Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Bordetella bronchiseptica Co-Infection in a Stem Cell Transplant Patient
Abstract
1. Introduction
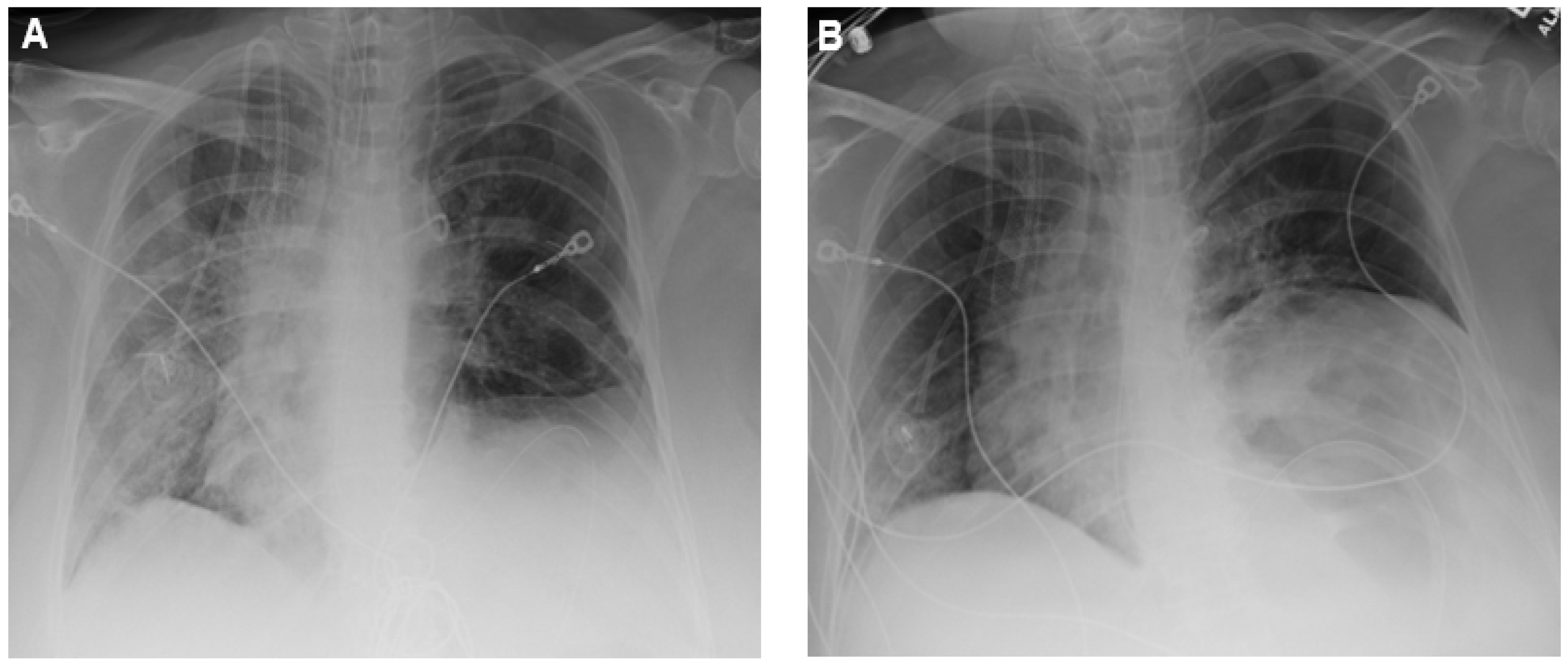
2. Case Presentation
3. Discussion
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 23, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Karaba, S.M.; Jones, G.; Helsel, T.; Smith, L.L.; Avery, R.; Dzintars, K.; Salinas, A.B.; Keller, S.C.; Townsend, J.L.; Klein, E.; et al. Prevalence of Co-infection at the Time of Hospital Admission in COVID-19 Patients, A Multicenter Study. Open Forum Infect. Dis. 2021, 8, ofaa578. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.R.; Mohan, S.; Cohen, D.J.; Husain, S.A.; Dube, G.K.; Ratner, L.E.; Arcasoy, S.; Aversa, M.M.; Benvenuto, L.J.; Dadhania, D.M.; et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am. J. Transpl. 2020, 20, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Shafiekhani, M.; Shekari, Z.; Boorboor, A.; Zare, Z.; Arabsheybani, S.; Azadeh, N. Bacterial and fungal co-infections with SARS-CoV-2 in solid organ recipients: A retrospective study. Virol. J. 2022, 19, 35. [Google Scholar] [CrossRef]
- Fung, M.; Babik, J.M. COVID-19 in immunocompromised hosts: What we know so far. Clin. Infect. Dis. 2021, 72, 340–350. [Google Scholar] [CrossRef]
- Woolfrey, B.F.; Moody, J.A. Human infections associated with Bordetella bronchiseptica. Clin. Microbiol. Rev. 1991, 4, 243–255. [Google Scholar] [CrossRef]
- Wernli, D.; Emonet, S.; Schrenzel, J.; Harbarth, S. Evaluation of eight cases of confirmed Bordetella bronchiseptica infection and colonization over a 15-year period. Clin. Microbiol. Infect. 2011, 17, 201–203. [Google Scholar] [CrossRef]
- Goebel, E.M.; Zhang, X.; Harvill, E.T. Bordetella pertussis infection or vaccination substantially protects mice against B. bronchiseptica infection. PLoS ONE 2009, 4, e6778. [Google Scholar] [CrossRef][Green Version]
- Patel, A.K.; Prescott-Focht, J.A.; Kunin, J.R.; Essmyer, C.E.; Rosado-de-Christenson, M.L. Imaging findings in human Bordetella bronchiseptica pneumonia. J. Thorac. Imaging 2011, 26, W146–W149. [Google Scholar] [CrossRef]
- García-de-la-Fuente, C.; Guzmán, L.; Cano, M.E.; Agüero, J.; Sanjuán, C.; Rodríguez, C.; Aguirre, A.; Martínez-Martínez, L. Microbiological and clinical aspects of respiratory infections associated with Bordetella bronchiseptica. Diagn. Microbiol. Infect. Dis. 2015, 82, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sameed, M.; Sullivan, S.; Marciniak, E.T.; Deepak, J. Chronic cough and cystic lung disease caused by Bordetella bronchiseptica in a patient with AIDS. BMJ Case Rep. 2019, 12, e228741. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Diano, D.; Allegrini, F.; Delmonte, A.; Fausti, V.; Cravero, P.; Marcantognini, G.; Frassineti, G.L. Bordetella bronchiseptica pneumonia in a patient with lung cancer; a case report of a rare infection. BMC Infect. Dis. 2017, 17, 644. [Google Scholar] [CrossRef] [PubMed]
- Faqihi, F.; Alharthy, A.; Pirompanich, P.; Noor, A.; Shahzad, A.; Nasim, N.; Balhamar, A.; Memish, Z.A.; Karakitsos, D. Co-infection of SARS-CoV-2 and Bordetella bronchiseptica in a young man with idiopathic non-cystic bronchiectasis and vitamin D(3) deficiency. Respir. Med. Case Rep. 2020, 31, 101203. [Google Scholar] [CrossRef]
- Nagarakanti, S.; Bishburg, E. Coinfection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Bordetella bronchiseptica Pneumonia in a Renal Transplant Patient. Cureus 2021, 13, e13113. [Google Scholar]
- Papantoniou, S.; Tsakiris, A.; Ladopoulos, T.; Kranidiotis, G.; Tamvakos, C. A Case of Bordetella bronchiseptica Bacteremia in a Patient With COVID-19: Brief Report. Cureus 2021, 13, e15976. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting. 2021. Available online: https://www.fda.gov/media/146217/download (accessed on 7 July 2021).
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 7 August 2022).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, Y.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Rath, B.A.; Register, K.B.; Wall, J.; Sokol, D.M.; Van Dyke, R.B. Persistent Bordetella bronchiseptica pneumonia in an immunocompetent infant and genetic comparison of clinical isolates with kennel cough vaccine strains. Clin. Infect. Dis. 2008, 46, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Szvalb, A.; Mori, N.; Viola, G.; Mulanovich, V. Bordetella bronchiseptica infection in cancer patients. In Proceedings of the 27th European Congress of Clinical Microbiology and Clinical Infectious Diseases, Vienna, Austria, 22–25 April 2017. Poster 2104. [Google Scholar]
| ATM | CAZ | CIP | IMI | MIN | P/T | T/C | TMP/SMZ |
|---|---|---|---|---|---|---|---|
| ≥64 (R) | 16 (I) | 1 (S) | 1 (S) | 0.5 (S) | ≤1/4 (S) | 8/2 (S) | 4/76 (R) |
| Age, Sex | Specimen | Underlying Conditions | Animal Exposure | Treatment | Outcome | |
|---|---|---|---|---|---|---|
| Faqihi et al. [14] | 30, M | Endotracheal aspirate | Idiopathic non-cystic bronchiectasis, vitamin D3 deficiency | Dog | Doxycycline | Cured |
| Nagarakanti et al. [15] | 48, M | Sputum | Renal transplant, chronic obstructive pulmonary diseases, hypertension, diabetes mellitus, obesity, gout, and obstructive sleep apnea | None | Azithromycin Piperacillin/tazobactam | Cured |
| Papantoniou et al. [16] | 47, M | Blood | None | Not reported | Piperacillin/tazobactam Meropenem | Cured |
| Pierce et al. | 50, F | Endotracheal aspirate | Hodgkin’s lymphoma, allogenic hematopoietic stem cell transplant, hypothyroidism, depression with anxiety, and gastroesophageal reflux disease | Cat | Levofloxacin Cefepime | Died |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierce, M.; Slipke, W.; Biagi, M. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Bordetella bronchiseptica Co-Infection in a Stem Cell Transplant Patient. Antibiotics 2022, 11, 1200. https://doi.org/10.3390/antibiotics11091200
Pierce M, Slipke W, Biagi M. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Bordetella bronchiseptica Co-Infection in a Stem Cell Transplant Patient. Antibiotics. 2022; 11(9):1200. https://doi.org/10.3390/antibiotics11091200
Chicago/Turabian StylePierce, Michael, Wendy Slipke, and Mark Biagi. 2022. "Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Bordetella bronchiseptica Co-Infection in a Stem Cell Transplant Patient" Antibiotics 11, no. 9: 1200. https://doi.org/10.3390/antibiotics11091200
APA StylePierce, M., Slipke, W., & Biagi, M. (2022). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Bordetella bronchiseptica Co-Infection in a Stem Cell Transplant Patient. Antibiotics, 11(9), 1200. https://doi.org/10.3390/antibiotics11091200





