Salphage: Salvage Bacteriophage Therapy for Recalcitrant MRSA Prosthetic Joint Infection
Abstract
:1. Introduction
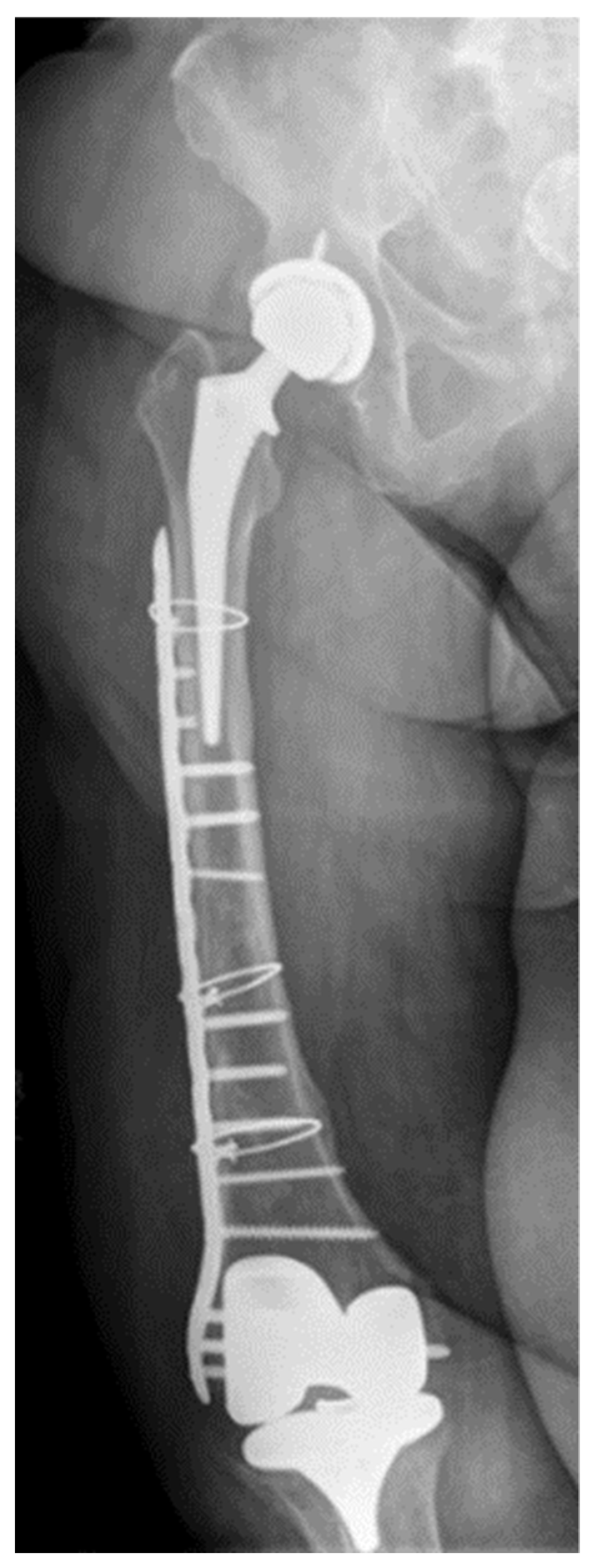
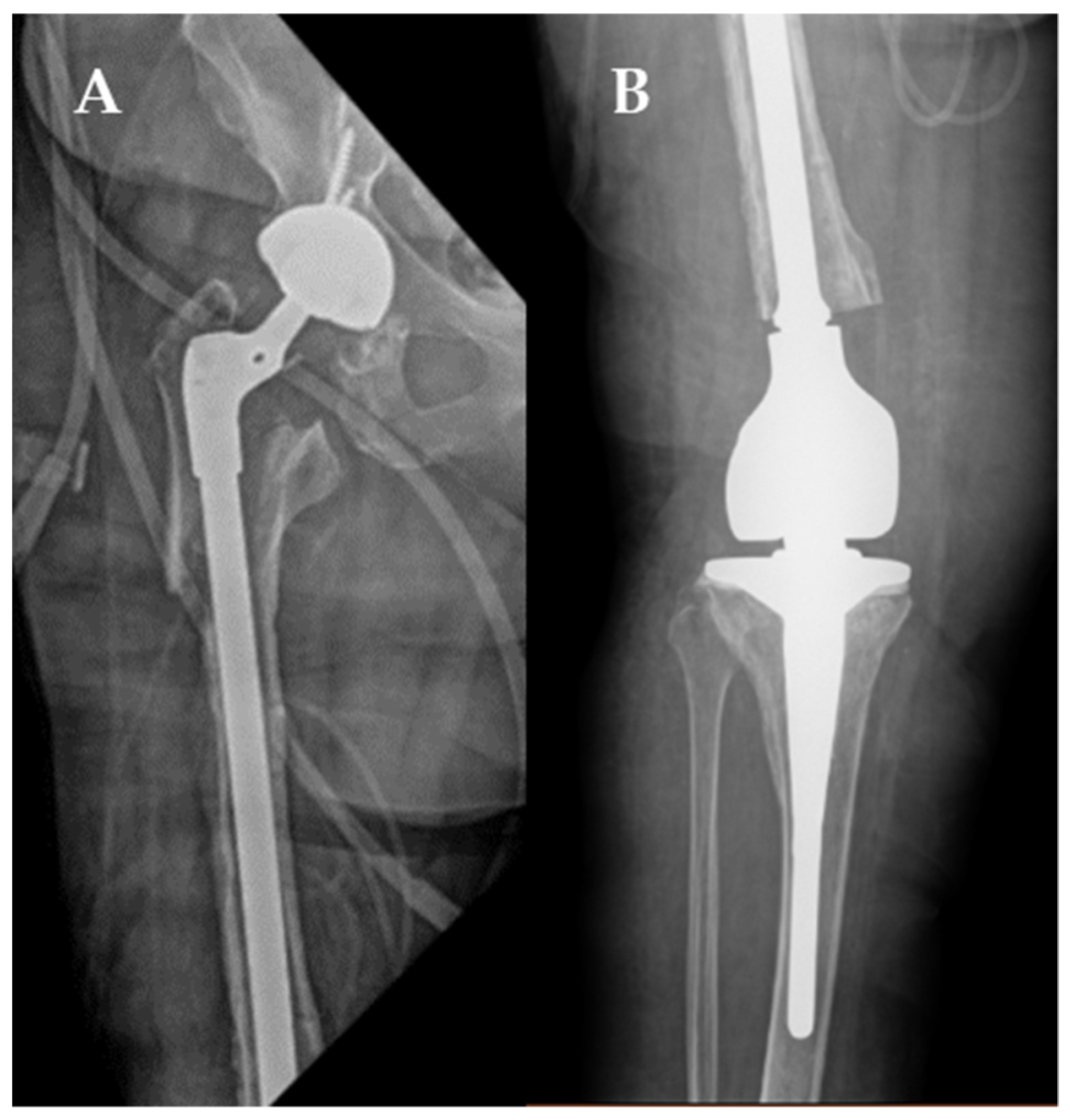
2. Case
3. Discussion
4. Materials and Methods
4.1. Bacteriophage Screening, Amplification and Purification
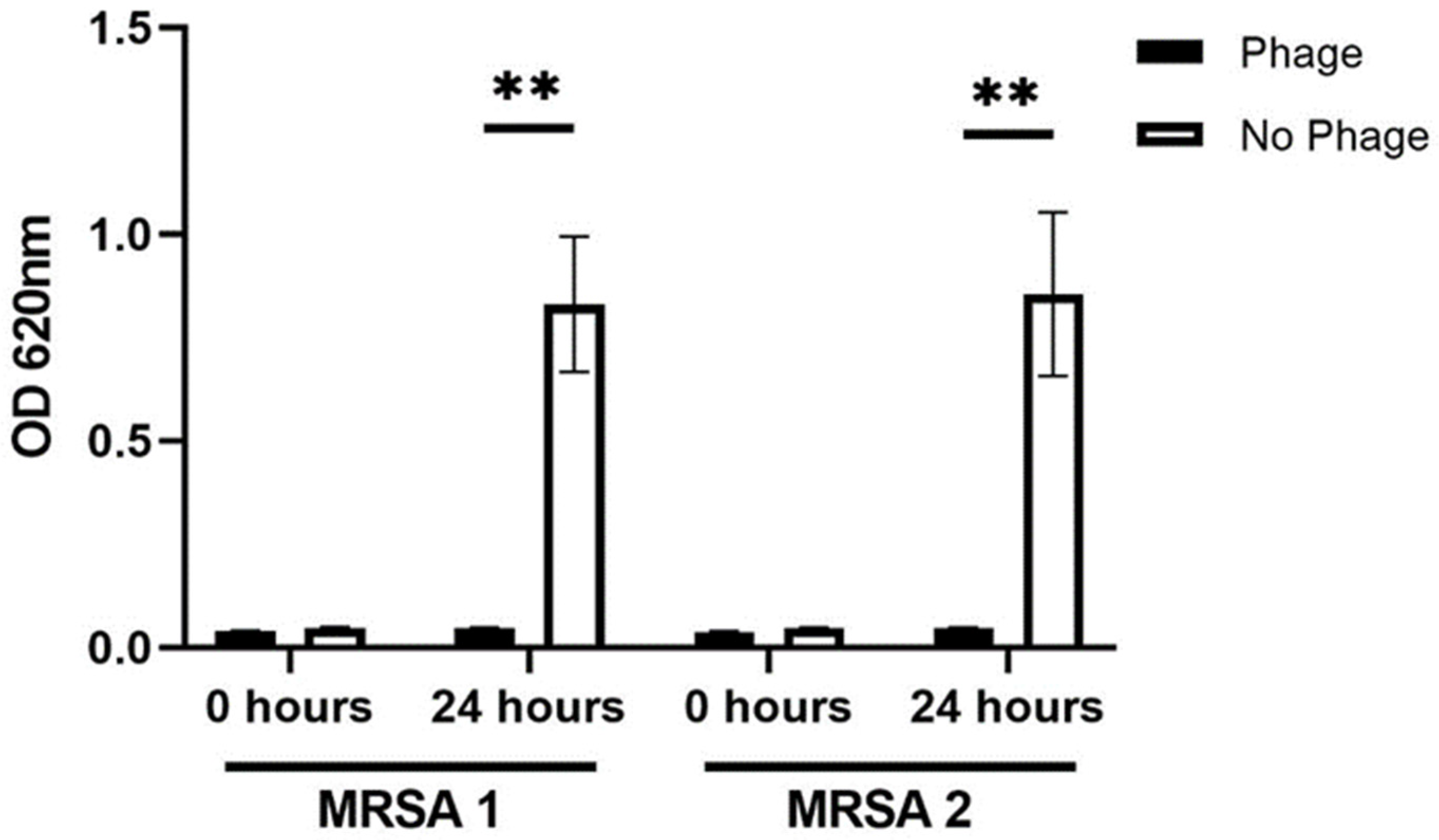
4.2. Testing for Bacteriophage Activity to the Two MRSA Morphologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natsuhara, K.M.; Shelton, T.J.; Meehan, J.P.; Lum, Z.C. Mortality During Total Hip Periprosthetic Joint Infection. J. Arthroplast. 2019, 34, S337–S342. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Goswami, K.; Li, W.T.; Tan, T.L.; Yayac, M.; Wang, S.H.; Parvizi, J. Is Treatment of Periprosthetic Joint Infection Improving Over Time? J. Arthroplast. 2020, 35, 1696–1702.e1. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.T. Bacteriophages and their enzymes in biofilm control. Curr. Pharm. Des. 2015, 21, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Doub, J.B.; Ng, V.Y.; Johnson, A.; Amoroso, A.; Kottilil, S.; Wilson, E. Potential Use of Adjuvant Bacteriophage Therapy With Debridement, Antibiotics, and Implant Retention Surgery to Treat Chronic Prosthetic Joint Infections. Open Forum Infect. Dis. 2021, 8, ofab277. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L. Biofilm-related disease. Expert Rev. Anti-Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Pestrak, M.J.; Gupta, T.T.; Dusane, D.H.; Guzior, D.V.; Staats, A.; Harro, J.; Horswill, A.R.; Stoodley, P. Investigation of synovial fluid induced Staphylococcus aureus aggregate development and its impact on surface attachment and biofilm formation. PLoS ONE 2020, 15, e0231791. [Google Scholar]
- de Mesy Bentley, K.L.; Trombetta, R.; Nishitani, K.; Bello-Irizarry, S.N.; Ninomiya, M.; Zhang, L.; Chung, H.L.; McGrath, J.L.; Daiss, J.L.; Awad, H.A.; et al. Evidence of Staphylococcus Aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. J. Bone Miner. Res. 2017, 32, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gustave, C.A.; Lustig, S.; Malatray, M.; Fevre, C.; Josse, J.; Petitjean, C.; Chidiac, C.; et al. Phage Therapy as Adjuvant to Conservative Surgery and Antibiotics to Salvage Patients With Relapsing S. aureus Prosthetic Knee Infection. Front. Med. 2020, 7, 570572. [Google Scholar] [CrossRef] [PubMed]
- Onsea, J.; Soentjens, P.; Djebara, S.; Merabishvili, M.; Depypere, M.; Spriet, I.; De Munter, P.; Debaveye, Y.; Nijs, S.; Vanderschot, P.; et al. Bacteriophage Application for Difficult-to-treat Musculoskeletal Infections: Development of a Standardized Multidisciplinary Treatment Protocol. Viruses 2019, 11, 891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gaillard, R.; Gustave, C.A.; Lustig, S.; Fevre, C.; Petitjean, C.; Leboucher, G.; Laurent, F.; et al. Case Report: Arthroscopic “Debridement Antibiotics and Implant Retention” With Local Injection of Personalized Phage Therapy to Salvage a Relapsing Pseudomonas Aeruginosa Prosthetic Knee Infection. Front. Med. 2021, 8, 569159. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Doub, J.B.; Wilson, E. Observed transaminitis with a unique bacteriophage therapy protocol to treat recalcitrant Staphylococcal biofilm infections. Infection 2022, 50, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Doub, J.B. Risk of Bacteriophage Therapeutics to Transfer Genetic Material and Contain Contaminants Beyond Endotoxins with Clinically Relevant Mitigation Strategies. Infect. Drug Resist. 2021, 14, 5629–5637. [Google Scholar] [CrossRef] [PubMed]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Doub, J.B.; Urish, K.; Lee, M.; Fackler, J. Impact of Bacterial Phenotypic Variation with Bacteriophage therapy: A Pilot Study with Prosthetic Joint Infection Isolates. Int. J. Infect. Dis. 2022, 119, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, N.; Brossaud, M.; Telles, F.; Sanchez, V.; Talaga, P.; Rokbi, B. Glycosylation of Staphylococcus aureus cell wall teichoic acid is influenced by environmental conditions. Sci. Rep. 2019, 9, 3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azam, A.H.; Tanji, Y. Peculiarities of Staphylococcus aureus phages and their possible application in phage therapy. Appl. Microbiol. Biotechnol. 2019, 103, 4279–4289. [Google Scholar] [CrossRef] [PubMed]
- Doub, J.B.; Ng, V.Y.; Wilson, E.; Corsini, L.; Chan, B.K. Successful Treatment of a Recalcitrant Staphylococcus epidermidis Prosthetic Knee Infection with Intraoperative Bacteriophage Therapy. Pharmaceuticals 2021, 14, 231. [Google Scholar] [CrossRef] [PubMed]
| Phage ID | Titer (PFU/mL) | Endotoxin (EU/Dose) | USP <71> Sterility | Staphylococcal Enterotoxin A (ng/mL) |
|---|---|---|---|---|
| Mallokai | 1 × 1010 | <1 | No Growth | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doub, J.B.; Ng, V.Y.; Lee, M.; Chi, A.; Lee, A.; Würstle, S.; Chan, B. Salphage: Salvage Bacteriophage Therapy for Recalcitrant MRSA Prosthetic Joint Infection. Antibiotics 2022, 11, 616. https://doi.org/10.3390/antibiotics11050616
Doub JB, Ng VY, Lee M, Chi A, Lee A, Würstle S, Chan B. Salphage: Salvage Bacteriophage Therapy for Recalcitrant MRSA Prosthetic Joint Infection. Antibiotics. 2022; 11(5):616. https://doi.org/10.3390/antibiotics11050616
Chicago/Turabian StyleDoub, James B., Vincent Y. Ng, Myounghee Lee, Andrew Chi, Alina Lee, Silvia Würstle, and Benjamin Chan. 2022. "Salphage: Salvage Bacteriophage Therapy for Recalcitrant MRSA Prosthetic Joint Infection" Antibiotics 11, no. 5: 616. https://doi.org/10.3390/antibiotics11050616
APA StyleDoub, J. B., Ng, V. Y., Lee, M., Chi, A., Lee, A., Würstle, S., & Chan, B. (2022). Salphage: Salvage Bacteriophage Therapy for Recalcitrant MRSA Prosthetic Joint Infection. Antibiotics, 11(5), 616. https://doi.org/10.3390/antibiotics11050616






