, D.; Uswege, M.; Robinson, H.M. Occurrence and Distribution of Sulfonamides, Tetracyclines and Quinolones in Livestock Manure in Morogoro Municipality, Tanzania. J. Zoonotic Dis. Public Health 2017, 1, 1–5. [Google Scholar]
- Kimera, Z.I.; Mdegela, R.H.; Mhaiki, C.J.; Karimuribo, E.D.; Mabiki, F.; Nonga, H.E.; Mwesongo, J. Determination of oxytetracycline residues in cattle meat marketed in the Kilosa district, Tanzania. Onderstepoort J. Veter-Res. 2015, 82, 1–5. [Google Scholar] [CrossRef]
- Azabo, R.; Mshana, S.; Matee, M.; Kimera, S.I. Antimicrobial usage in cattle and poultry production in Dar es Salaam, Tanzania: Pattern and quantity. BMC Veter-Res. 2022, 18, 1–12. [Google Scholar] [CrossRef]
- Katakweba, A.; Mtambo, M.; Olsen, J.; Muhairwe, A. Awareness of human health risks associated with the use of antimicrobials among livestock keepers and factors that contribute to selection of antibiotic resistance bacteria within livestock in Tanzania. Livestock Rural. Res. Dev. 2012, 24, 1–14. [Google Scholar]
- Ezekiel, P.; Mubito, F.; Shahada, M.; Kimanya, J.; Buza, N.; Mandela, A.T. Sulfonamide residues in commercial layer chicken eggs in Dar-es-Salaam, Tanzania. Am. J. Res. Commun. 2014, 2, 124–132. [Google Scholar]
- FAO; WHO. Codex Alimentarius Commission, Procedural Manual, Joint FAO/WHO Food Standard Programme; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2013; pp. 21–24. [Google Scholar]
- Ebrahimpour, B.; Yamini, Y.; Rezazadeh, M. A sensitive emulsification liquid phase microextraction coupled with on-line phase separation followed by HPLC for trace determination of sulfonamides in water samples. Environ. Monit. Assess. 2014, 187, 4162. [Google Scholar] [CrossRef]
- Malviya, R.; Bansal, V.; Pal, O.; Sharma, P. High performance liquid chromatography: A short review. J. Glob. Pharma Technol. 2010, 2, 22–26. [Google Scholar]
- Gaurav, A.; Gill, J.P.S.; Aulakh, R.S.; Bedi, J.S. ELISA based monitoring and analysis of tetracycline residues in cattle milk in various districts of Punjab. Vet. World 2014, 7, 26–29. [Google Scholar] [CrossRef]
- Shalaby, A.; Salama, N.; Abou-Raya, S.; Emam, W.; Mehaya, F. Validation of HPLC method for determination of tetracycline residues in chicken meat and liver. Food Chem. 2011, 124, 1660–1666. [Google Scholar] [CrossRef]
- Ramatla, T.; Ngoma, L.; Adetunji, M.; Mwanza, M. Evaluation of Antibiotic Residues in Raw Meat Using Different Analytical Methods. Antibiotics 2017, 6, 34. [Google Scholar] [CrossRef]
- El Tahir, Y.; Elshafie, E.I.; Asi, M.N.; Al-Kharousi, K.; Al Toobi, A.G.; Al-Wahaibi, Y.; Al-Marzooqi, W. Detection of Residual Antibiotics and Their Differential Distribution in Broiler Chicken Tissues Using Enzyme-Linked Immunosorbent Assay. Antibiotics 2021, 10, 1305. [Google Scholar] [CrossRef]
- Nonga, H.; Sungura, K.; Helena, A. Assessment of veterinary drug use and determination of antimicrobial residues in broiler chicken meat in Urban district, Zanzibar, Tanzania. Tanzania Vet. J. 2013, 28, 30–38. [Google Scholar]
- Sangeda, R.Z.; Baha, A.; Erick, A.; Mkumbwa, S.; Bitegeko, A.; Sillo, H.B.; Fimbo, A.M.; Chambuso, M.; Mbugi, E.V. Consumption Trends of Antibiotic for Veterinary Use in Tanzania: A Longitudinal Retrospective Survey from 2010–2017. Front. Trop. Dis. 2021, 2, 82. [Google Scholar] [CrossRef]
- Nonga, H.; Mariki, M.; Karimuribo, E.; Mdegela, R. Assessment of Antimicrobial Usage and Antimicrobial Residues in Broiler Chickens in Morogoro Municipality, Tanzania. Pak. J. Nutr. 2009, 8, 203–207. [Google Scholar] [CrossRef]
- Mdegela, R.H.; Mwakapeje, E.R.; Rubegwa, B.; Gebeyehu, D.T.; Niyigena, S.; Msambichaka, V.; Nonga, H.E.; Antoine-Moussiaux, N.; Folorunso, O. Antimicrobial Use, Residues, Resistance and Governance in the Food and Agriculture Sectors, Tanzania. Antibiotics 2021, 10, 454. [Google Scholar] [CrossRef]
- Mubito, E.; Shahada, F.; Kimanya, M.; Buza, J. Antimicrobial use in the poultry industry in Dar-es-Salaam, Tanzania and public health implications. Am. J. Res. Comm. 2014, 2, 51–63. [Google Scholar]
- Nanayakkara, A.K.; Boucher, H.W.; Fowler, V.G.; Jezek, A.; Outterson, K.; Greenberg, D.E. Antibiotic resistance in the patient with cancer: Escalating challenges and paths forward. CA Cancer J. Clin. 2021, 71, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Frye, J.G.; Jackson, C.R. Genetic mechanisms of antimicrobial resistance identified in Salmonella enterica, Escherichia coli, and Enteroccocus spp. isolated from U.S. food animals. Front. Microbiol. 2013, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M. Veterinary Drug Usage and Antimicrobial Resistance in Bacteria of Animal Origin. Basic Clin. Pharmacol. Toxicol. 2005, 96, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Mgaya, F.; Matee, M.; Muhairwa, A.; Hoza, A. Occurrence of Multidrug Resistant Escherichia coli in Raw Meat and Cloaca Swabs in Poultry Processed in Slaughter Slabs in Dar es Salaam, Tanzania. Antibiotics 2021, 10, 343. [Google Scholar] [CrossRef]
- Kimera, Z.; Mgaya, F.; Misinzo, G.; Mshana, S.; Moremi, N.; Matee, M. Multidrug-Resistant, Including Extended-Spectrum Beta Lactamase-Producing and Quinolone-Resistant, Escherichia coli Isolated from Poultry and Domestic Pigs in Dar es Salaam, Tanzania. Antibiotics 2021, 10, 406. [Google Scholar] [CrossRef]
- Sonola, V.; Katakweba, A.; Misinzo, G.; Matee, M. Occurrence of Multi-Drug-Resistant Escherichia coli in Chickens, Humans, Rodents and Household Soil in Karatu, Northern Tanzania. Antibiotics 2021, 10, 1137. [Google Scholar] [CrossRef]
- Frumence, G.; Mboera, L.; Sindato, C.; Durrance-Bagale, A.; Jung, A.-S.; Mshana, S.; Clark, T.; Legido-Quigley, H.; Matee, M. Practices and Challenges of Veterinary Paraprofessionals in Regards to Antimicrobial Use and Resistance in Animals in Dar Es Salaam, Tanzania. Antibiotics 2021, 10, 733. [Google Scholar] [CrossRef]
- Mshana, E.; Calvin, S.; Mecky, M.; Leonard, E. Antimicrobial Use and Resistance in Agriculture and Food Production Systems in Africa: A Systematic Review. Antibiotics 2021, 10, 976. [Google Scholar] [CrossRef]
- Tanzania’s AMR National Action Plan 2017–2022. Google Search. Available online: https://www.google.com/search?q=Tanzania%E2%80%99s+AMR+National+Action+Plan+2017-2022 (accessed on 18 January 2022).
- Sulfonamides (SAs) ELISA Kit. Shenzhen Lvshiyuan Biotechnology Co., Ltd., Version: 2016-01, Catalog No. LSY-10009. Available online: https://lsyu01.en.made-in-china.com/product/wbxQHsCDAIcu/China-Lsy-10009-Sulfonamides-SAS-Elisa-Test-Kit.html (accessed on 20 November 2021).
- Tetracyclines (TCs) ELISA Kit. Shenzhen Lvshiyuan Biotechnology Co., Ltd., Version: 2020-01, Catalog No. LSY-10006. Available online: https://lsyu01.en.made-in-china.com/product/wbxQHsCDAIcu/China-Lsy-10006-Tetracyclines-TCS-Elisa-Test-Kit.html (accessed on 20 November 2021).
| Median Concentration (IQR) | |||
|---|---|---|---|
| Chicken Processing Area | Manzese | Shekilango | p-Value |
| Tetracycline | 137.8 (35.48, 235.51) | 151.06 (59.53, 298.50) | 0.185 |
| Sulphonamides | 0.58 (0.25, 0.79) | 0.58 (0.13, 1.21) | 0.262 |
| SULPHONAMIDES COMPOUNDS | CROSS-REACTIVITY (%) |
|---|---|
| Sulfamerazine | 100 |
| Sulfadiazine | 130.2 |
| Sulfamonomethoxine | 181.8 |
| Sulfamethoxydizine | 194.8 |
| Sulfamethazine | 89.3 |
| Sulfisomidine | 70.6 |
| Sulfadimethoxine | 140.8 |
| Sulfadoxine | 132.1 |
| Sulfamethoxazole | 86.6 |
| Sulfadimoxine | 144.7 |
| Phthalylsulfathiazole | 73.9 |
| Sulfamethizole | 76.5 |
| Sulfathiazole | 103.3 |
| Sulfaquinoxaline | 59.4 |
| Sulfamethoxypyridazine | 108.6 |
| Sulfaclozine | 72.1 |
| Sulfachloropyridazine | 59.6 |
| Sulfabenzoy | 59.6 |
| TETRACYCLINE COMPOUNDS | |
| Doxycycline | 100 |
| Tetracycline | 150 |
| Minocycline | 92 |
| Pyrithione | 76 |
| Chlortetracycline | 75 |
| Demethylchromycin | 70 |
| Oxytetracycline | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulomi, W.J.; Mgaya, F.X.; Kimera, Z.; Matee, M.I. Determination of Sulphonamides and Tetracycline Residues in Liver Tissues of Broiler Chicken Sold in Kinondoni and Ilala Municipalities, Dar es Salaam, Tanzania. Antibiotics 2022, 11, 1222. https://doi.org/10.3390/antibiotics11091222
Ulomi WJ, Mgaya FX, Kimera Z, Matee MI. Determination of Sulphonamides and Tetracycline Residues in Liver Tissues of Broiler Chicken Sold in Kinondoni and Ilala Municipalities, Dar es Salaam, Tanzania. Antibiotics. 2022; 11(9):1222. https://doi.org/10.3390/antibiotics11091222
Chicago/Turabian StyleUlomi, Winstone J., Fauster X. Mgaya, Zuhura Kimera, and Mecky I. Matee. 2022. "Determination of Sulphonamides and Tetracycline Residues in Liver Tissues of Broiler Chicken Sold in Kinondoni and Ilala Municipalities, Dar es Salaam, Tanzania" Antibiotics 11, no. 9: 1222. https://doi.org/10.3390/antibiotics11091222
APA StyleUlomi, W. J., Mgaya, F. X., Kimera, Z., & Matee, M. I. (2022). Determination of Sulphonamides and Tetracycline Residues in Liver Tissues of Broiler Chicken Sold in Kinondoni and Ilala Municipalities, Dar es Salaam, Tanzania. Antibiotics, 11(9), 1222. https://doi.org/10.3390/antibiotics11091222






