An Observational Study of MDR Hospital-Acquired Infections and Antibiotic Use during COVID-19 Pandemic: A Call for Antimicrobial Stewardship Programs
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Objective
2.2. Data Collection
2.3. Microbiological Detection Methods
2.4. Statistical Analysis
3. Results
3.1. Microbiological Trends in Hospital
3.2. Antibiotic Use
Overall Antibiotic Use
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shbaklo, N.; Lupia, T.; De Rosa, F.G.; Corcione, S. Infection Control in the Era of COVID-19: A Narrative Review. Antibiotics 2021, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, F.; Palazzo, A.; Rosso, T.; Shbaklo, N.; Mussa, M.; Boglione, L.; Borgogno, E.; Rossati, A.; Pinna, S.M.; Scabini, S.; et al. Risk Factors for Mortality in COVID-19 Hospitalized Patients in Piedmont, Italy: Results from the Multicenter, Regional, CORACLE Registry. J. Clin. Med. 2021, 10, 1951. [Google Scholar] [CrossRef] [PubMed]
- Yap, F.H.Y.; Gomersall, C.; Fung, K.S.C.; Ho, P.L.; Ho, O.-M.; Lam, P.K.N.; Lam, D.T.C.; Lyon, D.J.; Joynt, G. Increase in Methicillin-Resistant Staphylococcus aureus Acquisition Rate and Change in Pathogen Pattern Associated with an Outbreak of Severe Acute Respiratory Syndrome. Clin. Infect. Dis. 2004, 39, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, Z.; Chen, Y.; Xiao, Y.; Huang, X.; Fan, X.-G. Bacterial and fungal infections in COVID-19 patients: A matter of concern. Infect. Control Hosp. Epidemiol. 2020, 41, 1124–1125. [Google Scholar] [CrossRef] [Green Version]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A.H. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Nieuwlaat, R.; Mbuagbaw, L.; Mertz, D.; Burrows, L.L.; E Bowdish, D.M.; Moja, L.; Wright, G.D.; Schünemann, H.J. Coronavirus Disease 2019 and Antimicrobial Resistance: Parallel and Interacting Health Emergencies. Clin. Infect. Dis. 2021, 72, 1657–1659. [Google Scholar] [CrossRef]
- van Duin, D.; Barlow, G.; Nathwani, D. The impact of the COVID-19 pandemic on antimicrobial resistance: A debate. JAC-Antimicrob. Resist. 2020, 2, dlaa053. [Google Scholar] [CrossRef]
- Abu-Rub, L.; Abdelrahman, H.; Johar, A.-R.; Alhussain, H.; Hadi, H.; Eltai, N. Antibiotics Prescribing in Intensive Care Settings during the COVID-19 Era: A Systematic Review. Antibiotics 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- EUCAST_Detection_of_Resistance_Mechanisms_170711.pdf. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 16 May 2022).
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R., Jr.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Corcione, S.; Shbaklo, N.; Vicentini, C.; Corradi, A.; Scabini, S.; Pinna, S.M.; Tarozzo, A.; Curtoni, A.; Cattel, F.; Cavallo, R.; et al. Impact of an empiric antimicrobial therapy manual on antimicrobial usage and multidrug resistant organism trends in a large Italian teaching hospital. Infect. Prev. Pract. 2021, 100187. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Dudoignon, E.; Caméléna, F.; Deniau, B.; Habay, A.; Coutrot, M.; Ressaire, Q.; Plaud, B.; Berçot, B.; Dépret, F. Bacterial Pneumonia in COVID-19 Critically Ill Patients: A Case Series. Clin. Infect. Dis. 2021, 72, 905–906. [Google Scholar] [CrossRef]
- Stevens, M.P.; Doll, M.; Pryor, R.; Godbout, E.; Cooper, K.; Bearman, G. Impact of COVID-19 on traditional healthcare-associated infection prevention efforts. Infect. Control Hosp. Epidemiol. 2020, 41, 946–947. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influ. Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacios, G.; Hornig, M.; Cisterna, D.; Savji, N.; Bussetti, A.V.; Kapoor, V.; Hui, J.; Tokarz, R.; Briese, T.; Baumeister, E.; et al. Streptococcus pneumoniae Coinfection Is Correlated with the Severity of H1N1 Pandemic Influenza. PLoS ONE 2009, 4, e8540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutiérrez-Ocampo, E.; Villamizar-Peña, R.; Holguin-Rivera, Y.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Bonilla-Aldana, D.K.; Franco-Paredes, C.; Henao-Martinez, A.F.; et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 34, 101623. [Google Scholar] [CrossRef]
- Huttner, B.; Catho, G.; Pano-Pardo, J.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Giacobbe, D.R.; Bruzzi, P.; Barisione, E.; Centanni, S.; Castaldo, N.; Corcione, S.; De Rosa, F.G.; Di Marco, F.; Gori, A.; et al. Clinical Management of Adult Patients with COVID-19 Outside Intensive Care Units: Guidelines from the Italian Society of Anti-Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Infect. Dis. Ther. 2021, 10, 1837–1885. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, G.; Corcione, S.; Sales, G.; Curtoni, A.; De Rosa, F.; Brazzi, L. Carbapenem-resistant Klebsiella pneumoniae in ICU-admitted COVID-19 patients: Keep an eye on the ball. J. Glob. Antimicrob. Resist. 2020, 23, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, M.; Burnett, E.; Castro-Sánchez, E.; Du Toit, B.; Figueiredo, R.; Gallagher, R.; Gotterson, F.; Kennedy, H.; Manias, E.; McEwen, J.; et al. Preparing nurses for COVID-19 response efforts through involvement in antimicrobial stewardship programmes. J. Hosp. Infect. 2020, 106, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Elbeddini, A.; Prabaharan, T.; Almasalkhi, S.; Tran, C. Pharmacists and COVID-19. J. Pharm. Policy Pract. 2020, 13, 1–4. [Google Scholar] [CrossRef]
| MDR | Total N (%) | Pre-COVID N (%) | COVID N (%) | p-Value |
|---|---|---|---|---|
| KPC-Kp | 723 (36.9) | 278 (14.2) | 445 (22.7) | |
| Respiratory | 111 (5.7) | 33 (1.7) | 78 (4) | <0.001 |
| BSI | 74 (3.8) | 33 (1.7) | 41 (2.1) | <0.001 |
| RS | 538 (27.4) | 212 (10.8) | 326 (16.6) | <0.001 |
| ESBL-E. coli | 398 (20.3) | 173 (8.8) | 225 (11.5) | |
| Respiratory | 45 (2.3) | 17 (0.9) | 28 (1.4) | <0.001 |
| BSI | 104 (5.3) | 48 (2.4) | 56 (2.9) | <0.001 |
| RS | 249 (12.7) | 108 (5.5) | 141 (7.2) | <0.001 |
| CR-AB | 225 (11.5) | 26 (1.4) | 199 (4.9) | |
| Respiratory | 80 (4.1) | 7 (0.4) | 73 (3.7) | <0.001 |
| BSI | 27 (1.4) | 4 (0.2) | 23 (1.2) | <0.001 |
| RS | 118 (6) | 15 (0.8) | 103 (5.3) | <0.001 |
| CR-PA | 127 (6.5) | 56 (2.9) | 71 (3.7) | |
| Respiratory | 104 (5.3) | 47 (2.4) | 57 (2.9) | <0.001 |
| BSI | 14 (0.7) | 5 (0.3) | 9 (0.5) | <0.001 |
| RS | 9 (0.5) | 4 (0.2) | 5 (0.3) | <0.001 |
| MRSA | 158 (8) | 1 (3.1) | 97 (5) | |
| Respiratory | 69 (3.5) | 24 (1.2) | 45 (2.3) | <0.001 |
| BSI | 89 (4.5) | 37 (1.9) | 52 (2.7) | <0.001 |
| CDI | 222 (11.3) | 92 (4.7) | 130 (6.6) | <0.001 |
| MDR | Total/1000 PD | Pre-COVID/1000 PD | COVID/1000 PD | IRR | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| KPC | 3.99 | 3.41 | 4.46 | 0.93 | 0.23–3.83 | 0.95 |
| Respiratory | 0.61 | 0.40 | 0.78 | 0.63 | 0.01–28.34 | 1 |
| BSI | 0.41 | 0.40 | 0.41 | 1.19 | 0.02–92.89 | 1 |
| RS | 2.97 | 2.60 | 3.27 | 0.97 | 0.19–4.95 | 1 |
| ESBL-E. coli | 2.19 | 2.12 | 2.26 | 1.15 | 0.18–7.47 | 1 |
| Respiratory | 0.25 | 0.21 | 0.28 | 0.92 | 0.03–262.60 | 0.97 |
| BSI | 0.57 | 0.59 | 0.56 | 1.29 | 0.03–49.86 | 0.90 |
| RS | 1.37 | 1.32 | 1.41 | 1.14 | 0.10–12.28 | 1 |
| A. baumanni | 1.24 | 0.32 | 2.00 | 0.20 | 0.04–8.161 | 0.60 |
| Respiratory | 0.44 | 0.09 | 0.73 | 0.15 | 0.04–153.1 | 1 |
| BSI | 0.15 | 0.05 | 0.23 | 0.27 | 0.06–4209 | 0.77 |
| RS | 0.65 | 0.18 | 1.03 | 0.21 | 0.02–31.91 | 1 |
| P. aeruginosa | 0.70 | 0.69 | 0.71 | 1.18 | 0.04–32.62 | 0.90 |
| Respiratory | 0.57 | 0.58 | 0.57 | 1.24 | 0.03–48.10 | 0.90 |
| BSI | 0.08 | 0.06 | 0.09 | 0.81 | 0.02–2494 | 0.96 |
| RS | 0.05 | 0.05 | 0.05 | 1.22 | 0.05–295,300 | 0.97 |
| MRSA | 0.87 | 0.75 | 0.97 | 0.94 | 0.05–19.24 | 19 |
| Respiratory | 0.38 | 0.29 | 0.45 | 0.79 | 0.07–83.78 | 1 |
| BSI | 0.49 | 0.45 | 0.52 | 1.05 | 0.02–57.18 | 1 |
| CDI | 1.22 | 1.13 | 1.30 | 1.06 | 0.09–13.21 | 1 |
| Ward | Estimate | SE | p-Value |
|---|---|---|---|
| All wards | |||
| 1st generation cephalosporins | |||
| Pre-COVID trend | −2.154 | 24.073 | 0.930 |
| Change in level | −539.527 | 132.812 | 0.001 |
| Change in trend | 12.972 | 24.777 | 0.609 |
| 2nd generation cephalosporins | |||
| Pre-COVID trend | −0.778 | 0.476 | 0.126 |
| Change in level | −2.537 | 2.710 | 0.366 |
| Change in trend | 0.797 | 0.495 | 0.131 |
| 3rd generation cephalosporins | |||
| Pre-COVID trend | −173.114 | 173.102 | 0.336 |
| Change in level | −690.663 | 945.501 | 0.478 |
| Change in trend | 159.432 | 176.906 | 0.384 |
| 4th generation cephalosporins | |||
| Pre-COVID trend | −19.712 | 21.995 | 0.386 |
| Change in level | 344.480 | 131.317 | 0.021 |
| Change in trend | 15.061 | 23.222 | 0.528 |
| 5th generation cephalosporins | |||
| Pre-COVID trend | 21.778 | 23.732 | 0.376 |
| Change in level | 531.828 | 134.128 | 0.002 |
| Change in trend | −46.451 | 24.593 | 0.081 |
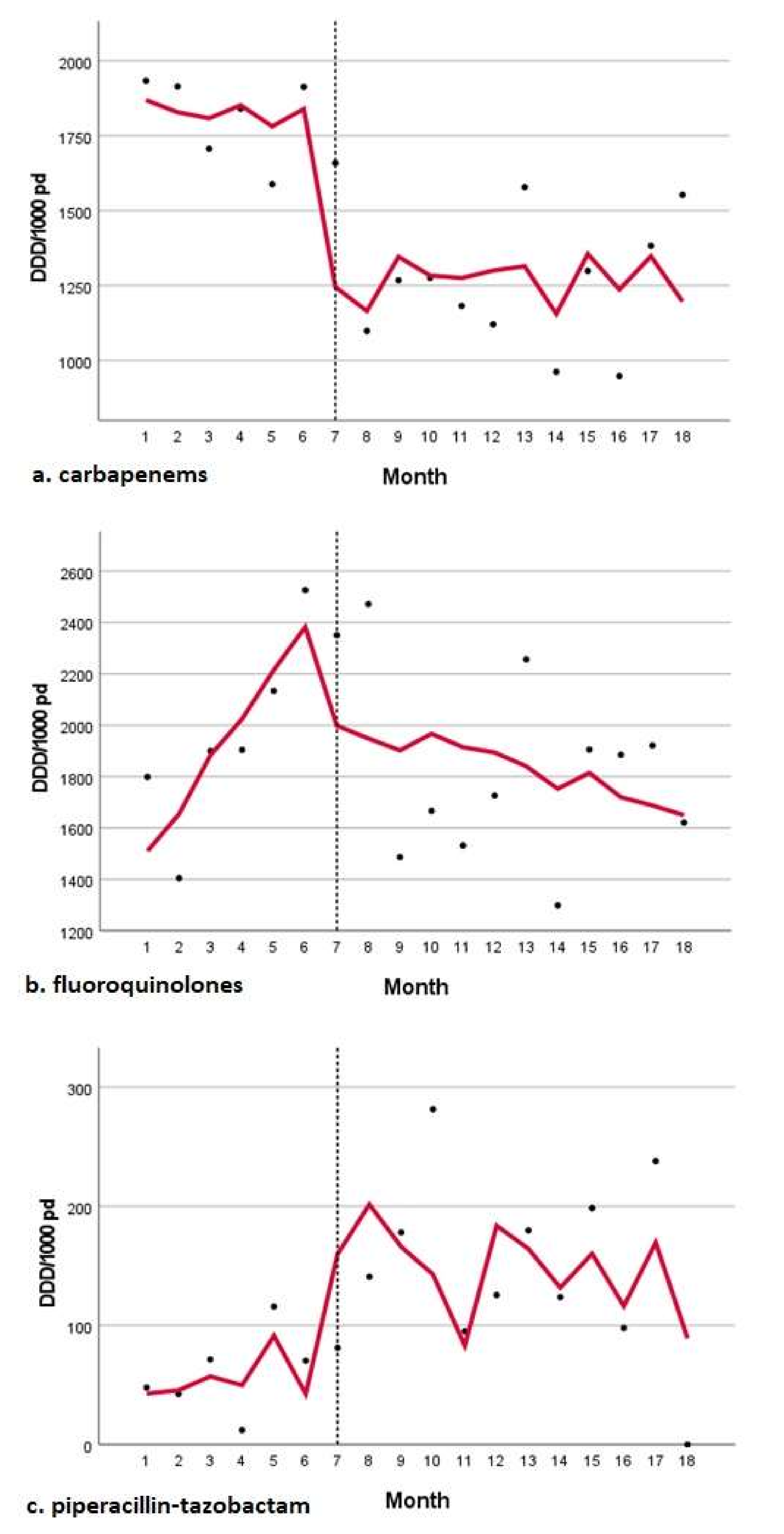
| Piperacillin–tazobactam | |||
| Pre-COVID trend | 5.576 | 11.369 | 0.632 |
| Change in level | 133.944 | 62.834 | 0.053 |
| Change in trend | −7.214 | 11.619 | 0.545 |
| Fluoroquinolones | |||
| Pre-COVID trend | 173.061 | 77.478 | 0.044 |
| Change in level | 893.653 | 449.492 | 0.068 |
| Change in trend | −204.120 | 81.122 | 0.026 |
| Vancomycin | |||
| Pre-COVID trend | −2.472 | 9.394 | 0.797 |
| Change in level | −50.358 | 52.584 | 0.356 |
| Change in trend | 1.013 | 9.720 | 0.919 |
| Carbapenems | |||
| Pre-COVID trend | −19.560 | 44.567 | 0.668 |
| Change in level | −564.805 | 247.041 | 0.040 |
| Change in trend | 15.009 | 45.425 | 0.746 |
| Caz-avi | |||
| Pre-COVID trend | 2.472 | 9.394 | 0.797 |
| Change in level | 50.358 | 52.584 | 0.356 |
| Change in trend | −1.013 | 9.720 | 0.919 |
| Colistin | |||
| Pre-COVID trend | 3.023 | 11.170 | 0.791 |
| Change in level | −87.292 | 67.925 | 0.221 |
| Change in trend | 6.923 | 11.923 | 0.571 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shbaklo, N.; Corcione, S.; Vicentini, C.; Giordano, S.; Fiorentino, D.; Bianco, G.; Cattel, F.; Cavallo, R.; Zotti, C.M.; De Rosa, F.G. An Observational Study of MDR Hospital-Acquired Infections and Antibiotic Use during COVID-19 Pandemic: A Call for Antimicrobial Stewardship Programs. Antibiotics 2022, 11, 695. https://doi.org/10.3390/antibiotics11050695
Shbaklo N, Corcione S, Vicentini C, Giordano S, Fiorentino D, Bianco G, Cattel F, Cavallo R, Zotti CM, De Rosa FG. An Observational Study of MDR Hospital-Acquired Infections and Antibiotic Use during COVID-19 Pandemic: A Call for Antimicrobial Stewardship Programs. Antibiotics. 2022; 11(5):695. https://doi.org/10.3390/antibiotics11050695
Chicago/Turabian StyleShbaklo, Nour, Silvia Corcione, Costanza Vicentini, Susanna Giordano, Denise Fiorentino, Gabriele Bianco, Francesco Cattel, Rossana Cavallo, Carla Maria Zotti, and Francesco Giuseppe De Rosa. 2022. "An Observational Study of MDR Hospital-Acquired Infections and Antibiotic Use during COVID-19 Pandemic: A Call for Antimicrobial Stewardship Programs" Antibiotics 11, no. 5: 695. https://doi.org/10.3390/antibiotics11050695
APA StyleShbaklo, N., Corcione, S., Vicentini, C., Giordano, S., Fiorentino, D., Bianco, G., Cattel, F., Cavallo, R., Zotti, C. M., & De Rosa, F. G. (2022). An Observational Study of MDR Hospital-Acquired Infections and Antibiotic Use during COVID-19 Pandemic: A Call for Antimicrobial Stewardship Programs. Antibiotics, 11(5), 695. https://doi.org/10.3390/antibiotics11050695









