The Molecular Detection of Class B and Class D Carbapenemases in Clinical Strains of Acinetobacter calcoaceticus-baumannii Complex: The High Burden of Antibiotic Resistance and the Co-Existence of Carbapenemase Genes
Abstract
:1. Introduction
2. Results
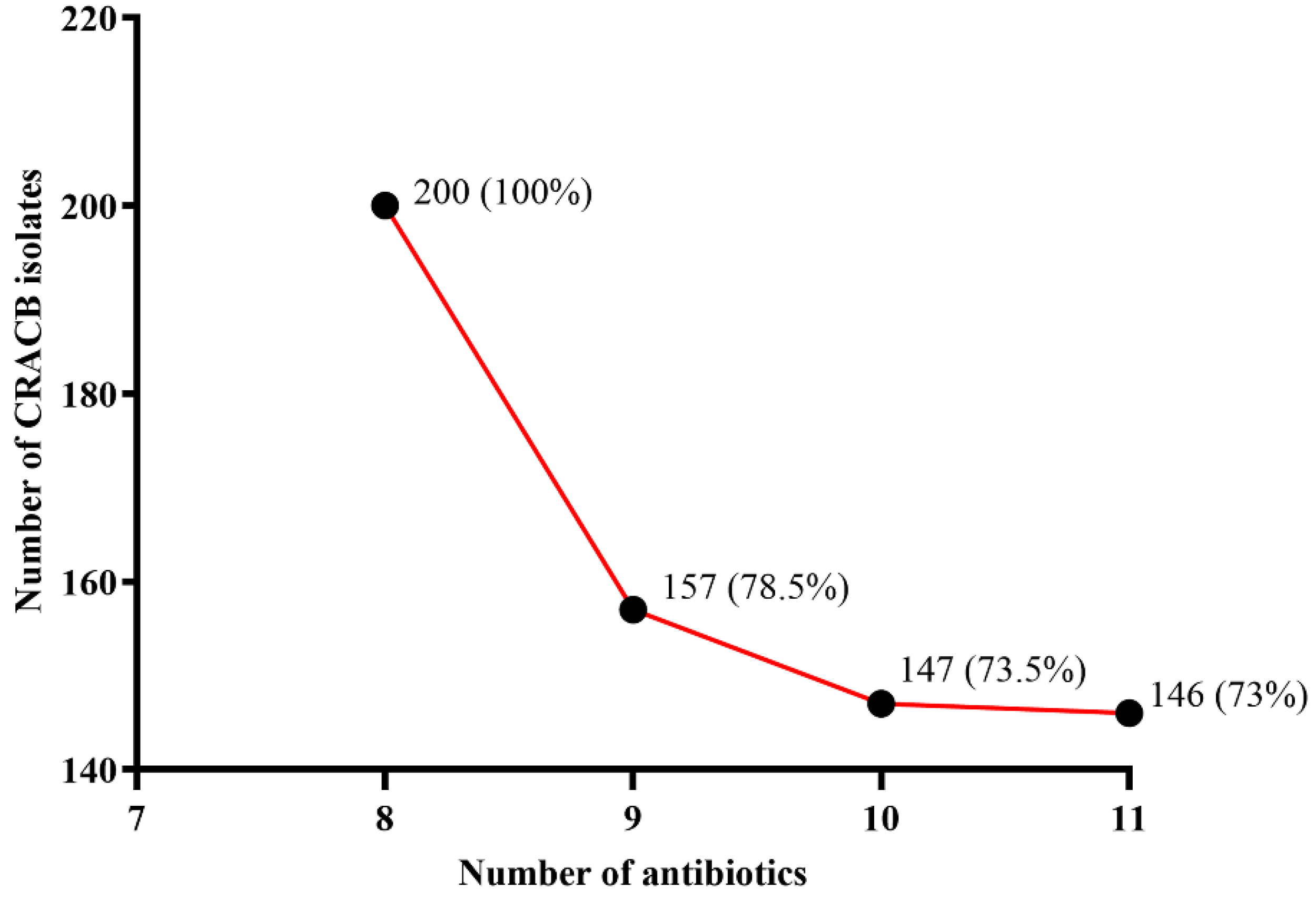
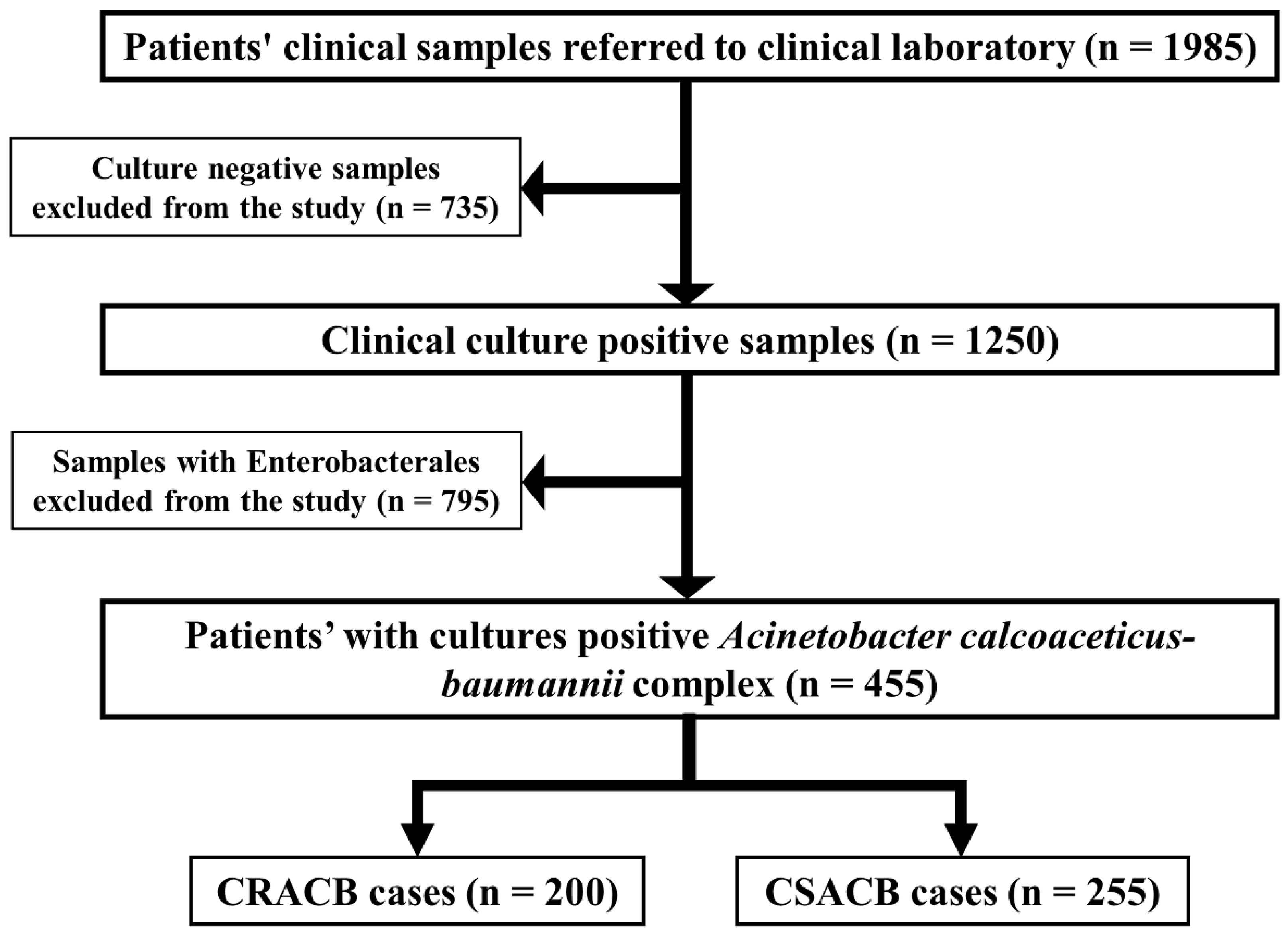
2.1. Prevalence of CRACB in the Clinical Setting
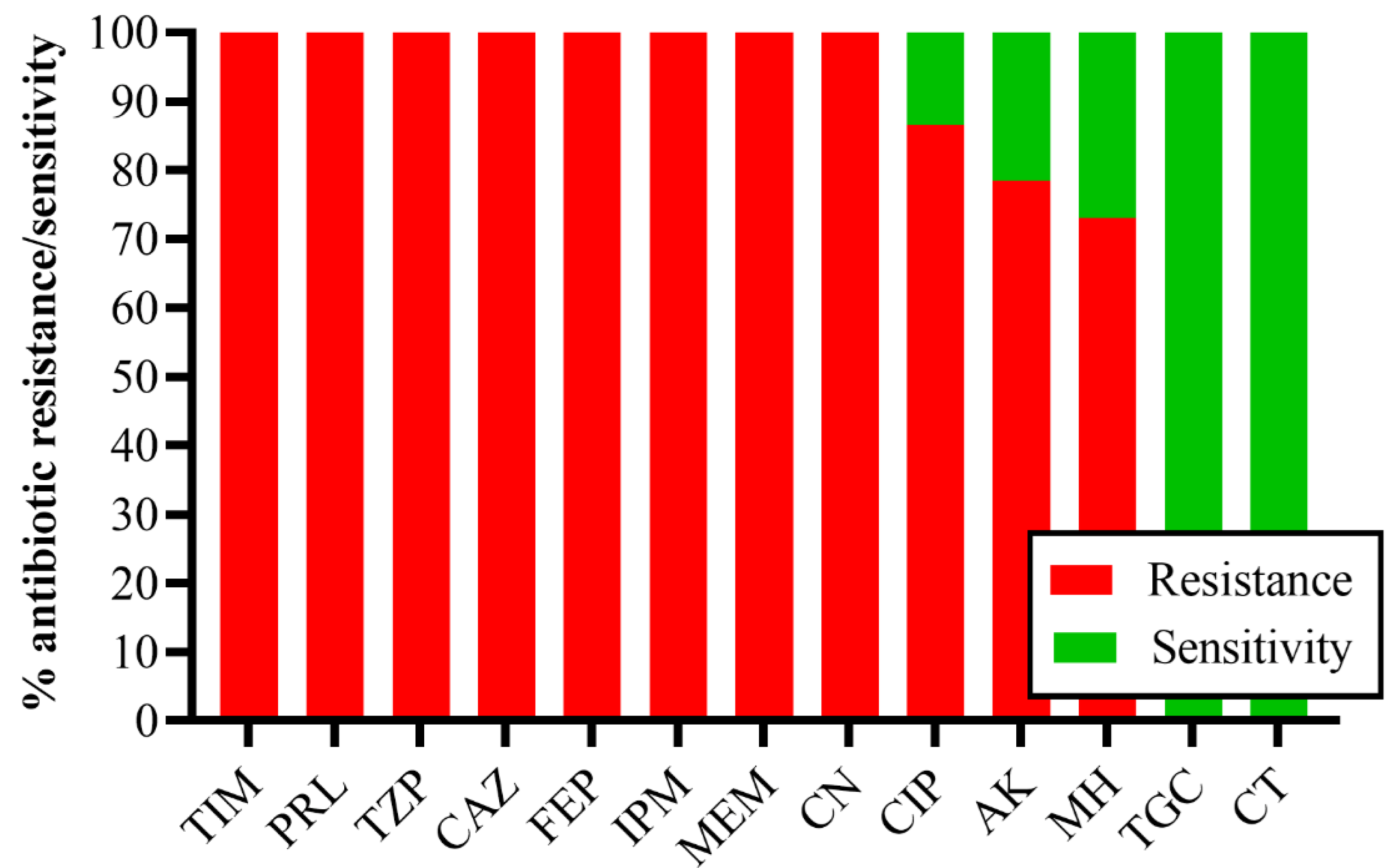
2.2. Minimum Inhibitory Concentration of Antibiotics against CRACB
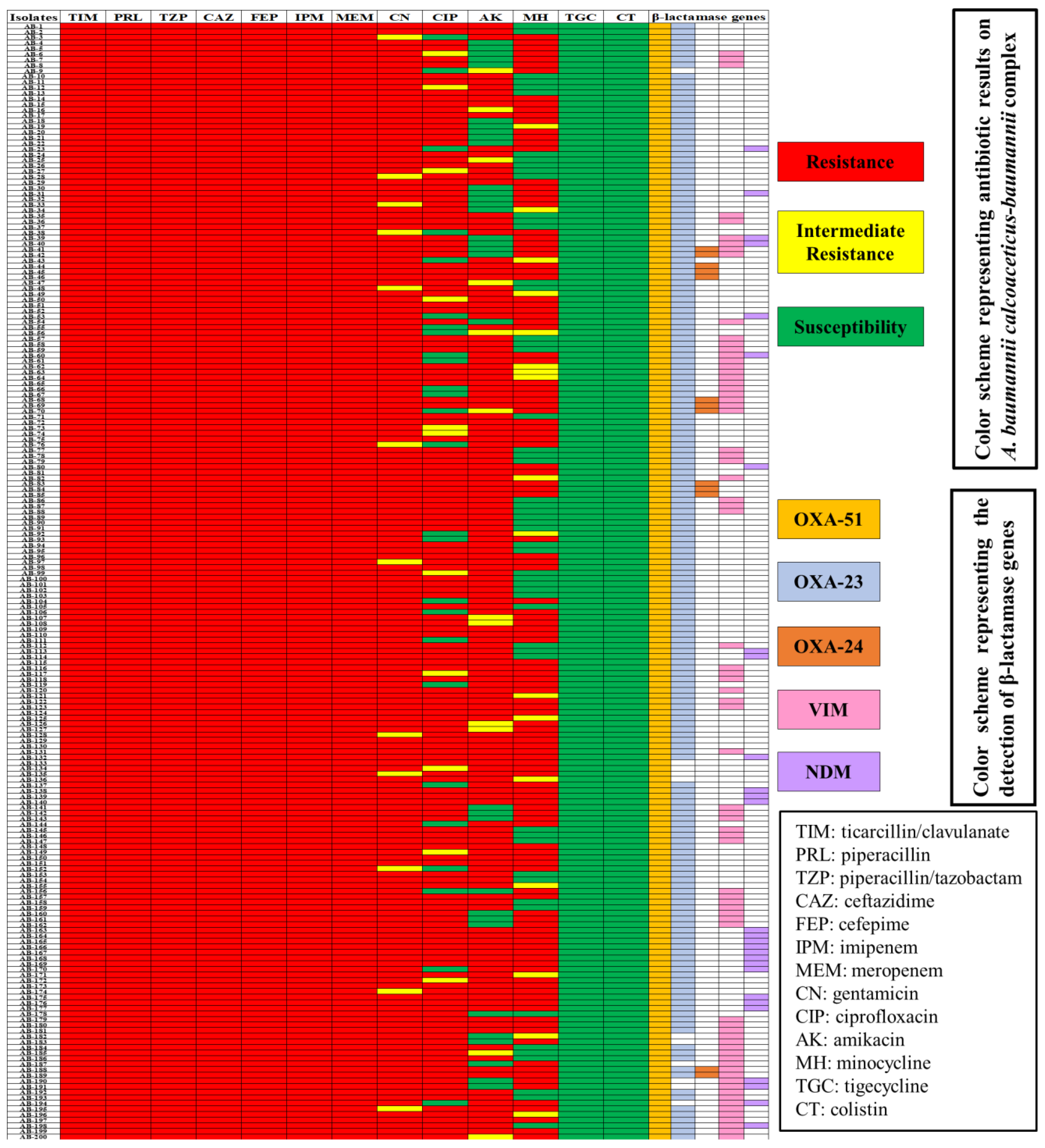
2.3. Detection of Carbapenemases
2.4. Clinical Information on CRACB
2.5. Genetic Determinants of Carbapenemase in CRACB
3. Discussion
4. Materials and Methods
4.1. Ethical Approval and Study Design
4.2. Case Definition
4.3. Confirmation of the CRACB Phenotypic
4.4. The Minimum Inhibitory Concentration of Antibiotics
4.5. Phenotypic Confirmation of Carbapenemase
4.6. Molecular Identification of Carbapenemase Genes
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wareth, G.; Linde, J.; Nguyen, N.H.; Nguyen, T.N.M.; Sprague, L.D.; Pletz, M.W.; Neubauer, H. WGS-Based Analysis of Carbapenem-Resistant Acinetobacter baumannii in Vietnam and Molecular Characterization of Antimicrobial Determinants and MLST in Southeast Asia. Antibiotics 2021, 10, 563. [Google Scholar] [CrossRef]
- Sharma, A.; Gaind, R. Development of Loop-Mediated Isothermal Amplification Assay for Detection of Clinically Significant Members of Acinetobacter calcoaceticus-baumannii Complex and Associated Carbapenem Resistance. Front. Mol. Biosci. 2021, 8, 659256. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Segala, F.V.; Bavaro, D.F.; Di Gennaro, F.; Salvati, F.; Marotta, C.; Saracino, A.; Murri, R.; Fantoni, M. Impact of SARS-CoV-2 Epidemic on Antimicrobial Resistance: A Literature Review. Viruses 2021, 13, 2110. [Google Scholar] [CrossRef]
- Cornejo-Juárez, P.; Cevallos, M.A.; Castro-Jaimes, S.; Castillo-Ramírez, S.; Velázquez-Acosta, C.; Martínez-Oliva, D.; Pérez-Oseguera, A.; Rivera-Buendía, F.; Volkow-Fernández, P. High mortality in an outbreak of multidrug resistant Acinetobacter baumannii infection introduced to an oncological hospital by a patient transferred from a general hospital. PLoS ONE 2020, 15, e0234684. [Google Scholar] [CrossRef]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 10 August 2022).
- Qamar, M.U.; Lopes, B.S.; Hassan, B.; Khurshid, M.; Shafique, M.; Atif Nisar, M.; Mohsin, M.; Nawaz, Z.; Muzammil, S.; Aslam, B.; et al. The present danger of New Delhi metallo-β-lactamase: A threat to public health. Future Microbiol. 2020, 15, 1759–1778. [Google Scholar] [CrossRef]
- Mancilla-Rojano, J.; Ochoa, S.A.; Reyes-Grajeda, J.P.; Flores, V.; Medina-Contreras, O.; Espinosa-Mazariego, K.; Parra-Ortega, I.; Rosa-Zamboni, D.; Castellanos-Cruz, M.D.C.; Arellano-Galindo, J.; et al. Molecular Epidemiology of Acinetobacter calcoaceticus-Acinetobacter baumannii Complex Isolated From Children at the Hospital Infantil de México Federico Gómez. Front. Microbiol. 2020, 11, 576673. [Google Scholar] [CrossRef]
- Ejaz, H.; Ahmad, M.; Younas, S.; Junaid, K.; Abosalif, K.O.A.; Abdalla, A.E.; Alameen, A.A.M.; Elamir, M.Y.M.; Bukhari, S.N.A.; Ahmad, N.; et al. Molecular Epidemiology of Extensively-Drug Resistant Acinetobacter baumannii Sequence Type 2 Co-Harboring bla (NDM) and bla (OXA) From Clinical Origin. Infect. Drug Resist. 2021, 14, 1931–1939. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [Green Version]
- Toleman, M.A.; Spencer, J.; Jones, L.; Walsh, T.R. blaNDM-1 is a chimera likely constructed in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 2773–2776. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)—Structure and function. J. Enzym. Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Ayobami, O.; Willrich, N.; Harder, T.; Okeke, I.N.; Eckmanns, T.; Markwart, R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: A systematic review and meta-analysis. Emerg. Microbes Infect. 2019, 8, 1747–1759. [Google Scholar] [CrossRef]
- Begum, S.; Hasan, F.; Hussain, S.; Ali Shah, A. Prevalence of multi drug resistant Acinetobacter baumannii in the clinical samples from Tertiary Care Hospital in Islamabad, Pakistan. Pak. J. Med. Sci. 2013, 29, 1253–1258. [Google Scholar] [CrossRef]
- Yadav, S.K.; Bhujel, R.; Hamal, P.; Mishra, S.K.; Sharma, S.; Sherchand, J.B. Burden of Multidrug-Resistant Acinetobacter baumannii Infection in Hospitalized Patients in a Tertiary Care Hospital of Nepal. Infect. Drug Resist. 2020, 13, 725–732. [Google Scholar] [CrossRef]
- Khamari, B.; Lama, M.; Pachi Pulusu, C.; Biswal, A.P.; Lingamallu, S.M.; Mukkirla, B.S.; Sahoo, A.K.; Dash, H.S.N.; Sharda, R.; Kumar, P.; et al. Molecular Analyses of Biofilm-Producing Clinical Acinetobacter baumannii Isolates from a South Indian Tertiary Care Hospital. Med. Princ. Pract. 2020, 29, 580–587. [Google Scholar] [CrossRef]
- Said, D.; Willrich, N.; Ayobami, O.; Noll, I.; Eckmanns, T.; Markwart, R. The epidemiology of carbapenem resistance in Acinetobacter baumannii complex in Germany (2014–2018): An analysis of data from the national Antimicrobial Resistance Surveillance system. Antimicrob. Resist. Infect. Control 2021, 10, 45. [Google Scholar] [CrossRef]
- Alavi-Moghadam, M.; Miri, M.; Mokhtari, M.; Kouchek, M.; Goharani, R.; Sistanzad, M.; Safari, S.; Solouki, M. Incidence of imipenem-resistant Acinetobacter baumannii in a general intensive care unit (ICU). Casp. J. Intern. Med. 2014, 5, 186–187. [Google Scholar]
- Hasan, B.; Perveen, K.; Olsen, B.; Zahra, R. Emergence of carbapenem-resistant Acinetobacter baumannii in hospitals in Pakistan. J. Med. Microbiol. 2014, 63, 50–55. [Google Scholar] [CrossRef]
- Shah, A.A.; Ali, Y.; Maqbool, A.; Abbasi, S.A. Phenotypic detection of extended-spectrum beta-lactamase in multidrug-resistant acinetobacter baumannii isolated in Fauji Foundation Hospital Rawalpindi. J. Pak. Med. Assoc. 2021, 71, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.; Saleem, S.; Ahmad, I. High prevalence of carbapenem-resistant Acinetobacter baumannii associated respiratory tract infections in Pakistani hospitals. J. Pak. Med. Assoc. 2020, 70, 1630–1632. [Google Scholar] [CrossRef] [PubMed]
- Strateva, T.; Sirakov, I.; Stoeva, T.; Stratev, A.; Dimov, S.; Savov, E.; Mitov, I. Carbapenem-resistant Acinetobacter baumannii: Current status of the problem in four Bulgarian university hospitals (2014–2016). J. Glob. Antimicrob. Resist. 2019, 16, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, L. Molecular Epidemiology and Mechanisms of Carbapenem-Resistant Acinetobacter baumannii Isolates from ICU and Respiratory Department Patients of a Chinese University Hospital. Infect. Drug Resist. 2021, 14, 743–755. [Google Scholar] [CrossRef]
- Waseem, H.; Ali, J.; Sarwar, F.; Khan, A.; Rehman, H.S.U.; Choudri, M.; Arif, N.; Subhan, M.; Saleem, A.R.; Jamal, A.; et al. Assessment of knowledge and attitude trends towards antimicrobial resistance (AMR) among the community members, pharmacists/pharmacy owners and physicians in district Sialkot, Pakistan. Antimicrob. Resist. Infect. Control 2019, 8, 67. [Google Scholar] [CrossRef]
- Saleem, Z.; Godman, B.; Azhar, F.; Kalungia, A.C.; Fadare, J.; Opanga, S.; Markovic-Pekovic, V.; Hoxha, I.; Saeed, A.; Al-Gethamy, M.; et al. Progress on the national action plan of Pakistan on antimicrobial resistance (AMR): A narrative review and the implications. Expert Rev. Anti Infect. Ther. 2022, 20, 71–93. [Google Scholar] [CrossRef]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Juárez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padró Alonzo, L.A.; Rivera Reséndiz, A.; Muleiro Álvarez, M.; Vega López, E.N.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, S.; Saleem, S.; Waheed, A.; Alvi, A.A. Molecular detection of blaOXA-23 gene and blaOXA-51 gene in carbapenem resistant strains of Acinetobacter baumannii in patients with ventilator associated pneumonia at tertiary care hospitals. J. Pak. Med. Assoc. 2021, 71, 2576–2581. [Google Scholar] [CrossRef]
- Guo, J.; Li, C. Molecular epidemiology and decreased susceptibility to disinfectants in carbapenem-resistant Acinetobacter baumannii isolated from intensive care unit patients in central China. J. Infect. Public Health 2019, 12, 890–896. [Google Scholar] [CrossRef]
- Ning, N.-Z.; Liu, X.; Bao, C.-M.; Chen, S.-M.; Cui, E.-B.; Zhang, J.-L.; Huang, J.; Chen, F.-H.; Li, T.; Qu, F.; et al. Molecular epidemiology of blaOXA-23-producing carbapenem-resistant Acinetobacter baumannii in a single institution over a 65-month period in north China. BMC Infect. Dis. 2017, 17, 14. [Google Scholar] [CrossRef]
- Chang, Y.; Luan, G.; Xu, Y.; Wang, Y.; Shen, M.; Zhang, C.; Zheng, W.; Huang, J.; Yang, J.; Jia, X.; et al. Characterization of carbapenem-resistant Acinetobacter baumannii isolates in a Chinese teaching hospital. Front. Microbiol. 2015, 6, 910. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Marotta, C.; Amicone, M.; Bavaro, D.F.; Bernaudo, F.; Frisicale, E.M.; Kurotschka, P.K.; Mazzari, A.; Veronese, N.; Murri, R.; et al. Italian young doctors’ knowledge, attitudes and practices on antibiotic use and resistance: A national cross-sectional survey. J. Glob. Antimicrob. Resist. 2020, 23, 167–173. [Google Scholar] [CrossRef]
- Association, W.M. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 8 May 2022).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical and Laboratory Standard Institute (CLSI): Wayne, PA, USA, 2022; Volume CLSI Supplement M100. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standard Institute (CLSI): Wayne, PA, USA, 2018; Volume CLSI Supplement M100. [Google Scholar]
- Shamsizadeh, Z.; Nikaeen, M.; Esfahani, B.N.; Mirhoseini, S.H.; Hatamzadeh, M.; Hassanzadeh, A. Detection of antibiotic resistant Acinetobacter baumannii in various hospital environments: Potential sources for transmission of Acinetobacter infections. Environ. Health Prev. Med. 2017, 22, 44. [Google Scholar] [CrossRef]
- Ejaz, H.; Alzahrani, B.; Hamad, M.F.S.; Abosalif, K.O.A.; Junaid, K.; Abdalla, A.E.; Elamir, M.Y.M.; Aljaber, N.J.; Hamam, S.S.M.; Younas, S. Molecular Analysis of the Antibiotic Resistant NDM-1 Gene in Clinical Isolates of Enterobacteriaceae. Clin. Lab. 2020, 66, 409–417. [Google Scholar] [CrossRef]

| Clinical Characteristic | CRACB (n = 200) | CSACB (n = 255) | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (Years) | |||||
| Range | 1–95 | 1–87 | |||
| Mean (±standard deviation) | 43.5 ± 17.5 | 46.8 ± 19.2 | 0.05 | ||
| Gender | |||||
| Male | 112 | 56 | 152 | 59.6 | 0.43 |
| Female | 88 | 44 | 103 | 40.3 | |
| Male to female ratio | 1.27: 1 | 1.47:1 | |||
| Clinical Sample | |||||
| Pus | 79 | 35.4 | 102 | 40.9 | 0.91 |
| Tracheal secretions | 47 | 23.5 | 59 | 23.1 | 0.92 |
| Sputum | 21 | 10.5 | 33 | 12.9 | 0.42 |
| Blood | 20 | 10 | 27 | 10.5 | 0.83 |
| CSF | 17 | 8.5 | 11 | 4.3 | 0.46 |
| Urine | 11 | 5.5 | 16 | 6.2 | 0.72 |
| Bile fluid | 5 | 2.5 | 7 | 2.7 | 0.87 |
| Hospital Ward | |||||
| ICU | 77 | 38.5 | 93 | 36.4 | 0.65 |
| MWW | 37 | 18.5 | 46 | 18 | 0.89 |
| FMW | 36 | 18 | 41 | 16 | 0.58 |
| CCU | 30 | 15 | 39 | 15.2 | 0.93 |
| OPD | 12 | 6 | 21 | 8.2 | 0.36 |
| OT | 8 | 4 | 15 | 5.8 | 0.34 |
| Clinical Source | Hospital Ward | |||||
|---|---|---|---|---|---|---|
| ICU | MMW | FMW | CCU | OPD | OT | |
| Pus (n = 79; 39.5%) | 31 (49.3%) | 16 (20.2%) | 12 (15%) | 12 (15%) | 7 (8.8%) | 1 (7.9%) |
| Tracheal secretions (n = 47; 23.5%) | 19 (40.4%) | 7 (14.8%) | 11 (23.4%) | 8 (17%) | 2 (4.2%) | - |
| Sputum (n = 21; 10.5%) | 6 (28.5%) | 7 (33.3%) | - | 2 (9.5%) | 1 (4.7%) | 5 (23.8%) |
| Blood (n = 20; 10%) | 12 (60%) | - | 1 (5%) | 5 (25%) | - | 2 (10%) |
| CSF (n = 17; 8.5%) | 3 (17.6%) | 3 (17.6%) | 8 (47%) | 2 (11.7%) | 1 (5.8%) | - |
| Urine (n = 11; 5.5%) | 3 (27.2%) | 4 (36.3%) | 2 (18%) | - | 2 (18%) | - |
| Bile fluid (n = 5; 2.5%) | 3 (60%) | 1 (20%) | 1 (20%) | - | - | - |
| Target Gene | Primer Sequence (5′-3′) | Size (bp) |
|---|---|---|
| blaOXA23-F | GATCGGATTGGAGAACCAGA | 501 |
| blaOXA23-R | ATTTCTGACCGCATTTCCAT | |
| blaOXA24-F | GGTTAGTTGGCCCCCTTAAA | 246 |
| blaOXA24-R | AGTTGAGCGAAAAGGGGATT | |
| blaOXA51-F | TAATGCTTTGATCGGCCTTG | 353 |
| blaOXA51-R | TGGATTGCACTTCATCTTGG | |
| blaVIM-F | GATGGTGTTTGGTCGCATAA | 390 |
| blaVIM-R | CGAATGCGCAGCACCA | |
| blaNDM-F | GGTTTGGCGATCTGGTTTTC | 699 |
| blaNDM-R | CGGAATGGCTCATCACGATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejaz, H.; Qamar, M.U.; Junaid, K.; Younas, S.; Taj, Z.; Bukhari, S.N.A.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmad, N.; Saleem, Z.; et al. The Molecular Detection of Class B and Class D Carbapenemases in Clinical Strains of Acinetobacter calcoaceticus-baumannii Complex: The High Burden of Antibiotic Resistance and the Co-Existence of Carbapenemase Genes. Antibiotics 2022, 11, 1168. https://doi.org/10.3390/antibiotics11091168
Ejaz H, Qamar MU, Junaid K, Younas S, Taj Z, Bukhari SNA, Abdalla AE, Abosalif KOA, Ahmad N, Saleem Z, et al. The Molecular Detection of Class B and Class D Carbapenemases in Clinical Strains of Acinetobacter calcoaceticus-baumannii Complex: The High Burden of Antibiotic Resistance and the Co-Existence of Carbapenemase Genes. Antibiotics. 2022; 11(9):1168. https://doi.org/10.3390/antibiotics11091168
Chicago/Turabian StyleEjaz, Hasan, Muhammad Usman Qamar, Kashaf Junaid, Sonia Younas, Zeeshan Taj, Syed Nasir Abbas Bukhari, Abualgasim E. Abdalla, Khalid O. A. Abosalif, Naveed Ahmad, Zikria Saleem, and et al. 2022. "The Molecular Detection of Class B and Class D Carbapenemases in Clinical Strains of Acinetobacter calcoaceticus-baumannii Complex: The High Burden of Antibiotic Resistance and the Co-Existence of Carbapenemase Genes" Antibiotics 11, no. 9: 1168. https://doi.org/10.3390/antibiotics11091168
APA StyleEjaz, H., Qamar, M. U., Junaid, K., Younas, S., Taj, Z., Bukhari, S. N. A., Abdalla, A. E., Abosalif, K. O. A., Ahmad, N., Saleem, Z., & Salem, E. H. M. (2022). The Molecular Detection of Class B and Class D Carbapenemases in Clinical Strains of Acinetobacter calcoaceticus-baumannii Complex: The High Burden of Antibiotic Resistance and the Co-Existence of Carbapenemase Genes. Antibiotics, 11(9), 1168. https://doi.org/10.3390/antibiotics11091168










