The Appropriateness of Empiric Treatment of Urinary Tract Infections in a Tertiary Teaching Hospital in Joran: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants, and Clinical Setting
2.2. Main Data Sources
2.3. Evaluation of the Appropriateness of the of Urinary Tract Infection Empiric Treatment
2.4. Ethical Consideration
2.5. Statistical Analysis
3. Results
3.1. Demographic and Medical Characteristics of the Study Sample
3.2. Empiric Antibiotic Prescribing
3.3. Urine Culture Testing and Susceptibility Testing
3.4. Evaluation of the Appropriateness of Urinary Tract Infection Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harbottle, H.; Thakur, S.; Zhao, S.; White, D. Genetics of antimicrobial resistance. Anim. Biotechnol. 2006, 17, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Tebano, G.; Mouelhi, Y.; Zanichelli, V.; Charmillon, A.; Fougnot, S.; Lozniewski, A.; Thilly, N.; Pulcini, C. Selective reporting of antibiotic susceptibility testing results: A promising antibiotic stewardship tool. Expert Rev. Anti-Infect. Ther. 2020, 18, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Davison, H.C.; Woolhouse, M.E.; Low, J.C. What is antibiotic resistance and how can we measure it? Trends Microbiol. 2000, 8, 554–559. [Google Scholar] [CrossRef]
- Byarugaba, D. Antimicrobial resistance in developing countries and responsible risk factors. Int. J. Antimicrob. Agents 2004, 24, 105–110. [Google Scholar] [CrossRef]
- Griffith, M.; Postelnick, M.; Scheetz, M. Antimicrobial stewardship programs: Methods of operation and suggested outcomes. Expert Rev. Anti-Infect. Ther. 2012, 10, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Kelly, L.J.; Thornsberry, C.; Jones, M.E.; Sahm, D.F. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob. Agents Chemother. 2002, 46, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Karavanaki, K.A.; Soldatou, A.; Koufadaki, A.M.; Tsentidis, C.; Haliotis, F.A.; Stefanidis, C.J. Delayed treatment of the first febrile urinary tract infection in early childhood increased the risk of renal scarring. Acta Paediatr. 2017, 106, 149–154. [Google Scholar] [CrossRef]
- Johnson, J.R.; Russo, T.A. Acute Pyelonephritis in Adults. N. Engl. J. Med. 2018, 378, 48–59. [Google Scholar] [CrossRef]
- Lee, C.-R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to minimize antibiotic resistance. Int. J. Environ. Res. Public Health 2013, 10, 4274–4305. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef]
- Carson, C.; Naber, K.G. Role of fluoroquinolones in the treatment of serious bacterial urinary tract infections. Drugs 2004, 64, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R. Implementation of strategies to control antimicrobial resistance. Chest 2001, 119, 405S–411S. [Google Scholar] [CrossRef] [PubMed]
- Tabak, Y.P.; Vankeepuram, L.; Ye, G.; Jeffers, K.; Gupta, V.; Murray, P.R. Blood culture turnaround time in US acute care hospitals and implications for laboratory process optimization. J. Clin. Microbiol. 2018, 56, e00500-18. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; Lychko, I.; Sobral, R.; Roque, A.C. Identification and antibiotic-susceptibility profiling of infectious bacteria. Biotechnol. J. 2019, 14, 1700750. [Google Scholar]
- LaPlante, K.; Cunha, C.; Morrill, H.; Rice, L.; Mylonakis, E. Antimicrobial Stewardship: Principles and Practice; CABI: Wallingford, UK, 2016. [Google Scholar]
- Harvey, R.A.; Clark, M.; Finkel, R.; Rey, J.; Whalen, K. Lippincott’s Illustrated Reviews: Pharmacology; Wolters Kluwer Health: Philadelphia, PA, USA, 2012; Volume 526. [Google Scholar]
- World Medical, A. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Hang, Y.; Luo, H.; Fang, X.; Xiao, Y.; Cao, X.; Zou, S.; Hu, X.; Hu, L.; et al. Impact of inappropriate empirical antibiotic treatment on clinical outcomes of urinary tract infections caused by Escherichia coli: A retrospective cohort study. J. Glob. Antimicrob. Resist. 2021, 26, 148–153. [Google Scholar] [CrossRef]
- Lodise, T.P.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Bonine, N.G. Antimicrobial resistance or delayed appropriate therapy—Does one influence outcomes more than the other among patients with serious infections due to carbapenem-resistant versus carbapenem-susceptible Enterobacteriaceae? Open Forum Infect. Dis. 2019, 6, ofz194. [Google Scholar] [CrossRef]
- O’Grady, M.C.; Barry, L.; Corcoran, G.D.; Hooton, C.; Sleator, R.D.; Lucey, B. Empirical treatment of urinary tract infections: How rational are our guidelines? J. Antimicrob. Chemother. 2019, 74, 214–217. [Google Scholar] [CrossRef]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar]
- Alharafsheh, A.; Alsheikh, M.; Ali, S.; Baraiki, A.; Alharbi, G.; Alhabshi, T.; Aboutaleb, A. A retrospective cross-sectional study of antibiotics prescribing patterns in admitted patients at a tertiary care setting in the KSA. Int. J. Health Sci. 2018, 12, 67–71. [Google Scholar]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M., Jr.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Tellado, J.M.; Sen, S.S.; Caloto, M.T.; Kumar, R.N.; Nocea, G. Consequences of inappropriate initial empiric parenteral antibiotic therapy among patients with community-acquired intra-abdominal infections in Spain. Scand. J. Infect. Dis. 2007, 39, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Tünger, Ö.; Dinç, G.; Özbakkaloglu, B.; Atman, Ü.C.; Algün, Ü. Evaluation of rational antibiotic use. Int. J. Antimicrob. Agents 2000, 15, 131–135. [Google Scholar] [CrossRef]
- Badaró, R.; Molinar, F.; Seas, C.; Stamboulian, D.; Mendonça, J.; Massud, J.; Nascimento, L.O. A multicenter comparative study of cefepime versus broad-spectrum antibacterial therapy in moderate and severe bacterial infections. Braz. J. Infect. Dis. 2002, 6, 206–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heyland, D.K.; Dodek, P.; Muscedere, J.; Day, A.; Cook, D.; Canadian Critical Care Trials Group. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit. Care Med. 2008, 36, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Li, N.Y.; Poh, G.Q.; Teng, G.C.W.; Chen, H.H.; Chan, D.S.G.; Chan, S.-P.; Tambyah, P.A.; Bagdasarian, N.; Wu, J.E. A prediction tool for the presence of ceftriaxone-resistant uropathogens upon hospital admission. Antibiotics 2020, 9, 316. [Google Scholar] [CrossRef] [PubMed]

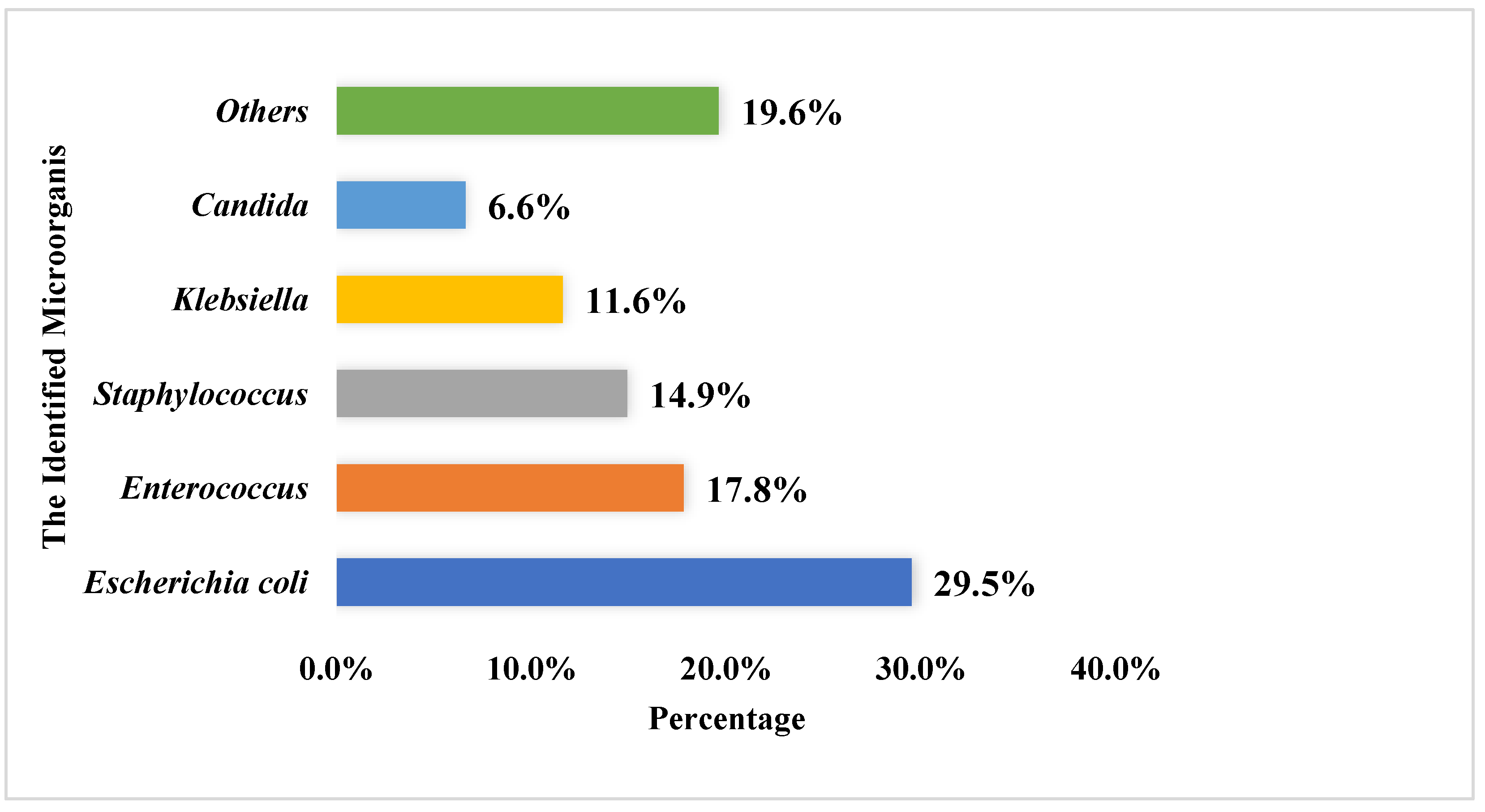

| Parameter | Results |
|---|---|
| Age in years, Median (IQR) | 64.0 (29.0) |
| Age categories (years), n (%) | |
| 232 (29.0) |
| 483 (60.4) |
| 85 (10.6) |
| Gender, n (%) | |
| 555 (69.4) |
| 245 (30.6) |
| Number of chronic medications, n (%) | |
| 178 (22.3) |
| 185 (23.1) |
| 437 (54.6) |
| Length of Stay, Median (IQR) | 12.0 (11.0) |
| Parameter | n (%) |
|---|---|
| The availability of susceptibility reporting# | |
| 409 (51.1) |
| 391 (48.8) |
| Number of patients with resistant microorganism# | 117 (14.6) |
| The most commonly prescribed antibiotics that have acquired the greatest resistance from bacteria ^ | |
| 48 (35.3) |
| 27 (19.9) |
| 21 (15.4) |
| 14 (10.3) |
| 7 (5.1) |
| 19 (14.0) |
| 136 |
| Inappropriateness of UTI Empiric Treatment | |
|---|---|
| Untreated condition | A 72-year-old male admitted to the JUH. The results of urine culture showed the presence of Klebsiella and Staphylococcus. The patient did not receive any intravenous antibiotics before the results of culture were available. |
| Incorrect antibacterial coverage | A-29-year-old female admitted to the JUH. The results of urine culture showed the presence of Pseudomonas aeruginosa. The patient received Cefazolin as an empiric antibiotic before the results of culture were available. The empiric antibiotic was not correct because of the lack of coverage. |
| Incorrect treatment (The identified pathogens were reported as resistant to the prescribed agents) | A 60-year-old female admitted to the JUH. The results of urine culture showed the presence of E. coli. The patient was given Ceftriaxone as an empiric antibiotic before results of culture were available. The empiric antibiotic was not correct because the identified pathogen was reported to be resistant to Ceftriaxone. |
| Inability to judge (Lack of susceptibility testing) Not performed at all
| A 76-year-old male admitted to the JUH. The results of urine culture showed the presence of E. coli. Meropenem was given to the patient as an empiric antibiotic before results of culture were available. We could not conclude the appropriateness of the use of Meropenem since no sensitivity test was performed. A 67-year-old male admitted to the JUH. The results of urine culture showed the presence of Acinetobacter. Colistin was given to the patient as an empiric antibiotic before results of the culture were available. We could not conclude the appropriateness of the use of Colistin since no sensitivity test was performed for this particular antibiotic. |
| Correct treatment (The identified pathogens were reported as susceptible to the prescribed agent) | An 87-year-old female admitted to the JUH. The urine culture results showed the presence of Staphylococcus. Vancomycin was given to the patient as an empiric antibiotic before the culture results were available. Depending on the results of the sensitivity test, the use of this antibiotic is considered appropriate. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhawaldeh, R.; Abu Farha, R.; Abu Hammour, K.; Alefishat, E. The Appropriateness of Empiric Treatment of Urinary Tract Infections in a Tertiary Teaching Hospital in Joran: A Cross-Sectional Study. Antibiotics 2022, 11, 629. https://doi.org/10.3390/antibiotics11050629
Alkhawaldeh R, Abu Farha R, Abu Hammour K, Alefishat E. The Appropriateness of Empiric Treatment of Urinary Tract Infections in a Tertiary Teaching Hospital in Joran: A Cross-Sectional Study. Antibiotics. 2022; 11(5):629. https://doi.org/10.3390/antibiotics11050629
Chicago/Turabian StyleAlkhawaldeh, Rama, Rana Abu Farha, Khawla Abu Hammour, and Eman Alefishat. 2022. "The Appropriateness of Empiric Treatment of Urinary Tract Infections in a Tertiary Teaching Hospital in Joran: A Cross-Sectional Study" Antibiotics 11, no. 5: 629. https://doi.org/10.3390/antibiotics11050629
APA StyleAlkhawaldeh, R., Abu Farha, R., Abu Hammour, K., & Alefishat, E. (2022). The Appropriateness of Empiric Treatment of Urinary Tract Infections in a Tertiary Teaching Hospital in Joran: A Cross-Sectional Study. Antibiotics, 11(5), 629. https://doi.org/10.3390/antibiotics11050629







