Abstract
Analytical methods for the drug substance and degradation products (DPs) are validated by performing forced degradation studies. Forced degradation studies of Velpatasvir (VEL) drug substance and Velpatasvir copovidone solid dispersion (VEL-CSD) were performed under the stressed alkaline, acidic, oxidative and thermal conditions according to ICH guidelines ICH Q1A (R2). VEL is labile to degrade in stressed alkaline, acidic, and oxidative conditions. It is also photolabile and degraded during photostability studies as described by ICH Q1B, and showed no degradation on exposure to extreme temperature when protected from light. A sensitive stability indicating HPLC-UV method was developed and validated for the separation of VEL and eight DPs. The DPs of VEL are separated using gradient elution of mobile phase containing 0.05% Trifluoroacetic acid (TFA) and methanol over symmetry analytical column C18 (250 mm × 4.6 mm, 5 µm) with a flow rate of 0.8 mL min−1. Simultaneous detection of all DPs and VEL was performed on UV detector at 305 nm. The performance parameters like precision, specificity and linearity of the method were validated using reference standards as prescribed by ICHQ2 (R1). Limits of quantification and limits of detection were determined from calibration curve using the expression 10δ/slope and 3δ/slope respectively. The proposed method is stability-indicating and effectively applied to the analysis of process impurities and DPs in VEL drug substance and VEL-CSD.
1. Introduction
Hepatitis C is considerable global health problem and the infected population of Hepatitis C Virus (HCV) is more than 170 to 200 million worldwide [1,2,3]. Velpatasvir (VEL) is a new molecule and a novel second-generation direct acting antiviral (DAA) for the inhibition of HCV. VEL has potent activity against all genotypes 1–6 of HCV and low toxicity EC50 values (6–130 pM). VEL 100 mg fixed dose combination tablets with Sofosbuvir 400 mg received marketing authorization from USFDA in June 2016 [4,5,6,7]. VEL is chemically carbamic acid with molecular formula C49H54N8O8 and structural formula shown in Figure 1A. It is a white to off-white, crystalline, non-hygroscopic, solid and belongs to BCS Class-IV having low pH dependent solubility and low permeability. To enhance the bioavailability of drug product, the pure drug of VEL is processed to copovidone solid dispersion and then used in pharmaceutical formulations. The bulk material of VEL is therefore supplied in the form of VEL-CSD containing VEL 50% w/w instead of pure dug substance [8,9]. Hence, the availability of stability indicating analytical method and forced degradation studies of VEL-CSD is the matter of concern.
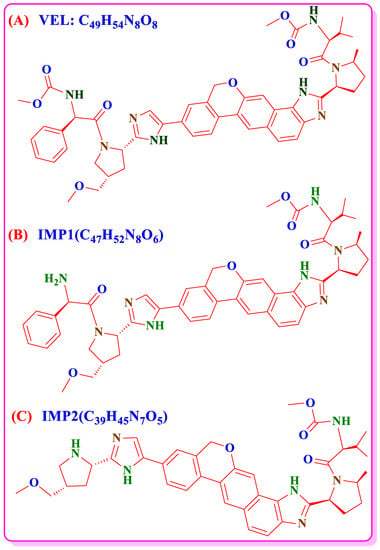
Figure 1.
Chemical structures of (A) VEL, (B) process IMP-1 and (C) process IMP-2.
The adverse effects of impurities and DPs due to manufacturing process, and uncontrolled storage conditions of intermediate and finished pharmaceutical products are crucial. There are strict guidelines to enhance the therapeutic effects and limit the adverse effects due to process impurities (IMPs) and DPs in pharmaceutical preparations [10,11]. The chemical stability of the drug substance is evaluated under the influence of various stressed environmental conditions to study the new molecules and its DPs. An efficient and validated stability indicating method is used to separate and quantify the impurities and DPs in the drug substance and pharmaceutical preparations [12,13].
Upon the literature survey, VEL has two major IMPs (Figure 1B,C) and eight DPs (Figure 2A–H) and no stability indicating HPLCUV method is available to quantify these degradation products in VEL-CSD. The official pharmacopeial monograph is also not available for the analysis of drug substance and pharmaceutical formulations. Very few analytical methods, focusing on simultaneous analysis of VEL and Sofosbuvir combined formulation, are reported in literature [13,14,15,16,17,18] Stability indicating methods for the analysis of VEL pure drug substance and degradation studies are also reported [19,20,21,22,23] but have limited information and discrepancies with published literature of FDA and MHRA [24,25]. As per MHRA public assessment report, VEL is photolabile, however it is reported stable in forced degradation studies published on UPLC [26]. The published methods exhibit certain disadvantages because of low sensitivity and are not applicable to the analysis of IMPs and degradation products in VEL-CSD. Similarly, the reported methods published for the analysis of DPs in VEL drug substance are not properly validated using reference standards of impurities and DPs. To the best of our knowledge, no study is reported till date to describe the forced degradation studies and validated stability indicating HPLC-UV method for the IMPs and DPs of VEL-CSD. For the purpose, we performed forced degradation studies of VEL-CSD as per the published guidelines of ICHQ1A (R2) [27,28]. Photostability testing [29] and all DPs were separated using simple and sensitive HPLC-UV method. The analytical method was accurately authenticated according to the guidelines ICHQ2 (R1) [30,31] using reference standards of IMPs and DPs. The proposed method is simple, precise and accurate and can easily be used for routine analysis in pharmaceutical testing and research laboratories.
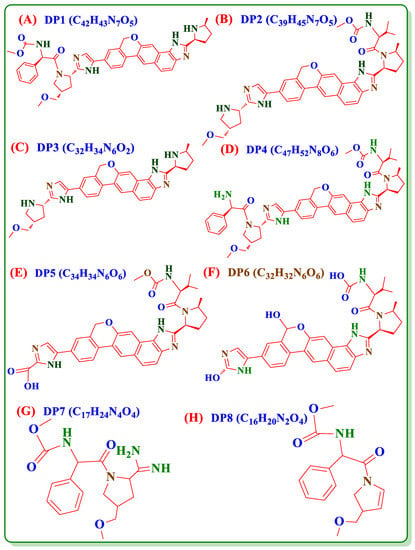
Figure 2.
Chemical structures of degradation products of VEL (A) DP1, (B) DP2, (C) DP3, (D) DP4, (E) DP5, (F) DP6, (G) DP7 and (H) DP8.
2. Experimental
2.1. Materials and Chemicals
Reference standard of VEL 99.6% and VEL-CSD containing VEL 49.68% was provided by Anhui Yellen Pharmaceutical Co., Ltd., Hefei, China. Reference standards of IMPs i.e., IMP1 (C47H52N8O6) and IMP2 (C39H45N7O5), DPS i.e., DP1 (C42H43N7O5), DP2 (C39H45N7O5), DP3 (C32H34N6O2), DP4 (C47H52N8O6), DP5 (C34H34N6O6), DP6 (C32H32N6O6), DP7 (C17H24N4O4) and DP8 (C16H20N2O4) all purity < 84.0% was provided by Nantong Chanyoo Pharmatech Co. Ltd. (Nantong, China). Methanol HPLC grade, trifluoroacetic acid, sodium hydroxide, hydrochloric acid (37% w/v) and hydrogen peroxide solution (30% w/v) were purchased from Sigma-Aldrich (Darmstadt, Germany). Known excipients copovidone, croscarmellose sodium, microcrystalline cellulose, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol and purified talc were provided by Genome Pharmaceuticals Pvt. Ltd. (Rawalpindi, Pakistan).
2.2. Instrumentation
Cecil low pressure quaternary gradient HPLC system comprised Adept CE-4104 pump, Adept CE 4200 variable wavelength UV detector, CE 4040 Solvent Degasser and CE4800-100 auto sampler by Cecil Instruments Limited, Peterborough, UK. The system was controlled by power stream chromatography manager version 4.2. Symmetry analytical column 250 mm × 4.6 mm, 5 µm, packing C18 (Waters, Milford, MA, USA) was used for analysis. Other equipment including UV-Visible spectrophotometer Shimadzu UV-2450, controlled by UV probe version 2.42, Climatic chamber for thermal and photo stability studies with florescent light of 1.2 milli lux h m−2 and UV light of 200 watt h m−2 (China), analytical balance AT-201 Mettler Toledo (Greifensee, Switzerland), ultrasonic bath SONOREX (Bandelin, Bandelin, Germany), Millipore vacuum filtration assembly and Milli-Q water distillation system (Millipore, Burlington, MA, USA) were also used in the studies.
2.3. Reference and Sample Stock Solutions
Stock solution of VEL reference standard 0.1 mg mL−1 was prepared by dissolving accurately 10.0 mg VEL in 100 mL methanol. The sample solution was prepared taking 20.3 mg VEL-CSD equivalent to 10 mg of VEL and dissolved in 100 mL methanol. The stock solutions 0.1 mg mL−1 of each impurity and DPs were also prepared in the same way taking equivalent quantity of 5 mg each and dissolved in 50 mL methanol separately. All the stock solutions were stored in refrigerator 2–8 °C in amber colored volumetric flask protected from light. The stock solutions were further diluted 10 µg mL−1 VEL and 0.01 µg mL−1 (0.1%) each impurity in method validation studies.
2.4. Forced Degradation Studies
Solutions of drug substance and copovidone solid dispersion equivalent to VEL 0.5 mg mL−1 subjected to hydrolysis in acidic and alkaline conditions using 5 M HCl and 1 M sodium hydroxide solutions [30]. Acidic, alkaline and neutral solutions were refluxed separately for 4 h and 8 h and the hydrolytic DPs were determined using the same validated procedure (Figure 3). The solutions of drug substance VEL and VEL-CSD having same concentration were kept in 10% H2O2 at room temperature and the extent of degradation were checked after 4 h and 8 h. Following ICH IB guidelines [28,29], VEL drug substance and VEL-CSD were exposed to 200 W hm2 UV light (320 to 400 nm) and 1.2 milli lux h m−2 visible light (400 to 800 nm) at 40 °C using photo stability chamber. The effect of heat and humidity on the drug substance was studied, exposing the VEL drug substance and VEL-CSD to dry heat 105 °C ± 5 °C and 80 °C/75 ± 5% RH for 24 h. The stability of solutions stored at room temperature (15–25 °C) and refrigerator (02–08 °C) for 7 days was also established, comparing the results with the results of reference standards’ freshly prepared solutions.
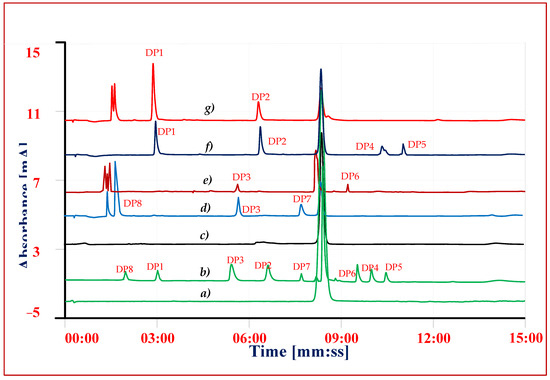
Figure 3.
Chromatograms of forced degradation studies and reference standards (a) VEL reference standard (b) VEL composite reference standards, (c) VEL-CSD thermal study, (d) Acid degradation (e) Alkaline degradation, (f) Oxidative degradation (g) Photolytic degradation.
2.5. Optimization of Chromatographic Conditions
The chromatographic conditions for separation of impurities and DPs from VEL were optimized using gradient chromatographic system. First of all, the detection wavelength was studied on UV-Visible spectrophotometer and all the solutions 10 µg mL−1 were scanned from 200 nm to 400 nm. As the chemical structures of all impurities are closely similar to that of VEL, so the similar UV absorbance spectrum and absorption maxima 305 ± 3 nm were obtained (Figure 4). The optimum wavelength 305 nm was selected as suitable wavelength for simultaneous analysis of all DPs and IMPs. Now, the selection of the chromatographic conditions appropriate for separation of DPs and impurities having similar structure was a big challenge. The performance of different stationary phases i.e., Octyl Silica C8 (Waters), Octadecyl Silica C18 (Waters) and phenyl-hexyl (Accucore) columns was compared using same mobile phase with same gradient programs. The separation of composite reference solution containing all IMPs and DPs were studied using combination of different buffers ranging from pH 1.2 to 8.0 with methanol or acetonitrile as organic modifiers. Using C8 analytical column, the resolution of ingredients was quite difficult, and the impurities were not resolved from VEL at any pH described above. The resolution of all ingredients at acidic pH over C18 and phenyl-hexyl analytical columns was good, however the retention time of VEL and two DPs was more than 20 min. To achieve good resolution of each component in minimum run time, C18 analytical column was selected for further optimization. The gradient program for elution of acidic mobile phase A (0.1% TFA) with B (menthol) was adjusted to get optimal resolution in run time less than 15 min. Good separation was achieved (Figure 5 and Figure 6) using gradient elution: 0–2 min: 10% B, 2–6 min: 10–50% B, 6–12 min: 50–90% B, 12–13 min: 90–10% B, and 13–15 min: 10% B at a flow rate of 0.8 mL/min over C18 symmetry analytical column equilibrated at 30 °C. To validate the optimized conditions, all the performance parameters of the analytical method i.e., accuracy and recovery, precisions and repeatability, specificity and linearity were evaluated according to the prescribed limits and criteria of ICHQ1A (R2) guidelines.
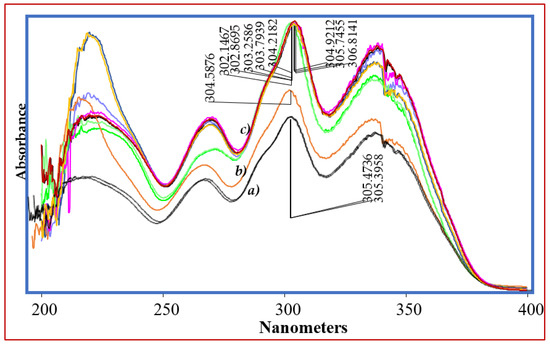
Figure 4.
UV absorbance spectrum of VEL, IMPs and DPs.
3. Results and Discussion
3.1. Method Validation
Replicate injections (n = 5) of the reference composite solution containing VEL 10 µg mL−1 and impurities and degradation products each 0.1 µg mL−1 were analyzed on the optimized chromatographic conditions. Values for the system suitability parameters i.e., relative standard deviation (RSD), theoretical plates (N), symmetry factor (As) and retention factor (Ko) were calculated from the resultant chromatograms. The results of RSD values were crosschecked against the acceptable limits for the drug substance (±2%) and impurities (±10.0%). All the peaks were symmetrical with the value of As near to 1.0 and the resolution of each component calculated with Relative Retention Time (tRR). The values theoretical plates for VEL were more than 3000 and Ko was more than 2.0 (Table 1).

Table 1.
System suitability studies.
3.2. Recovery Studies
The recovery and accuracy were checked (n = 5) for six concentration levels ranging from 20 to 120% of the sample solutions of VEL 0.1 mg mL−1 (2.0, 4.0, 6.0, 8.0, 10.0 and 12.0 µg mL−1). To assure the detection and reporting threshold as prescribed by ICH guidelines Q3B (R2), each solution was spiked with the level of 0.05% of individual impurity and degradation product. VEL was recovered from the sample solution 98.0 ± 2% and the recovery for each impurity and DP was ±20% of the applied concentration (Table 2). The RSD value for recovery of VEL and each impurity was also within the acceptable limits ±2% and ±10% respectively.

Table 2.
Results of Recovery and Accuracy.
3.3. Specificity
To evaluate the interference of other ingredients or excipients on the response and recovery of VEL and degrading products, the specificity of the method was checked. The recovery of VEL and degrading products was evaluated in the presence of excipients by adding an appropriate level of known excipients to the spiked composite solution. The response of the composite samples (n = 5) was compared with the response and individual reference solutions. It is evident from the chromatogram shown in (Figure 5) that each ingredient has its specific retention time, and there was no interference of other ingredients on the response of drug substance or any impurity.
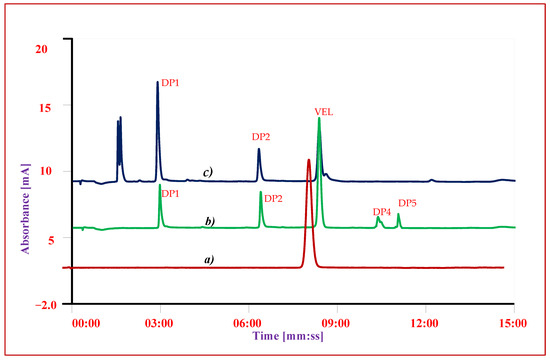
Figure 5.
Chromatogram of VEL-CSD (a) Reference standard of VEL (b) Oxidative stress condition (c) Photolytic stressed studies.
3.4. Precision
To assure the repeatability of results, replicates (n = 6) of impurity spiked three concentration levels (low, medium and high) corresponding to 20, 60 and 120% of the sample solutions of VEL 0.1 mg mL−1 were analyzed. For repeatability, the RSD values of the results of same sample analyzed on the same equipment and same day were examined against the prescribed limits. The intraday precision was established, analyzing the same solution on different days and different equipment. For this purpose, the prepared sample solutions were provided to the pharmaceutical testing laboratory to analyze according to the optimized chromatographic conditions. The values of RSD for the responses i.e., peak area and retention time of VEL and impurities were less than 2.0% for the drug substance and 10.0% for all DPs respectively.
3.5. Robustness
To demonstrate the effect of small changes in the chromatographic conditions on results, robustness studies were performed. The values of optimized chromatographic conditions were deliberately changed by ±2% and the consequence of these deviations were observed in the results of replicates (n = 6) of reference solutions. The results of the chromatographic response were checked against the values obtained using validated optimized chromatographic conditions. It was observed that the influence of the small changes in the chromatographic condition was negligible and the value of RSD for the peak area and retention time compared with standard values was less than 2.0%.
3.6. Linearity and Range
A linear relationship of the response and concentration is required to get a reportable range of an analytical method. Seven composite reference solutions varying from low concentration to high concentration, i.e., 10 to 120% of VEL 0.1 mg mL were prepared and analyzed on the optimized chromatographic conditions. The linearity of each individual component was examined using linear regression equation (A = slop C + Y intercept) from the graph of response plotted at on X axis verses concentration on Y axis. To establish the minimum detectable and quantifiable response limits (LOD and LOQ) for each analyte in the sample, the slope of the Calibration curve was determined. Based on the standard deviation of the response and slope, the values of LOD and LOQ shown in Table 3, were estimated using expression (3.3δ/slope) and (10δ/slope) respectively.

Table 3.
Precision and linearity studies.
3.7. Forced Degradation Studies
Sample solutions after degradation studies were diluted to 0.1 mg mL−1 and analyzed using validated HPLC-UV method. The overall level of degradation was quantified by comparing the results of sample solution with freshly prepared reference solution (Table 4 and Figure 7). VEL both, pure drug substance and VEL-CSD, were hydrolyzed 16–20% in alkaline and acidic conditions and oxidized about 37–40% on exposure to oxidative stressed conditions. VEL is photolabile and the pure drug substance was degraded more than 15% during storage in photo-stability chamber for 7 days. VEL-CSD was also degraded during photolytic degradation studies, but the level of degradation was less than VEL pure drug i.e., 9.4% (Figure 6). The effect of dry heat and humidity on VEL and VEL-CSD was negligible and there was no degradation detected. Similarly, the solutions of VEL pure drug substance and VEL-CSD stored in amber color flasks at room temperature and refrigerator, were stable and showed no degradation during studies.
To identify and quantify the individual DP obtained in the studies, the results of sample solution were compared with results of composite reference solution. In overall studies DP1 (C42H43N7O5, Figure 2A), DP2 (C39H45N7O5, Figure 2B) and DP3 (C32H34N6O2, Figure 2C) were the major determined degradation product. DP1 and DP2 were quantified in both oxidative studies and photolytic studies; however, DP4 (C47H52N8O6, Figure 2D) and DP5 (C34H34N6O6, Figure 2E) were only detected in oxidative studies. DP3 was quantified in both alkaline and acidic conditions while DP6 (C32H32N6O6, Figure 2F) was detected only in alkaline and DP7 (C17H24N4O4, Figure 2G) and DP8 (C16H20N2O4, Figure 2H) in acidic condition. The results showed that VEL degraded to 4 DPs i.e., DP1, DP2, DP4 and DP5 in oxidative studies and into 2 DPs i.e., DP1 and DP2 during photolytic stress. On exposure to acidic stress, VEL hydrolyzed into 3 DPs i.e., DP3, DP7 and DP8 while in alkaline condition it degraded into 2 DPs i.e., DP3 and DP6.
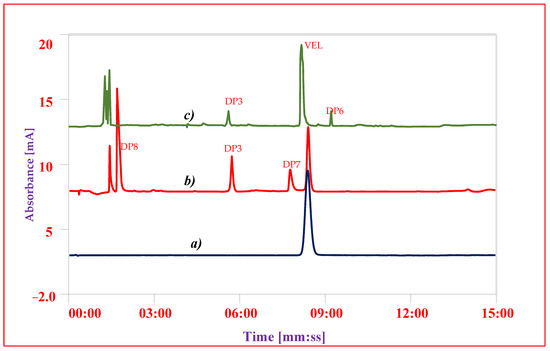
Figure 6.
Chromatograms of VEL-CSD (a) Reference standard of VEL, (b) Hydrolysis in acidic condition (c) Hydrolysis in alkaline condition.
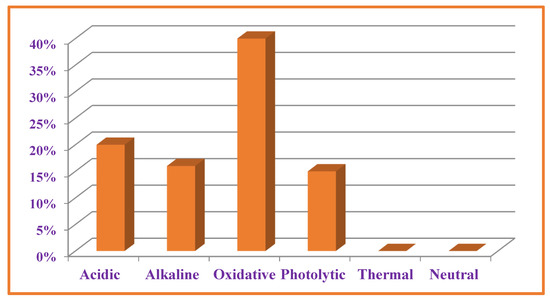
Figure 7.
Degradation of VEL-CSD in different stressed conditions.

Table 4.
Assay of VEL and VEL-CSD after exposure to stressed studies.
Table 4.
Assay of VEL and VEL-CSD after exposure to stressed studies.
| Medium | Material | At Refrigerator (02–08 °C) Protected from Light for 7 Days | At Room Temperature (15–25 °C) Protected from Light for 7 Days | At 80 °C Refluxed for 4 h Protected from Light | At 80 °C Refluxed for 8 h Protected from Light | At 40 °C) Exposed to 1.2 Milli lux h m−2 of Fluorescence Light for 7 Days | At 80 °C/75 ± 5% RH Protected from Light and Moisture for 24 h | At 105 °C Temperature Protected from Light and Moisture for 24 h |
|---|---|---|---|---|---|---|---|---|
| In neutral condition | VEL | 100.13 ± 0.27 0.28% | 98.14 ± 0.74 0.69% | 97.51 ± 0.84 0.74% | 98.27 ± 1.18 1.65% | - | - | - |
| VEL-CSD | 99.44 ± 0.79 0.65% | 99.73 ± 1.14 0.79% | 98.69 ± 1.17 1.20% | 98.66 ± 1.12 1.24% | - | - | - | |
| Acid Hydrolysis (5M HCl) | VEL | - | - | 87.26 ± 3.24 2.47% | 79.84 ± 4.46 5.12% | - | - | - |
| VEL-CSD | - | 90.41 ± 2.37 3.10% | 94.56 ± 3.49 2.18% | - | ||||
| Alkaline hydrolysis (1M sodium hydroxide solutions) | VEL | - | - | 90.51 ± 2.29 1.77% | 87.98 ± 3.87 3.21% | - | - | - |
| VEL-CSD | - | - | 92.36 ± 2.24 1.96% | 89.12 ± 1.14 1.25% | - | - | - | |
| Oxidative conditions (10% H2O2) | VEL | - | - | 76.66 ± 3.21 2.45% | 79.64 ± 5.18 4.75% | - | - | - |
| VEL-CSD | 91.33 ± 6.41 4.82% | 83.45 ± 4.79 2.87 | - | - | - | |||
| Photolytic condition | VEL | - | - | 84.16 ± 2.11 1.98% | - | - | ||
| VEL-CSD | - | - | 91.64 ± 1.75 154% | - | - | |||
| Thermal stress | VEL | - | - | 99.54 ± 0.78 0.78% | 98.71 ± 0.24 0.24% | 98.27 ± 0.67 0.68% | 99.10 ± 0.25 0.25% | 99.16 ± 1.16 1.15% |
| VEL-CSD | - | 98.21 ± 01.31 1.67% | 100.47 ± 1.61 1.52% | 99.44 ± 0.75 0.38% | 98.91 ± 0.31 0.46% | 99.22 ± 0.78 0.80% |
4. Conclusions
Stability indicating HPLC-UV method for the analysis of VEL-CSD was developed and validated using reference standards as per guidelines presented by ICH Q1A (R2). Forced degradation studies were performed on VEL drug substance and VEL-CSD. The reported IMPs and all DPs were separated and quantified using the same validated method. The specificity of the method for DPS and IMPs was conformed using reference standards from the manufacturer. The recovery of VEL in VEL-CSD and samples of stressed studies was determined and extent of degradation was evaluated accordingly. It was concluded from the results of validation studies that the developed method is specific, precise, and accurate for the intended purpose. HPLC-UV is readily available in all pharmaceutical research and testing laboratories. This validated method will help the researchers and analysts to assure the quality of novel and lifesaving antiviral drug VEL and VEL-CSD during routine quality control analysis.
Author Contributions
Conceptualization, B.Z. and W.H.; Methodology, B.Z.; Validation, B.Z.; Formal Analysis, A.M.; Investigation, A.K.; Resources, D.A.A.; Writing—original draft preparation, N.A.; writing—review and editing, A.K.; Supervision, W.H.; Project Administration, M.B.; Funding acquisition, M.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key laboratory for special resource development and medicinal research in Jiangsu Province, project number LPRK202101.
Acknowledgments
The authors are thankful to Genome Pharmaceuticals Pvt. Ltd. for providing pharmaceutical excipients and for their support in this work. Also, we are pleased to acknowledge the support from key laboratory for special resource development and medicinal research in Jiangsu Province, project number LPRK202101.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ugah, U.I.; Alo, M.N.; Gloria, U.C. Epidemiology of hepatitis b virus, hepatitis C virus and human immunodeficiency virus co-infection among assymptomatic persons resident in Alex Ekwueme federal university Ndufu-alike. Sci. Afr. 2021, 14, 00985. [Google Scholar] [CrossRef]
- Thomas, D.L.; Seeff, L.B. Natural history of hepatitis C. Clin. Liver Dis. 2005, 9, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, L.; Wedemeyer, H. New Treatments for Chronic Hepatitis B Virus/Hepatitis D Virus Infection. Clin. Liver Dis. 2021, 25, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Maus, A.; Strait, L.; Zhu, D. Nanoparticles as delivery vehicles for antiviral therapeutic drugs. Eng. Regen. 2021, 2, 31–46. [Google Scholar] [CrossRef]
- Naggie, S.; Patel, K.; McHutchison, J. Hepatitis C virus directly acting antivirals: Current developments with NS3/4A HCV serine protease inhibitors. J. Antimicrob. Chemother. 2010, 65, 2063–2069. [Google Scholar] [CrossRef]
- Wei, L.; Lim, S.G.; Xie, Q.; Văn, K.N.; Piratvisuth, T.; Huang, Y.; Wu, S.; Xu, M.; Tang, H.; Cheng, J.; et al. Sofosbuvir–velpatasvir for treatment of chronic hepatitis C virus infection in Asia: A single-arm, open-label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2019, 4, 127–134. [Google Scholar] [CrossRef]
- FDA Approves Epclusa for Treatment of Chronic Hepatitis C Virus Infection. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-epclusa-treatment-chronic-hepatitis-c-virus-infection#:~:text=The%20U.S.%20Food%20and%20Drug,combination%20with%20the%20drug%20ribavirin (accessed on 13 November 2021).
- Yang, Y.; Lv, Y.; Shen, C.; Shi, T.; He, H.; Qi, J.; Dong, X.; Zhao, W.; Lu, Y.; Wu, W. In vivo dissolution of poorly water-soluble drugs: Proof of concept based on fluorescence bioimaging. Acta. Pharm. Sin. B 2021, 11, 1056–1068. [Google Scholar] [CrossRef]
- Devane, J.; Butler, J. The impact of in vitro-in vivo relationships on product development. Pharm. Technol. 1997, 21, 146–159. [Google Scholar]
- Bari, S.B.; Kadam, B.R.; Jaiswal, Y.S.; Shirkhedkar, A.A. Impurity profile: Significance in active pharmaceutical ingredient. Eurasian J. Anal. Chem. 2007, 2, 32–53. [Google Scholar] [CrossRef]
- Maggio, R.M.; Calvo, N.L.; Vignaduzzo, S.E.; Kaufman, T.S. Pharmaceutical impurities and degradation products uses and applications of NMR techniques. J. Pharm. Biomed. Anal. 2014, 101, 102–122. [Google Scholar] [CrossRef]
- Singh, S.; Junwal, M.; Modhe, G.; Tiwari, H.; Kurmi, M.; Parashar, N.; Sidduri, P. Forced degradation studies to assess the stability of drugs and products. TrAC Trends Anal. Chem. 2013, 49, 71–88. [Google Scholar] [CrossRef]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y. Development of forced degradation and stability indicating studies of drugs—A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamal, R.M.; Gawad, S.A.; Belal, F.F.; Moustapha, M.E. Selective and sensitive spectrofluorimetric quantification of velpatasvir in presence of sofosbuvir. Application to their co-formulated tablet. RSC Adv. 2018, 8, 32909–32915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustapha, M.E.; El-Gamal, R.M.; Belal, F.F. Two novel UPLC methods utilizing two different analytical columns and different detection approaches for the simultaneous analysis of velpatasvir and sofosbuvir: Application to their co-formulated tablet. BMC Chem. 2019, 13, 118. [Google Scholar] [CrossRef]
- Kamal, A.H.; Mabrouk, M.M.; Bebawy, L.I.; Mekky, M.A. Spectrophotometric and robust UPLC methods for simultaneous determination of velpatasvir and sofosbuvir in their tablet. Microchem. J. 2019, 149, 103996. [Google Scholar] [CrossRef]
- Rezk, M.R.; Monir, H.H.; Marzouk, H.M. Spectrophotometric assessment of the brand-new antiviral combination: Sofosbuvir and velpatasvir in their pure forms and pharmaceutical formulation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 159–166. [Google Scholar] [CrossRef]
- Zaman, B.; Waseem, H. Development of Stability Indicating HPLC-UV Method for Determination of Process Impurities and Degradation Products in Sofosbuvir and Velpatasvir Tablets. Pharm. Chem. J. 2021, 54, 1295–1305. [Google Scholar] [CrossRef]
- Bandla, J.; Ganapaty, S. Stability indicating RP-HPLC method development and validation for the simultaneous determination of Sofosbuvir and Velpatasvir in tablet dosage forms. Indian J. Pharm. Biol. Res. 2017, 5, 10–16. [Google Scholar] [CrossRef]
- Omar, M.A.; Abdel-Lateef, M.A.; Ali, R.; Derayea, S.M. Study on fluorescence properties of HCV antiviral (velpatasvir) and its fluorimetric determination in presence of sofosbuvir; application to stability study and human plasma. J. Lumin. 2018, 33, 1249–1256. [Google Scholar] [CrossRef]
- Priyanka, K.; Vinutha, K.; Sridevi, P.; Ramya, B.; Bhagavan Raju, M. A Stability Indicating RP-HPLC method for simultaneous estimation of Velpatasvir and Sofosbuvir in its bulk and tablet dosage form. Am. J. Pharmtech Res. 2018, 8, 129–139. [Google Scholar] [CrossRef]
- Rao, P.V.; Rao, A.L.; Prasad, S. Validated Stability Indicating RP-HPLC method for estimation of antiviral class of drugs Sofosbuvir and Velpatasvir in combination and its comparison with reported methods. Res. J. Pharm. Technol. 2018, 11, 5425–5430. [Google Scholar] [CrossRef]
- Vanaja, B.; Vageesh, N.M.; Kistayya, C.; Urukundu, V. RP-HPLC method development and validation for simultaneous estimation of sofosbuvir and velpatasvir in pure and pharmaceutical dosage form. Innov. Int. J. Med. Pharm. Sci. 2018, 3, 45–48. [Google Scholar]
- European Medicine Agency, Assessment Report Sofosbuvir/Velpatasvir. Available online: https://www.ema.europa.eu/en/documents/assessment-report/epclusa-epar-public-assessment-report_en.pdf (accessed on 14 October 2021).
- U.S. Food and Drug Administration. Chemistry Review Sofosbuvir/Velpatasvir. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208341Orig1s000TOC.cfm (accessed on 14 October 2021).
- Swain, D.; Yadav, A.S.; Sasapu, C.; Akula, V.; Samanthula, G. UPLC Separation of forced degradation and process related impurities of Velpatasvir and structure elucidation by online LC-Quadrupole-Time of flight-Tandem mass Spectrometry. Microchem. J. 2020, 155, 104657. [Google Scholar] [CrossRef]
- Stability Testing of New Drug Substances and Products. Available online: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf (accessed on 7 February 2021).
- Impurities in New Drug Products. Q3B (R2), Current Step. Available online: https://database.ich.org/sites/default/files/Q3B%28R2%29%20Guideline.pdf (accessed on 7 February 2021).
- Guideline IHT. Photostability testing of new drug substance and products. Fed. Regist. 1996, 62, 27115–27122. [Google Scholar]
- Zaman, B.; Hassan, W. Development of stability indicating HPLC–UV method for determination of daclatasvir and characterization of forced degradation products. Chromatographia 2018, 81, 785–797. [Google Scholar] [CrossRef]
- Validation of Analytical Procedures: Text and Methodology Q2 (R1). Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 7 February 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).