Comparative In Vitro Activity of Ceftolozane/Tazobactam against Clinical Isolates of Pseudomonas aeruginosa and Enterobacterales from Five Latin American Countries
Abstract
1. Introduction
2. Results
2.1. Susceptibility Profile for Phenotypic Subsets
2.2. Activity of C/T and Comparator Agents against P. aeruginosa
2.3. Activity of C/T and Comparator Agents against Enterobacterales
3. Discussion
4. Materials and Methods
4.1. Sampling Sites and Organisms
4.2. Antimicrobial Susceptibility Testing
4.3. Phenotypic Subsets
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, K.; Horwich-Scholefield, S.; Epson, E. Carbapenem and cephalosporin resistance among enterobacteriaceae in healthcare- associated infections, california, usa1. Emerg. Infect. Dis. 2019, 25, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Sucher, A.J.; Chahine, E.B.; Cogan, P.; Fete, M. Ceftolozane/Tazobactam: A New Cephalosporin and β-Lactamase Inhibitor Combination. Ann. Pharmacother. 2015, 49, 1046–1056. [Google Scholar] [CrossRef]
- Moya, B.; Zamorano, L.; Juan, C.; Pérez, J.L.; Ge, Y.; Oliver, A. Activity of a new cephalosporin, CXA-101 (FR264205), against β-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob. Agents Chemother. 2010, 54, 1213–1217. [Google Scholar] [CrossRef]
- Murano, K.; Yamanaka, T.; Toda, A.; Ohki, H.; Okuda, S.; Kawabata, K.; Hatano, K.; Takeda, S.; Akamatsu, H.; Itoh, K.; et al. Structural requirements for the stability of novel cephalosporins to AmpC β-lactamase based on 3D-structure. Bioorganic Med. Chem. 2008, 16, 2261–2275. [Google Scholar] [CrossRef]
- Toda, A.; Ohki, H.; Yamanaka, T.; Murano, K.; Okuda, S.; Kawabata, K.; Hatano, K.; Matsuda, K.; Misumi, K.; Itoh, K.; et al. Synthesis and SAR of novel parenteral anti-pseudomonal cephalosporins: Discovery of FR264205. Bioorganic Med. Chem. Lett. 2008, 18, 4849–4852. [Google Scholar] [CrossRef]
- Jean, S.S.; Chang, Y.C.; Lin, W.C.; Lee, W.S.; Hsueh, P.R.; Hsu, C.W. Epidemiology, treatment, and prevention of nosocomial bacterial pneumonia. J. Clin. Med. 2020, 9, 275. [Google Scholar] [CrossRef]
- Hong, M.C.; Hsu, D.I.; Bounthavong, M. Ceftolozane/tazobactam: A novel antipseudomonal cephalosporin and β-lactamase-inhibitor combination. Infect. Drug Resist. 2013, 6, 215–223. [Google Scholar] [CrossRef]
- Giancola, S.E.; Mahoney, M.V.; Bias, T.E.; Hirsch, E.B. Critical evaluation of ceftolozane–tazobactam for complicated urinary tract and intra-abdominal infections. Ther. Clin. Risk Manag. 2016, 12, 787–797. [Google Scholar] [CrossRef][Green Version]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Shortridge, D.; Pfaller, M.A.; Streit, J.M.; Flamm, R.K. Antimicrobial activity of ceftolozane/tazobactam tested against contemporary (2015–2017) Pseudomonas aeruginosa isolates from a global surveillance programme. J. Glob. Antimicrob. Resist. 2020, 21, 60–64. [Google Scholar] [CrossRef]
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin Infect Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef]
- Wright, H.; Bonomo, R.A.; Paterson, D.L. New agents for the treatment of infections with Gram-negative bacteria: Restoring the miracle or false dawn? Clin. Microbiol. Infect. 2017, 23, 704–712. [Google Scholar] [CrossRef]
- Teo, J.Q.-M.; Lim, J.C.; Tang, C.Y.; Lee, S.J.Y.; Tan, S.H.; Sim, J.H.C.; Ong, R.T.-H.; Kwa, A.L.H. Ceftolozane/Tazobactam Resistance and Mechanisms in Carbapenem-Nonsusceptible Pseudomonas aeruginosa. mSphere 2021, 6, e01026-20. [Google Scholar] [CrossRef]
- Sader, H.S.; Farrell, D.J.; Castanheira, M.; Flamm, R.K.; Jones, R.N. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011-12). J. Antimicrob. Chemother. 2014, 69, 2713–2722. [Google Scholar] [CrossRef]
- Walkty, A.; Karlowsky, J.A.; Adam, H.; Baxter, M.; Lagacé-Wiens, P.; Hoban, D.J.; Zhanel, G.G. In vitro activity of ceftolozane-tazobactam against Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals in the CANWARD study, 2007 to 2012. Antimicrob. Agents Chemother. 2013, 57, 5707–5709. [Google Scholar] [CrossRef]
- Farrell, D.J.; Sader, H.S.; Flamm, R.K.; Jones, R.N. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalised patients with pneumonia in US and European medical centres (2012). Int. J. Antimicrob. Agents 2014, 43, 533–539. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Shortridge, D.; Sader, H.S.; Gales, A.; Castanheira, M.; Flamm, R.K. Ceftolozane-tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing healthcare-associated infections in Latin America: Report from an antimicrobial surveillance program (2013–2015). Braz. J. Infect. Dis. 2017, 21, 627–637. [Google Scholar] [CrossRef]
- García-Betancur, J.C.; Appel, T.M.; Esparza, G.; Gales, A.C.; Levy-Hara, G.; Cornistein, W.; Vega, S.; Nuñez, D.; Cuellar, L.; Bavestrello, L.; et al. Update on the epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev. Anti. Infect. Ther. 2021, 19, 197–213. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters-EUCAST. Available online: http://www.eucast.org (accessed on 13 March 2022).
- Fernández-Esgueva, M.; López-Calleja, A.I.; Mulet, X.; Fraile-Ribot, P.A.; Cabot, G.; Huarte, R.; Rezusta, A.; Oliver, A. Characterization of AmpC β-lactamase mutations of extensively drug-resistant Pseudomonas aeruginosa isolates that develop resistance to ceftolozane/tazobactam during therapy. Enferm. Infecc. Microbiol. Clin. 2020, 38, 474–478. [Google Scholar] [CrossRef]
- Fournier, D.; Carrière, R.; Bour, M.; Grisot, E.; Triponney, P.; Muller, C.; Lemoine, J.; Jeannot, K.; Plésiat, P.; GERPA Study Group. Mechanisms of resistance to ceftolozane/tazobactam in pseudomonas aeruginosa: Results of the GERPA multicenter study. Antimicrob. Agents Chemother. 2021, 65, e01117-20. [Google Scholar] [CrossRef]
- Wi, Y.M.; Greenwood-Quaintance, K.E.; Schuetz, A.N.; Ko, K.S.; Peck, K.R.; Song, J.H.; Patel, R. Activity of Ceftolozane-Tazobactam against Carbapenem- Resistant, Non-Carbapenemase-Producing Pseudomonas aeruginosa and Associated Resistance Mechanisms. Antimicrob. Agents Chemother. 2018, 62, e01970-17. [Google Scholar] [CrossRef]
- Hirsch, E.B.; Brigman, H.V.; Zucchi, P.C.; Chen, A.; Anderson, J.C.; Eliopoulos, G.M.; Cheung, N.; Gilbertsen, A.; Hunter, R.C.; Emery, C.L.; et al. Ceftolozane-tazobactam and ceftazidime-avibactam activity against β-lactam-resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Enterobacterales clinical isolates from U.S. medical centres. J. Glob. Antimicrob. Resist. 2020, 22, 689–694. [Google Scholar] [CrossRef]
- Shiju, K.S.; Pallam, G.; Mandal, J.; Jindal, B.; Kumaravel, S. Use of fosfomycin combination therapy to treat multidrug-resistant urinary tract infection among paediatric surgical patients–a tertiary care centre experience. Access Microbiol. 2020, 2, 10–12. [Google Scholar] [CrossRef]
- Lob, S.H.; DePestel, D.D.; DeRyke, C.A.; Kazmierczak, K.M.; Young, K.; Motyl, M.R.; Sahm, D.F. Ceftolozane/Tazobactam and Imipenem/Relebactam Cross-Susceptibility among Clinical Isolates of Pseudomonas aeruginosa from Patients with Respiratory Tract Infections in ICU and Non-ICU Wards-SMART United States 2017–2019. Open Forum Infect. Dis. 2021, 8, ofab320. [Google Scholar] [CrossRef]
- Casellas, J.M. Resistencia a los antibacterianos en América Latina: Consecuencias para la infectología. Rev. Panam. Salud Publica/Pan Am. J. Public Heal. 2011, 30, 519–528. [Google Scholar]
- Moreno-Switt, A.I.; Rivera, D.; Caipo, M.L.; Nowell, D.C.; Adell, A.D. Antimicrobial Resistance in Water in Latin America and the Caribbean: Available Research and Gaps. Front. Vet. Sci. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Rojas, L.J.; Weinstock, G.M.; De La Cadena, E.; Diaz, L.; Rios, R.; Hanson, B.M.; Brown, J.S.; Vats, P.; Phillips, D.S.; Nguyen, H.; et al. An analysis of the epidemic of klebsiella pneumoniae carbapenemase-producing k. pneumoniae: Convergence of two evolutionary mechanisms creates the ‘perfect storm.’ J. Infect. Dis. 2018, 217, 82–92. [Google Scholar] [CrossRef]
- Correa, A.; Montealegre, M.C.; Mojica, M.F.; Maya, J.J.; Rojas, L.J.; De La Cadena, E.P.; Ruiz, S.J.; Recalde, M.; Rosso, F.; Quinn, J.P.; et al. First report of a Pseudomonas aeruginosa isolate coharboring KPC and VIM carbapenemases. Antimicrob. Agents Chemother. 2012, 56, 5422–5423. [Google Scholar] [CrossRef] [PubMed]
- Escandón-Vargas, K.; Reyes, S.; Gutiérrez, S.; Villegas, M.V. The epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev. Anti. Infect. Ther. 2017, 15, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Losito, A.R.; Raffaelli, F.; del Giacomo, P.; Tumbarello, M. New Drugs for the Treatment of Pseudomonas aeruginosa Infections with Limited Treatment Options: A Narrative Review. Antibiotics 2022, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Coyne, A.J.K.; el Ghali, A.; Holger, D.; Rebold, N.; Rybak, M.J. Therapeutic Strategies for Emerging Multidrug-Resistant Pseudomonas aeruginosa. Infect. Dis. Ther. 2022, 11, 661–682. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Shortridge, D.; Sader, H.S.; Castanheira, M.; Flamm, R.K. Ceftolozane/tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing healthcare-associated infections in the Asia-Pacific region (minus China, Australia and New Zealand): Report from an Antimicrobial Surveillance Pr. Int. J. Antimicrob. Agents 2018, 51, 181–189. [Google Scholar] [CrossRef]
- Livermore, D.M.; Mushtaq, S.; Meunier, D.; Hopkins, K.L.; Hill, R.; Adkin, R.; Chaudhry, A.; Pike, R.; Staves, P.; Woodford, N.; et al. Activity of ceftolozane/tazobactam against surveillance and ‘problem’ Enterobacteriaceae, Pseudomonas Aeruginosa and non-fermenters from the British Isles. J. Antimicrob. Chemother. 2017, 72, 2278–2289. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Young, K.; Motyl, M.R.; Sahm, D.F. Activity of ceftolozane/tazobactam against Gram-negative isolates from patients with lower respiratory tract infections–SMART United States 2018–2019. BMC Microbiol 2021, 21, 4–11. [Google Scholar] [CrossRef]
- Yin, D.; Wu, S.; Yang, Y.; Shi, Q.; Dong, D.; Zhu, D.; Hu, F. Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the In Vitro Activities of Ceftazidime-Avibactam and Ceftolozane-Tazobactam against Clinical Isolates of Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e02431-18. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Shortridge, D.; Sader, H.S.; Flamm, R.K.; Castanheira, M. Ceftolozane–tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing healthcare-associated infections in Australia and New Zealand: Report from an Antimicrobial Surveillance Program (2013–2015). J. Glob. Antimicrob. Resist. 2017, 10, 186–194. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Bassetti, M.; Duncan, L.R.; Castanheira, M. Ceftolozane/tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing urinary tract and intraabdominal infections in Europe: Report from an antimicrobial surveillance programme (2012-15). J. Antimicrob. Chemother. 2017, 72, 1386–1395. [Google Scholar] [CrossRef]
- Castanheira, M.; Mills, J.C.; Farrell, D.J.; Jones, R.N. Mutation-Driven β-Lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob. Agents Chemother. 2014, 58, 6844–6850. [Google Scholar] [CrossRef]
- Grupper, M.; Sutherland, C.; Nicolau, D.P. Multicenter Evaluation of CeftazidimeAvibactam and Ceftolozane-Tazobactam Inhibitory Activity against MeropenemNonsusceptible Pseudomonas aeruginosa from Blood, Respiratory Tract, and Wounds. Antimicrob. Agents Chemother. 2017, 61, e00875-17. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Kim, T.J., Jr.; Lewis, J.S., II; Limbago, B.; Bobenchik, A.M.; Mathers, A.J.; Campeau, S.; Mazzulli, T.; Cullen, S.K.; Satlin, M.; et al. Performance Standards for Antimicrobial Testing; CLSI Supplement M100, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Pfennigwerth, N.; Kaminski, A.; Korte-Berwanger, M.; Pfeifer, Y.; Simon, M.; Werner, G.; Jantsch, J.; Marlinghaus, L.; Gatermann, S.G. Evaluation of six commercial products for colistin susceptibility testing in Enterobacterales. Clin. Microbiol. Infect. 2019, 25, 1385–1389. [Google Scholar] [CrossRef]
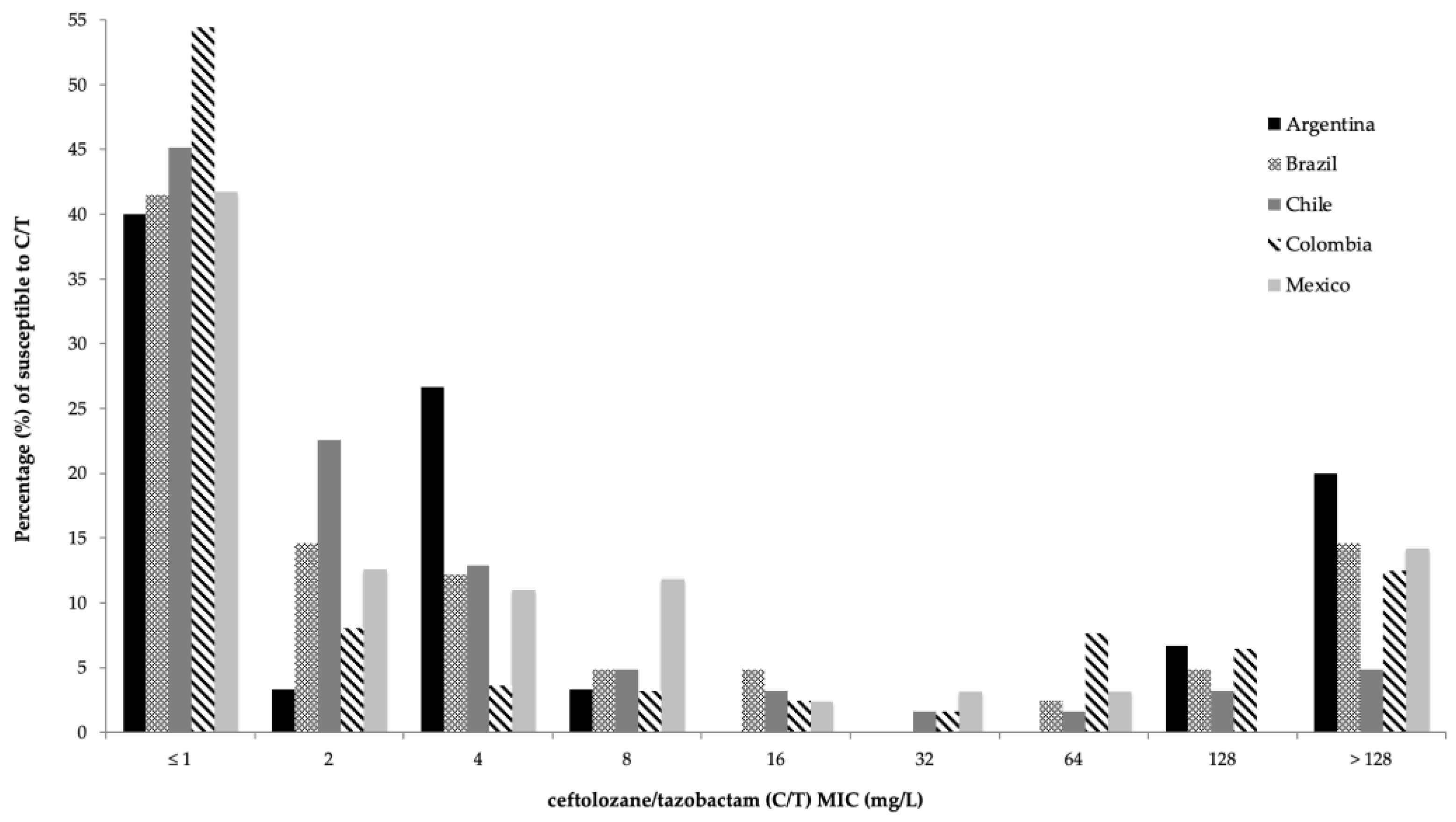
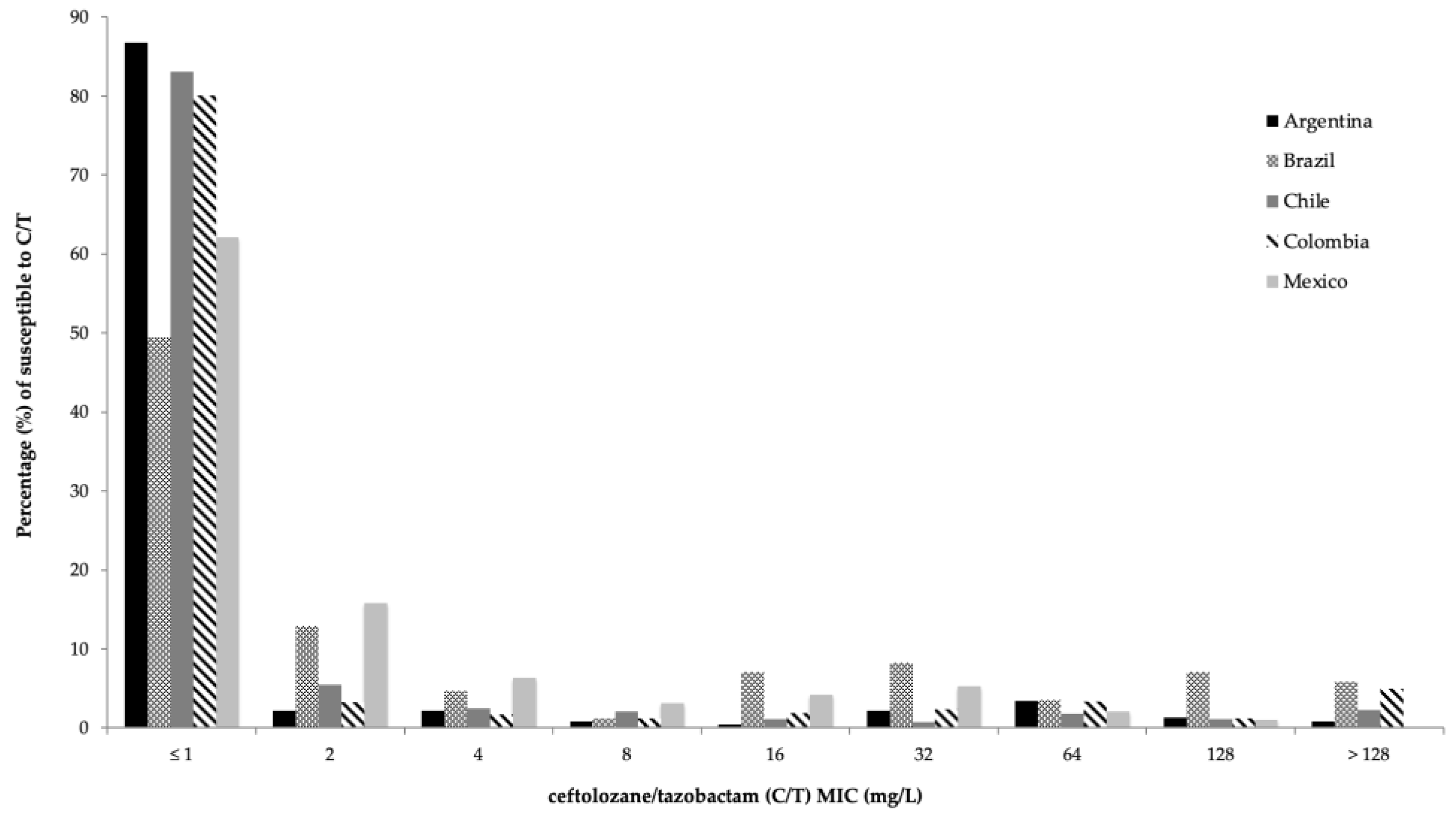
| Percentage of Susceptibility (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organism | Number of Isolates | C/T | CRO | CTX | CAZ | TZP | ETP | IMI | MEM | DOR | TGC | FOS |
| Pseudomonas aeruginosa | 508 | 68.1 | NA | NA | 53.1 | 54.5 | NA | 10.8 | 36.4 | 38.6 | NA | 72.2 ** |
| piperacillin/tazobactam-NS | 231 | 36.8 | NA | NA | 10.9 | - | NA | 3.46 | 10.8 | 13.4 | NA | 62.8 ** |
| ceftazidime-NS | 238 | 35.7 | NA | NA | - | 13.4 | NA | 14.7 | 21.0 | 26.1 | NA | 40.8 ** |
| meropenem-NS | 272 | 46.7 | NA | NA | 30.9 | 29.8 | NA | 1.1 | - | 12.5 | NA | 64.3 ** |
| Escherichia coli | 1409 | 93.2 | 60.3 | 60.9 | 71.8 | 90.3 | 90.3 | 94.3 | 95.5 | 95.7 | 95.6 | 94.8 |
| ESBL non-CRE phenotype * | 425 | 95.3 | - | 3.5 | 33.4 | 90.3 | - | 99.5 | 100.0 | 100.0 | 98.1 | 90.8 |
| Klebsiella pneumoniae | 610 | 68.7 | 44.9 | 46.9 | 50.0 | 59.2 | 70.0 | 71.0 | 76.6 | 77.7 | 84.4 | 92.5 |
| ESBL non-CRE phenotype * | 153 | 79.7 | - | 8.5 | 24.2 | 52.3 | - | 97.4 | 99.3 | 100.0 | 96.1 | 94.8 |
| Serratia marcescens | 91 | 71.4 | 46.2 | 42.9 | 62.6 | 69.2 | 70.3 | 73.6 | 78.0 | 76.9 | 80.2 | 94.5 |
| ertapenem-susceptible | 64 | 92.2 | 64.0 | 59.4 | 85.9 | 90.6 | - | 93.7 | 100.0 | 98.4 | 90.6 | 100.0 |
| Enterobacter cloacae complex | 112 | 62.5 | 30.4 | 30.4 | 42.9 | 51.8 | 63.4 | 71.4 | 79.5 | 80.4 | 89.3 | 79.5 |
| ertapenem-susceptible | 71 | 84.5 | 45.1 | 45.1 | 63.4 | 79.4 | - | 90.1 | 100.0 | 98.59 | 94.4 | 83.1 |
| Klebsiella aerogenes | 30 | 73.3 | 56.7 | 56.7 | 66.7 | 83.3 | 73.3 | 83.3 | 83.3 | 83.33 | 90.0 | 96.6 |
| ertapenem-susceptible | 22 | 90.9 | 77.3 | 72.7 | 86.4 | 95.4 | - | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Percentage of Susceptibility (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organism | Number of Isolates | C/T | CAZ | TZP | IMI | MEM | DOR | FOS * | |
| Pseudomonas aeruginosa | 30 | 70.0 | 33.3 | 40.0 | 30.0 | 40.0 | 53.3 | 53.3 | |
| piperacillin/tazobactam-NS | 18 | 50.0 | 5.6 | - | 5.6 | 5.6 | 22.2 | 38.9 | |
| Argentina | meropenem-susceptible | 12 | 66.7 | 91.7 | 91.7 | 75.0 | 100.0 | 100.0 | 75.0 |
| meropenem-NS | 18 | 50.0 | 11.1 | 5.6 | 0.0 | - | 22.2 | 38.9 | |
| ceftazidime-NS | 20 | 55.0 | - | 15.0 | 20.0 | 15.0 | 35.0 | 45.0 | |
| Pseudomonas aeruginosa | 41 | 68.3 | 49.6 | 46.3 | 12.2 | 26.8 | 29.3 | 73.2 | |
| piperacillin/tazobactam-NS | 22 | 50.0 | 40.9 | - | 13.6 | 13.6 | 9.1 | 40.9 | |
| Brazil | meropenem-susceptible | 11 | 100.0 | 90.9 | 72.7 | 36.4 | 100.0 | 72.7 | 72.7 |
| meropenem-NS | 30 | 56.7 | 53.3 | 36.7 | 3.3 | - | 13.3 | 73.3 | |
| ceftazidime-NS | 15 | 33.3 | - | 13.3 | 6.7 | 6.7 | 6.7 | 66.7 | |
| Pseudomonas aeruginosa | 62 | 80.6 | 56.5 | 58.1 | 41.9 | 62.9 | 66.1 | 83.9 | |
| piperacillin/tazobactam-NS | 26 | 61.5 | 11.5 | - | 26.9 | 30.8 | 34.6 | 80.8 | |
| Chile | meropenem-susceptible | 39 | 92.3 | 76.9 | 79.5 | 64.1 | 100.0 | 97.4 | 84.6 |
| meropenem-NS | 23 | 60.9 | 21.7 | 21.7 | 4.3 | - | 13.0 | 82.6 | |
| ceftazidime-NS | 27 | 55.6 | - | 14.8 | 25.9 | 33.3 | 37.0 | 85.2 | |
| Pseudomonas aeruginosa | 248 | 66.1 | 54.8 | 58.9 | 30.2 | 47.2 | 52.4 | 80.2 | |
| piperacillin/tazobactam-NS | 102 | 23.5 | 7.8 | - | 4.9 | 9.8 | 15.7 | 76.5 | |
| Colombia | meropenem-susceptible | 117 | 96.6 | 97.5 | 103.3 | 71.5 | 100.0 | 70.8 | 65.1 |
| meropenem-NS | 131 | 38.9 | 26.7 | 29.8 | 0.8 | - | 13.7 | 73.3 | |
| ceftazidime-NS | 112 | 26.8 | - | 16.1 | 11.6 | 14.3 | 21.4 | 75.9 | |
| Pseudomonas aeruginosa | 127 | 64.4 | 49.6 | 50.4 | 25.2 | 44.9 | 42.5 | 55.1 | |
| piperacillin/tazobactam-NS | 63 | 39.7 | 6.3 | - | 14.3 | 28.6 | 27.0 | 39.7 | |
| Mexico | meropenem-susceptible | 57 | 84.2 | 64.9 | 45.6 | 56.1 | 100.0 | 86.0 | 68.4 |
| meropenem-NS | 70 | 50.0 | 37.1 | 35.7 | 0.0 | - | 7.1 | 44.3 | |
| ceftazidime-NS | 64 | 37.5 | - | 3.1 | 15.6 | 31.3 | 31.3 | 40.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Betancur, J.C.; De La Cadena, E.; Mojica, M.F.; Hernández-Gómez, C.; Correa, A.; Radice, M.A.; Castañeda-Méndez, P.; Jaime-Villalon, D.A.; Gales, A.C.; Munita, J.M.; et al. Comparative In Vitro Activity of Ceftolozane/Tazobactam against Clinical Isolates of Pseudomonas aeruginosa and Enterobacterales from Five Latin American Countries. Antibiotics 2022, 11, 1101. https://doi.org/10.3390/antibiotics11081101
García-Betancur JC, De La Cadena E, Mojica MF, Hernández-Gómez C, Correa A, Radice MA, Castañeda-Méndez P, Jaime-Villalon DA, Gales AC, Munita JM, et al. Comparative In Vitro Activity of Ceftolozane/Tazobactam against Clinical Isolates of Pseudomonas aeruginosa and Enterobacterales from Five Latin American Countries. Antibiotics. 2022; 11(8):1101. https://doi.org/10.3390/antibiotics11081101
Chicago/Turabian StyleGarcía-Betancur, Juan Carlos, Elsa De La Cadena, María F. Mojica, Cristhian Hernández-Gómez, Adriana Correa, Marcela A. Radice, Paulo Castañeda-Méndez, Diego A. Jaime-Villalon, Ana C. Gales, José M. Munita, and et al. 2022. "Comparative In Vitro Activity of Ceftolozane/Tazobactam against Clinical Isolates of Pseudomonas aeruginosa and Enterobacterales from Five Latin American Countries" Antibiotics 11, no. 8: 1101. https://doi.org/10.3390/antibiotics11081101
APA StyleGarcía-Betancur, J. C., De La Cadena, E., Mojica, M. F., Hernández-Gómez, C., Correa, A., Radice, M. A., Castañeda-Méndez, P., Jaime-Villalon, D. A., Gales, A. C., Munita, J. M., & Villegas, M. V. (2022). Comparative In Vitro Activity of Ceftolozane/Tazobactam against Clinical Isolates of Pseudomonas aeruginosa and Enterobacterales from Five Latin American Countries. Antibiotics, 11(8), 1101. https://doi.org/10.3390/antibiotics11081101






