Prediction of Potential Natural Antibiotics Plants Based on Jamu Formula Using Random Forest Classifier
Abstract
:1. Introduction
2. Results
2.1. Preliminary Screening Using Several Machine Learning Methods
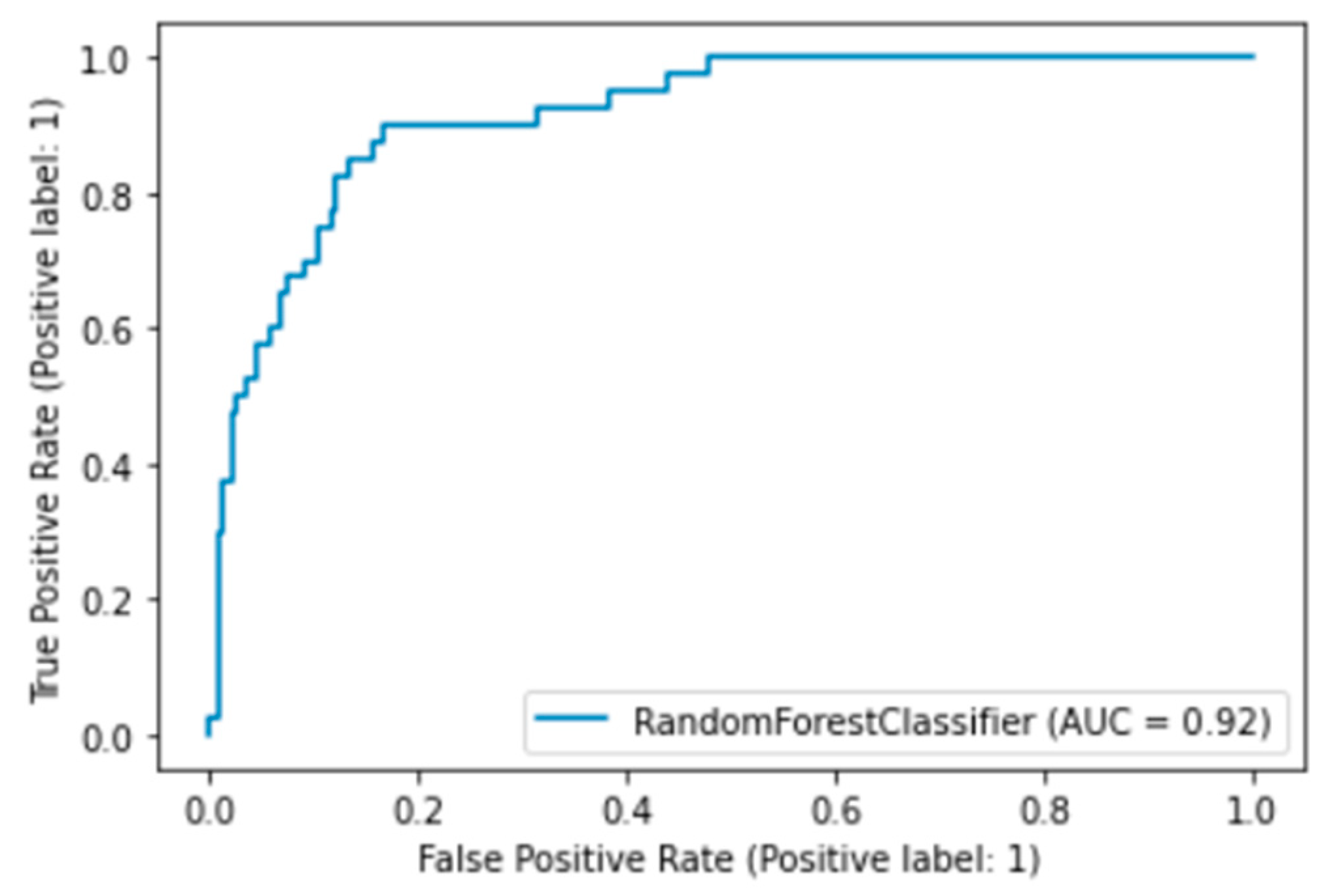
2.2. Tuning Model Parameters for Random Forest
2.3. Identification and Validation of Important Plants
| Name of Plant | Habitat | Pharmacological Activities | References |
|---|---|---|---|
| Clerodendrom squamatum | Indonesia | Staphylococcus aureus, Escherichia coli and Salmonella typhi bacteria | [20,21] |
| Prunus cerasus | United States of America, Turkey, Russia, Serbia, Hungary, Iran, Austria, Azerbaijan, Germany, and Indonesia | Antibacterial activity | [22] |
| Borreria hispida | Indonesia | Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli | [23] |
| Coptis chinensis | China | Escherichia coli | [24,25] |
| Cassia alata | Indonesia | Dermathophilus congolensis, Staphylococcus aureus, Corynebacterium parvum, Actinomyces bovis, and Clostridium septicum | [26] |
| Brucea javanica | Indonesia | Streptococcus pyogenes | [27] |
| Aglaia odorata | Indonesia and China | Bacillus cereus ATCC 11778, Staphylococcus aureus ATCC 25923, Acinetobacter baumannii ATCC 19606 and Escherichia coli ATCC 25922 | [28] |
| Costus speciosus | Indonesia | Antibacterial, antifungal, anticholinesterase, antioxidant, antihyperglycemic, anti-inflammatory, analgesic, antipyretic, antidiuretic, larvicidal, anti-stress and estrogenic activity | [29] |
| Stachytarpheta jamaicensis | Indonesia | Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Proteus vulgaris, Klebsiella aerogenes, Proteus mirabilisand Candida albicans. | [30,31] |
| Trichosanthes kirilowii | China | Bacillus cereus, Escherichia coli, and Streptococcus faecalis. | [32] |
| Prunus armeniaca L. | US, Turkey, and Indonesia | Antimicrobial, antimutagenic, inhibiting enzymes, cardioprotective, anti-inflammatory and antinociceptive | [33] |
| Fritillariae cirrhosae bulbus | China | Antitussive, expectorant, analgesic, anti-cancer, anti-inflammatory, and antioxidative. | [34] |
| Scaphium affinis | Indonesia | Used to treat acute cough, sore throat, hemorrhoids, and increase female fertility | - |
| Pueraria lobata | China | Antioxidant, antiglycation, skin generation, and melanogenesis | [35] |
- Clerodendrom squamatum or better known as sesewanua leaf by the people of North Sulawesi, Indonesia, has often been used as a traditional medicine to treat fever, fractures, and swelling [18]. As stated by [19], sesewanua leaf extract using 96% ethanol by the Kirby and Bauer diffusion method could inhibit the growth of Staphylococcus aureus, Escherichia coli, and Salmonella typhi bacteria. This can be attributed to a scientific basis to support our prediction that this plant is useful as a natural antibiotic.
- Prunus cerasus or sour cherry were also predicted as natural antibiotic candidates in our study. This plant grows in so many countries including Poland, the United States of America, Turkey, Russia, Serbia, Hungary, Iran, Austria, Azerbaijan, Germany, and Indonesia. This plant is usually called cherry kersen in Indonesia which is used as a decoration for cakes. It helps in lowering blood pressure, regulating sugar levels, and strengthening our immune system. Research [22] states that it can obstruct the growth of bacteria which justifies our prediction result that this plant is a natural antibiotic.
- Borreria hispida, commonly known as gempur batu, is a plant that belongs to the family rubiaceae and the genus Borreria has been used by the Indonesian people as a medicinal plant, especially to treat kidney diseases. To emphasize the hypothesis of the research results that Borreria hispida can be used as a candidate for natural antibiotics, this plant should exhibit the function of prohibiting bacterial growth or killing bacteria. According to [23], the extracts of this plant can be used against Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli using the agar disc diffusion method.
- Coptis chinensis is one of the drugs found in traditional Chinese medicine commonly known as Huanglian. The extracts of this plant possess strong properties to hinder bacterial growth. Furthermore, it is also used as a medicine for dysentery, cholera, leukemia, diabetes, and lung cancer [24]. Plants produce berberine alkaloids, coptisine, and palmatine which can slow down the growth of Escherichia coli [25]. Additionally, referring to the KNApSAck family database, it can be said this plant has biological activity as antibacterial and/or antibiotics.
- Cassia alata, a plant with extreme effectiveness is commonly known as ketepeng cina in Indonesia. This plant has several names according to various regions in Indonesia. For example, it is called kupang leaf in the Malay area, ki manila in the Sunda area, kupang-kupang in Madura, and ketepeng cina in east and central Java. The leaves of this plant are traditionally used to treat scurvy and malaria. According to [26], the contents of Cassia alata leaf can inhibit the growth of Dermathophilus congolensis, Staphylococcus aureus, Corynebacterium parvum, Actinomyces bovis, and Clostridium septicum. This plant has biological activity as antibacterial or antibiotics according to the KNApSAck family database.
- Brucea javanica is commonly known as buah makasar or amber merica with a bitter taste and is classified as toxic. However, this plant is used as a medicine to prevent dysentery, diarrhea, and malaria. As stated in [27], the potions of its fruits produced a new antibacterial compound for Streptococcus pyogenes bacteria where the effective compound is the bitter-tasting alkaloid called brucine. This reference can be utilized as reasoning for predicting this plant as a candidate for natural antibiotics in this study.
- Aglaia odorata or commonly known as pacar cina is a plant that has efficacies such as healing bloating, throat, cough, ulcer, and also speeding up of labor. According to [28], stem-derived essential oil from this plant can slow down the growth of Gram-positive and Gram-negative bacteria such as Bacillus cereus ATCC 11778, Staphylococcus aureus ATCC 25923, Acinetobacter baumannii ATCC 19606 and Escherichia coli ATCC 25922. Referring to the TCM database, it is explained that this plant can cure abscess disease. Abscess disease is a painful collection of pus, usually emanating from a bacterial infection.
- Costus speciosus is a plant that has a height of about 0.5–3 m with a humid and shady living habit. In Indonesia, this plant has many names such as pancing, pempung tawar, poncang-pancing, tubu-tubu and so on. Traditionally this plant is used for various diseases such as kidney disease, stomach ulcer, urinary tract infection, and liver constriction. From [29], we came to know that this plant has several pharmacological activities such as antibacterial, antifungal, anticholinesterase, antioxidant, antihyperglycemic, anti-inflammatory, analgesic, antipyretic, antidiuretic, larvicidal, antistress and estrogenic activity.
- Stachytarpheta jamaicensis or commonly known as pecut kuda, is a wild plant commonly found in Indonesia and has diverse efficacy as per the beliefs of Indonesian people. According to [30], this plant is habitually used to treat digestive, allergic, and respiratory diseases namely asthma, cold, flu, and cough. The plant extracts can be used as an inhibitor for the growth of the following bacteria and fungus: Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Proteus vulgaris, Klebsiella aerogenes, Proteus mirabilis and Candida albicans [31]. In KNApSAck family database it is recorded that this plant has biological activity as antibacterial and/or antibiotics.
- Trichosanthes kirilowii belongs to the cucurbitaceae family which has effectiveness against abscess disease according to the TCM database. This abscess disease is generally caused by a bacterial infection and, therefore, it can be concluded that this plant has a direct or indirect relationship in prohibiting bacterial growth. Referring to [32], this plant produces a compound 1-C-(p-Hydroxyphenyl)-Glycerol which can hamper bacterial growth of Bacillus cereus, Escherichia coli, and Streptococcus faecalis.
- Prunus armeniaca L. is a medicinal plant commonly known as apricot and is normally eaten because of its delicious taste. In addition, this plant can also be used as medicine due to properties such as antimicrobial, antimutagenic, inhibiting enzymes, cardioprotective, anti-inflammatory, and antinociceptive. This plant is rich in polysaccharides, polyphenols, fatty acids, sterol derivatives, carotenoids, cyanogenic glucosides, and volatile components that make this plant produce a pleasant aroma [33].
- Fritillariae cirrhosae bulbus, a medicinal plant known as chuan bei mu in China, has been used as medicine for a long time for remedies against cough and phlegm. This plant has biological activities such as antitussive, expectorant, analgesic, anticancer, anti-inflammatory, and antioxidative. Moreover, this plant has therapeutic effects on many diseases such as cancer, acute lung injury, chronic obstructive, pulmonary diseases, asthma, Parkinson’s disease, and diabetes [34]. Thus, we assume that it has potential as natural antibiotic for its anti-inflammatory attribute.
- Scaphium affinis is a plant from Indonesia which goes by popular names such as tempayang or semangkuk. It appears brown and is shaped like melinjo seed. As per the traditional belief, this plant can treat diseases such as fever, acute cough, sore throat, hemorrhoids, and increase female fertility.
- Pueraria lobata is one of the plants that has usefulness based in traditional Chinese medicine. A common name for this plant is kudzu in the continent of Asia. This plant is used in the preparation of many foods and cosmetics. In addition, this plant also has potential for biological activities such as antioxidant, antiglycation, skin generation, and melanogenesis inhibitory [35].
3. Discussion
4. Materials and Methods
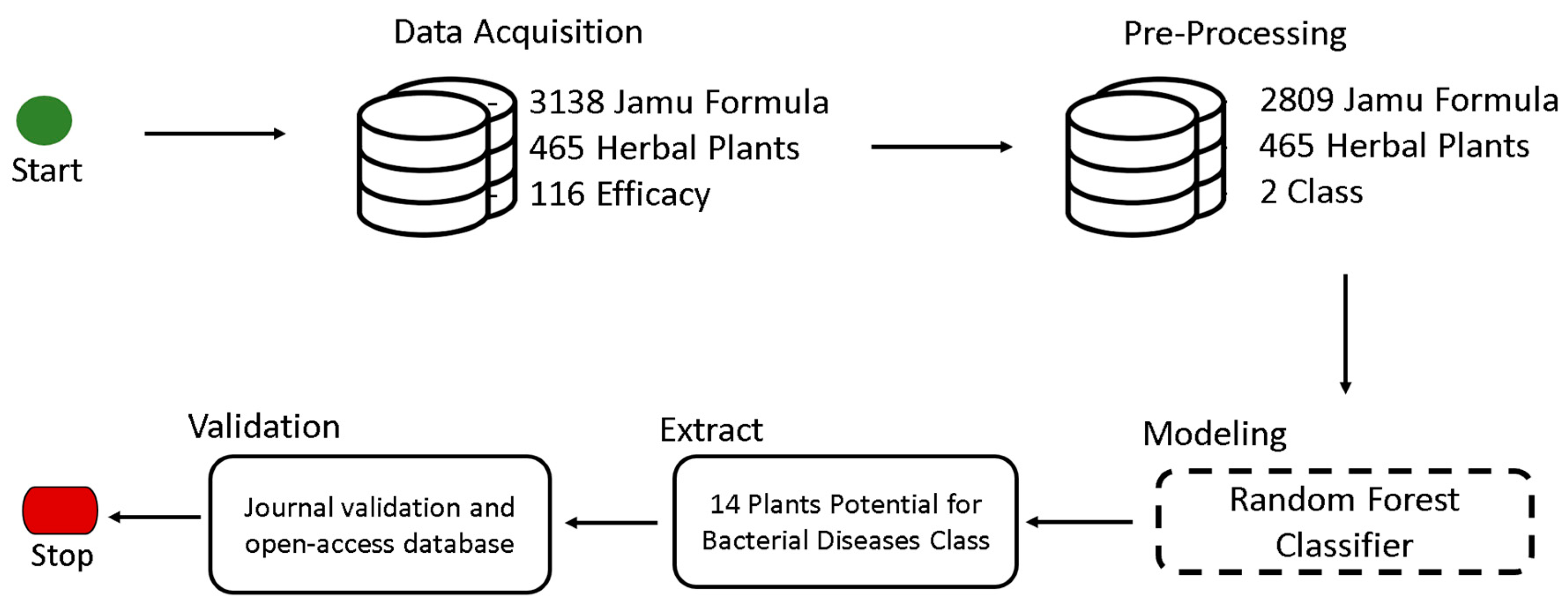
4.1. Data Acquisition
4.2. Pre-Processing
4.3. Modeling
4.4. Extraction
4.5. Validation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasution, A.K.; Wijaya, S.H.; Kusuma, W.A. Prediction of drug-target interaction on Jamu formulas using machine learning approaches. In Proceedings of the International Conference on Advanced Computer Science and Information Systems (ICACSIS), Bali, Indonesia, 12–13 October 2019. [Google Scholar]
- Khan, I.; Abbas, T.; Anjum, K.; Abbas, S.Q.; Shagufta, B.I.; Ali Shah, S.A.; Akhter, N. Antimicrobial potential of aqueous extract of Camellia sinensis against representative microbes. Pak. J. Pharm. Sci. 2019, 32, 2. [Google Scholar]
- Ippolito, G.; Leone, S.; Lauria, F.N.; Nicastri, E.; Wenzel, R.P. Methicillin-resistant Staphylococcus aureus: The superbug. Int. J. Infect. Dis. 2010, 14, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 30 October 2021).
- Goode, O.; Smith, A.; Zarkan, A.; Cama, J.; Invergo, B.M.; Belgami, D.; Caño-Muñiz, S.; Metz, J.; O’Neill, P.; Jeffries, A.; et al. Persister Escherichia coli cells have a lower intracellular pH than susceptible cells but maintain their pH in response to antibiotic treatment. mBio 2021, 12, e00909–e00921. [Google Scholar] [CrossRef] [PubMed]
- Wagley, S.; Morcrette, H.; Kovacs-Simon, A.; Yang, Z.R.; Power, A.; Tennant, R.K.; Love, J.; Murray, N.; Tiball, R.W.; Butter, C. Bacterial dormancy: A subpopulation of viable but non-culturable cells demonstrates better fitness for revival. PLoS Pathog. 2021, 17, e1009194. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, L.; Bollen, C.; Wilmaerts, D.; Louwagie, E.; Herpels, P.; Matthay, P.; Khodaparast, L.; Khodaparast, L.; Rousseau, F.; Schymkowitz, J.; et al. The dynamic transition of persistence toward the viable but nonculturable state during stationary phase is driven by protein aggregation. mBio 2021, 12, e00703–e00721. [Google Scholar] [CrossRef]
- Łapińska, U.; Voliotis, M.; Lee, K.K.; Campey, A.; Stone, M.R.L.; Tuck, B.; Phestang, W.; Zhang, B.; Tseneva, A.K.; Blascovich, M.A.T.; et al. Fast bacterial growth reduces antibiotic accumulation and efficacy. eLife 2022, 11, e74062. [Google Scholar] [CrossRef]
- Bamford, R.A.; Smith, A.; Metz, J.; Glover, G.; Titball, R.W.; Pagliara, S. Investigating the physiology of viable but non-culturable bacteria by microfluidics and time-lapse microscopy. BMC Biol. 2017, 15, 1–21. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Hopkins, S. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- David, L.; Brata, A.M.; Mogosan, C.; Pop, C.; Czako, Z.; Muresan, L.; Ismaiel, A.; Dumitrascu, D.I.; Leucuta, D.C.; Stanculete, M.F. Artificial Intelligence and Antibiotic Discovery. Antibiotics 2021, 10, 1376. [Google Scholar] [CrossRef]
- Siltrakool, B.; Berrou, I.; Griffiths, D.; Alghamdi, S. Antibiotics’ Use in Thailand: Community Pharmacists’ Knowledge, Attitudes and Practices. Antibiotics 2021, 10, 137. [Google Scholar] [CrossRef]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; BloomAckermann, Z. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feretzakis, G.; Loupelis, E.; Sakagianni, A.; Kalles, D.; Martsoukou, M.; Lada, M.; Skarmoutsou, N.; Christopoulos, C.; Valakis, K.; Velenza, A.; et al. Using machine learning techniques to aid empirical antibiotic therapy decisions in the intensive care unit of a general hospital in Greece. Antibiotics 2020, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, R.B.; Fonseca, L.P.; Calado, C.R.C. Simultaneous elucidation of antibiotic mechanism of action and potency with high-throughput Fourier-transform infrared (FTIR) spectroscopy and machine learning. Appl. Microbiol. Biotechnol. 2021, 105, 1269–1286. [Google Scholar] [CrossRef] [PubMed]
- Zoffmann, S.; Vercruysse, M.; Benmansour, F.; Maunz, A.; Wolf, L.; Marti, R.B.; Heckel, T.; Ding, H.; Truong, H.H.; Prummer, M. Machine learning-powered antibiotics phenotypic drug discovery. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Orfali, R.; Perveen, S.; AlAjmI, M.F.; Ghaffar, S.; Rehman, M.T.; AlanzI, A.R.; Gamea, S.B.; Essa Khwayri, M. Antimicrobial Activity of Dihydroisocoumarin Isolated from Wadi Lajab Sediment-Derived Fungus Penicillium chrysogenum: In Vitro and In Silico Study. Molecules 2022, 27, 3630. [Google Scholar] [CrossRef]
- Johnston, C.W.; Skinnider, M.A.; Dejong, C.A.; Rees, P.N.; Chen, G.M.; Walker, C.G.; Magarvey, N.A. Assembly and clustering of natural antibiotics guides target identification. Nat. Chem. Biol. 2016, 12, 233–239. [Google Scholar] [CrossRef]
- Afendi, F.M.; Okada, T.; Yamazaki, M.; Hirai-Morita, A.; Nakamura, Y.; Nakamura, K.; Ikeda, S.; Takahasi, G.; Amin, M.d.A.U.; Darusman, L.K.; et al. KNApSAcK family databases: Integrated metabolite–plant species databases for multifaceted plant research. Plant Cell Physiol. 2012, 53, e1. [Google Scholar] [CrossRef]
- Pratasik, M.C.M.; Yamlean, P.V.Y.; Wiyono, W.I. Formulasi dan uji stabilitas fisik sediaan krim ekstrak etanol daun sesewanua (Clerodendron squamatum Vahl.). Pharmacon 2019, 8, 261–267. [Google Scholar] [CrossRef]
- Kumakauw, V.V.; Simbala, H.E.I.; Mansauda, K.L.R. Aktivitas antibakteri ekstrak etanol daun sesewanua (Clerodendron Squamatum Vahl.) terhadap Bakteri Staphylococcus aureus Escherichia coli dan Salmonella typhi. J. MIPA 2020, 9, 86–90. [Google Scholar] [CrossRef]
- Coccia, A.; Carraturo, A.; Mosca, L.; Masci, A.; Bellini, A.; Campagnaro, M.; Lendaro, E. Effects of methanolic extract of sour cherry (Prunus cerasus L.) on microbial growth. Int. J. Food Sci. Technol. 2021, 47, 1620–1629. [Google Scholar] [CrossRef]
- Rajasudha, V.; Anburaj, G.; Manikandan, R. Effect of various extracts of the leaves of Borreria hispida (Linn) on antibacterial activity. Meth 2016, 25, 26. [Google Scholar]
- O’Neill, M.A.; Vine, G.J.; Beezer, A.E.; Bishop, A.H.; Hadgraft, J.; Labetoulle, C.; Walker, M.; Bowler, P.G. Antimicrobial properties of silver-containing wound dressings: A microcalorimetric study. Int. J. Pharm. 2003, 263, 61–68. [Google Scholar] [CrossRef]
- Yan, D.; Jin, C.; Xiao, X.H.; Dong, X.P. Antimicrobial properties of berberines alkaloids in Coptis chinensis Franch by microcalorimetry. Biochem. Biophys. Methods 2008, 70, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Makinde, A.A.; Igoli, J.O.; Ta’ama, L.; Shaibu, S.J.; Garba, A. Antimicrobial activity of Cassia alata. Afr. J. Biotechnol. 2003, 6, 1509–1510. [Google Scholar]
- Sornwatana, T.; Roytrakul, S.; Wetprasit, N.; Ratanapo, S. Brucin, an antibacterial peptide derived from fruit protein of fructus bruceae, Brucea javanica (L.) Merr. Appl. Microbiol. 2013, 57, 129–136. [Google Scholar] [CrossRef]
- Joycharat, N.; Thammavong, S.; Voravuthikunchai, S.P.; Plodpai, P.; Mitsuwan, W.; Limsuwan, S.; Subhadhirasakul, S. Chemical constituents and antimicrobial properties of the essential oil and ethanol extract from the stem of Aglaia odorata Lour. Nat. Prod. Res. 2014, 28, 2169–2172. [Google Scholar] [CrossRef]
- Pawar, V.; Pawar, P. Costus speciosus: An Important Medicinal Plant. Net 2014, 3, 28–33. [Google Scholar]
- Liew, P.M.; Yong, Y.K. Stachytarpheta jamaicensis (L.) Vahl: From traditional usage to pharmacological evidence. Evid.-Based Complement. Altern. Med. 2016, 2016, 7842340. [Google Scholar] [CrossRef]
- Idu, M.; Omogbai, E.K.I.; Aghimien, G.E.; Amaechina, F.; Timothy, O.; Omonigho, S.E. Preliminary phytochemistry, antimicrobial properties and acute toxicity of Stachytarpheta jamaicensis (L.) Vahl. leaves. Trends Med. Res. 2007, 2, 193–198. [Google Scholar]
- Jang, K.C.; Lee, J.H.; Kim, S.C.; Song, E.Y.; Ro, N.Y.; Moon, D.Y.; Park, K.H. Antibacterial and radical scavenging activities of 1-C-(p-hydroxyphenyl)-glycerol from Trichosanthes kirilowii. J. Appl. Biol. Chem. 2007, 50, 17–21. [Google Scholar]
- Erdogan-Orhan, I.; Kartal, M. Insights into research on phytochemistry and biological activities of Prunus armeniaca L. (apricot). Food Res. Int. 2011, 44, 1238–1243. [Google Scholar]
- Chen, T.; Zhong, F.; Yao, C.; Chen, J.; Xiang, Y.; Dong, J.; Ma, Y. A systematic review on traditional uses, sources, phytochemistry, pharmacology, pharmacokinetics, and toxicity of fritillariae cirrhosae bulbus. Evid.-Based Complement. Altern. Med. 2020, 2020, 1536534. [Google Scholar]
- Tungmunnithum, D.; Intharuksa, A.; Sasaki, Y. A Promising View of Kudzu Plant, Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep: Flavonoid phytochemical compounds, taxonomic data, traditional uses and potential biological activities for future cosmetic application. Cosmetics 2020, 7, 12. [Google Scholar]
- Wojityrzka, R.D.; Dziedzic, A.; Kepa, M.; Kubina, R.; Kabala, D.A.; Mularz, T.; Idzik, D. Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative Straphylococcus strain in vitro. Molecules 2014, 19, 6583–6596. [Google Scholar]
- Anzaku, A.A.; Akyala, J.I.; Juliet, A.; Obianuju, E.C. Antibacterial activity of lauric acid on some selected clinical isolates. Ann. Clin. Lab. Res. 2017, 5, 2. [Google Scholar]
- Xiong, J.; Li, S.; Wang, W.; Hong, Y.; Tang, K.; Luo, Q. Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves. Food Chem. 2013, 1, 327–333. [Google Scholar]
- Cong, S.; Tong, Q.; Peng, Q.; Shen, T.; Zhu, X.; Xu, Y.; Qi, S. In vitro anti-bacterial activity of diosgenin on Porphyromonas gingivalis and Prevotella intermedia. Mol. Med. Rep. 2020, 6, 5392–5398. [Google Scholar]
- Kharwar, R.N.; Verma, V.C.; Kumar, A.; Gond, S.K.; Harper, J.K.; Hess, W.M.; Lobkovosky, E.; Ma, C.; Ren, Y.; Strobel, G.A. Javanicin, an antibacterial naphthaquinone from an endophytic fungus of neem, Chloridium sp. Curr. Microbiol. 2009, 3, 233–238. [Google Scholar] [CrossRef]
- Tadesse, A.A.; Muhammed, B.L.; Zeleke, M.A. Chrysophanol from the Roots of Kniphofia Insignis and Evaluation of Its Antibacterial Activities. J. Chem. 2022, 2022, 5884309. [Google Scholar]
- Babu, K.S.; Babu, T.H.; Srinivas, P.V.; Kishore, K.H.; Murthy, U.S.N.; Rao, J.M. Synthesis and biological evaluation of novel C (7) modified chrysin analogues as antibacterial agents. Bioorg. Med. Chem. Lett. 2006, 1, 221–224. [Google Scholar] [CrossRef]
- Wijaya, S.H.; Batubara, I.; Nishioka, T.; Altaf-Ul-Amin, M.; Kanaya, S. Metabolomic studies of Indonesian jamu medicines: Prediction of jamu efficacy and identification of important metabolites. Mol. Inform. 2017, 36, 1700050. [Google Scholar]
- Chand, S. On tuning parameter selection of lasso-type methods-a monte carlo study. In Proceedings of the 2012 9th International Bhurban Conference on Applied Sciences & Technology (IBCAST), Islamabad, Pakistan, 9–12 January 2012. [Google Scholar]

| Model | Accuracy | Balanced Accuracy | ROC-AUC | F1-Score | Required Time |
|---|---|---|---|---|---|
| RandomForestClassifier | 0.81 | 0.79 | 0.79 | 0.80 | 0.75 |
| ExtraTreesClassifier | 0.79 | 0.78 | 0.78 | 0.79 | 0.76 |
| LGBMClassifier | 0.78 | 0.76 | 0.76 | 0.77 | 0.26 |
| BaggingClassifier | 0.76 | 0.75 | 0.75 | 0.76 | 0.50 |
| XGBClassifier | 0.77 | 0.75 | 0.75 | 0.77 | 1.13 |
| DecisionTreeClassifier | 0.75 | 0.75 | 0.75 | 0.75 | 0.13 |
| NuSVC | 0.76 | 0.74 | 0.74 | 0.75 | 2.77 |
| KNeighborsClassifier | 0.73 | 0.73 | 0.73 | 0.74 | 1.68 |
| NearestCentroid | 0.73 | 0.73 | 0.73 | 0.73 | 0.10 |
| AdaBoostClassifier | 0.75 | 0.73 | 0.73 | 0.75 | 0.71 |
| ExtraTreeClassifier | 0.73 | 0.73 | 0.73 | 0.73 | 0.07 |
| LogistciRegression | 0.74 | 0.72 | 0.72 | 0.74 | 0.15 |
| LinearSVC | 0.74 | 0.72 | 0.72 | 0.74 | 1.72 |
| LinearDiscriminantAnalysis | 0.74 | 0.72 | 0.72 | 0.73 | 0.21 |
| BernouliNB | 0.73 | 0.71 | 0.71 | 0.72 | 0.09 |
| SGDClassifier | 0.72 | 0.70 | 0.70 | 0.72 | 0.19 |
| Parameter Name | Parameter Value |
|---|---|
| n_estimators | 200, 400, …, 2000 |
| min_samples_split | 2, 5, 10 |
| min_samples_leaf | 1, 2, 4 |
| max_features | ‘auto’, ‘sqrt’ |
| max_depth | 10, 20, …, 110 |
| bootstrap | “True”, “False” |
| Fold | Accuracy | Recall | Precision |
|---|---|---|---|
| 1 | 87.90% | 87.90% | 86.21% |
| 2 | 89.32% | 89.32% | 88.36% |
| 3 | 87.90% | 87.90% | 86.30% |
| 4 | 88.26% | 88.26% | 88.33% |
| 5 | 91.10% | 91.10% | 90.54% |
| 6 | 89.32% | 89.32% | 88.59% |
| 7 | 88.26% | 88.26% | 86.68% |
| 8 | 87.90% | 87.90% | 86.15% |
| 9 | 87.90% | 87.90% | 86.15% |
| 10 | 90.00% | 90.00% | 89.15% |
| Min | 87.90% | 87.90% | 86.15% |
| Avg | 88.79% | 88.79% | 87.65% |
| Std | 1.05% | 1.05% | 1.48% |
| Jamu Formula | Plants | Class Label | ||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | … | P465 | ||
| J1 | 0 | 0 | 0 | … | 0 | 0 |
| J2 | 0 | 0 | 0 | … | 0 | 1 |
| J3 | 1 | 1 | 1 | … | 1 | 0 |
| … | … | … | … | … | … | … |
| J2809 | 1 | 1 | 0 | … | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasution, A.K.; Wijaya, S.H.; Gao, P.; Islam, R.M.; Huang, M.; Ono, N.; Kanaya, S.; Altaf-Ul-Amin, M. Prediction of Potential Natural Antibiotics Plants Based on Jamu Formula Using Random Forest Classifier. Antibiotics 2022, 11, 1199. https://doi.org/10.3390/antibiotics11091199
Nasution AK, Wijaya SH, Gao P, Islam RM, Huang M, Ono N, Kanaya S, Altaf-Ul-Amin M. Prediction of Potential Natural Antibiotics Plants Based on Jamu Formula Using Random Forest Classifier. Antibiotics. 2022; 11(9):1199. https://doi.org/10.3390/antibiotics11091199
Chicago/Turabian StyleNasution, Ahmad Kamal, Sony Hartono Wijaya, Pei Gao, Rumman Mahfujul Islam, Ming Huang, Naoaki Ono, Shigehiko Kanaya, and Md. Altaf-Ul-Amin. 2022. "Prediction of Potential Natural Antibiotics Plants Based on Jamu Formula Using Random Forest Classifier" Antibiotics 11, no. 9: 1199. https://doi.org/10.3390/antibiotics11091199
APA StyleNasution, A. K., Wijaya, S. H., Gao, P., Islam, R. M., Huang, M., Ono, N., Kanaya, S., & Altaf-Ul-Amin, M. (2022). Prediction of Potential Natural Antibiotics Plants Based on Jamu Formula Using Random Forest Classifier. Antibiotics, 11(9), 1199. https://doi.org/10.3390/antibiotics11091199










