Treatment Outcome in Patients with Mycobacterium abscessus Complex Lung Disease: The Impact of Tigecycline and Amikacin
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Mycobacterium Abscessus Complex Clinical Strains
2.3. Antimicrobial Susceptibility Testing (AST)
2.4. Administration and Assessment of Effective Treatment Regimens
2.5. Chest Radiographical Scoring
2.6. Assessment of Outcomes
2.7. Statistical Analyses
3. Results
3.1. Patient Enrollment
3.2. Patient Characteristics
3.3. Antimicrobial Susceptibility Testing
3.4. Treatment Prognosis and Modalities
3.5. Effect of Antimicrobial Agents on Microbiology and Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Tan, C.K.; Chou, C.H.; Hsu, H.L.; Liao, C.H.; Huang, Y.T.; Yang, P.C.; Luh, K.T.; Hsueh, P.R. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerg. Infect. Dis. 2010, 16, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Field, S.K.; Cowie, R.L. Lung disease due to the more common nontuberculous mycobacteria. Chest 2006, 129, 1653–1672. [Google Scholar] [CrossRef]
- Chien, J.Y.; Lai, C.C.; Sheng, W.H.; Yu, C.J.; Hsueh, P.R. Pulmonary infection and colonization with nontuberculous mycobacteria, Taiwan, 2000–2012. Emerg. Infect. Dis. 2014, 20, 1382–1385. [Google Scholar] [CrossRef] [Green Version]
- Honda, J.R.; Knight, V.; Chan, E.D. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin. Chest Med. 2015, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Myung, W.; Koh, W.-J.; Moon, S.M.; Jhun, B.W. Epidemiology of Nontuberculous Mycobacterial Infection, South Korea, 2007–2016. Emerg. Infect. Dis. 2019, 25, 569–572. [Google Scholar] [CrossRef]
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Manabu, A.; Mitarai, S.; Hasegawa, N. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef] [Green Version]
- Jhun, B.W.; Moon, S.M.; Jeon, K.; Kwon, O.J.; Yoo, H.; Carriere, K.C.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: A 15-year follow-up study. Eur. Respir. J. 2020, 55, 1900798. [Google Scholar] [CrossRef]
- Diel, R.; Ringshausen, F.; Richter, E.; Welker, L.; Schmitz, J.; Nienhaus, A. Microbiological and Clinical Outcomes of Treating Non-Mycobacterium Avium Complex Nontuberculous Mycobacterial Pulmonary Disease: A Systematic Review and Meta-Analysis. Chest 2017, 152, 120–142. [Google Scholar] [CrossRef]
- Weng, Y.W.; Huang, C.K.; Sy, C.L.; Wu, K.S.; Tsai, H.C.; Lee, S.S. Treatment for Mycobacterium abscessus complex-lung disease. J. Formos. Med. Assoc. 2020, 119 (Suppl. 1), S58–S66. [Google Scholar] [CrossRef]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F.; Leitch, A.; Loebinger, M.R.; Milburn, H.J.; Nightingale, M.; et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72, ii1–ii64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline: Executive Summary. Clin. Infect. Dis. 2020, 71, e1–e36. [Google Scholar] [CrossRef] [PubMed]
- van Ingen, J.; Wagner, D.; Gallagher, J.; Morimoto, K.; Lange, C.; Haworth, C.S.; Floto, R.A.; Adjemian, J.; Prevots, D.R.; Griffith, D.E. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur. Respir. J. 2017, 49, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, N.; Dalcolmo, M.P.; Daley, C.L.; Eather, G.; Gayoso, R.; Hasegawa, N.; Jhun, B.W.; Koh, W.J.; Namkoong, H.; Park, J.; et al. Mycobacterium abscessus pulmonary disease: Individual patient data meta-analysis. Eur. Respir. J. 2019, 54, 1801991. [Google Scholar] [CrossRef]
- Ferro, B.; Srivastava, S.; Deshpande, D.; Pasipanodya, J.G.; van Soolingen, D.; Mouton, J.W.; van Ingen, J.; Gumbo, T. Tigecycline Is Highly Efficacious against Mycobacterium abscessus Pulmonary Disease. Antimicrob. Agents Chemother. 2016, 60, 2895–2900. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.W.; Chen, J.H.; Hu, S.T.; Huang, W.C.; Lee, Y.C.; Huang, C.C.; Shen, G.H. Synergistic activities of tigecycline with clarithromycin or amikacin against rapidly growing mycobacteria in Taiwan. Int. J. Antimicrob. Agents 2013, 41, 218–223. [Google Scholar] [CrossRef]
- Wallace, R.J., Jr.; Dukart, G.; Brown-Elliott, B.A.; Griffith, D.E.; Scerpella, E.G.; Marshall, B. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J. Antimicrob. Chemother. 2014, 69, 1945–1953. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Levin, A.; Kasperbauer, S.H.; Huitt, G.A.; Daley, C.L. Efficacy and safety of tigecycline for Mycobacterium abscessus disease. Respir. Med. 2019, 158, 89–91. [Google Scholar] [CrossRef]
- Zelazny, A.M.; Root, J.M.; Shea, Y.R.; Colombo, R.E.; Shamputa, I.C.; Stock, F.; Conlan, S.; McNulty, S.; Brown-Elliott, B.A.; Wallace, R.J., Jr.; et al. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J. Clin. Microbiol. 2009, 47, 1985–1995. [Google Scholar] [CrossRef] [Green Version]
- Woods, G.L.; Brown-Elliott, B.A.; Conville, P.S.; Desmond, E.P.; Hall, G.S.; Lin, G.; Pfyffer, G.E.; Ridderhof, J.C.; Siddiqi, S.H.; Wallace, R.J., Jr.; et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]
- Lin, C.H.; Shu, C.C.; Hsu, C.L.; Cheng, S.L.; Wang, J.Y.; Yu, C.J.; Lee, L.N. The trend and the disease prediction of vascular endothelial growth factor and placenta growth factor in nontuberculous mycobacterial lung disease. Sci. Rep. 2016, 6, 37266. [Google Scholar] [CrossRef] [Green Version]
- van Ingen, J.; Aksamit, T.; Andrejak, C.; Bottger, E.C.; Cambau, E.; Daley, C.L.; Griffith, D.E.; Guglielmetti, L.; Holland, S.M.; Huitt, G.A.; et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: An NTM-NET consensus statement. Eur. Respir. J. 2018, 51, 1800170. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.C.; Sun, P.L.; Wu, T.L.; Wang, L.H.; Yang, C.H.; Hung, S.I.; Kuo, A.J.; Liu, T.P.; Lu, J.J.; Chiu, C.H.; et al. Antimicrobial resistance in Mycobacterium abscessus complex isolated from patients with skin and soft tissue infections at a tertiary teaching hospital in Taiwan. J. Antimicrob. Chemother. 2017, 72, 2782–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, C.C.; Lee, C.H.; Hsu, C.L.; Wang, J.T.; Wang, J.Y.; Yu, C.J.; Lee, L.N. Clinical characteristics and prognosis of nontuberculous mycobacterial lung disease with different radiographic patterns. Lung 2011, 189, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, L.; Mao, Y.; Ye, M.; Guo, Q.; Zhang, Y.; Xu, L.; Zhang, Z.; Li, B.; Chu, H. Clinical Efficacy and Adverse Effects of Antibiotics Used to Treat Mycobacterium abscessus Pulmonary Disease. Front. Microbiol. 2019, 10, 1977. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Jeon, K.; Kim, H.; Kwon, O.J.; Huh, H.J.; Lee, N.Y.; Daley, C.L.; Koh, W.-J.; Jhun, B.W. Outcomes of inhaled amikacin-containing multidrug regimens for Mycobacterium abscessus pulmonary disease. Chest 2021, 160, 436–445. [Google Scholar] [CrossRef]

| Total (N = 71), | Microbiology Failure (N = 43) | Microbiology Cure (N = 28) | p Value | Treatment Failure (N = 44) | Treatment Success (N = 27) | p Value | |
|---|---|---|---|---|---|---|---|
| Age, years | 63.6 ± 13.07 | 64.3 ± 13.6 | 62.5 ± 12.2 | 0.68 | 64.1 ± 13.6 | 62.9 ± 12.4 | 0.69 |
| Gender, female | 47 (66.2) | 30 (69.8) | 17 (60.7) | 0.43 | 30 (68.2) | 17 (62.9) | 0.65 |
| Body weight, Kg | 50.4 ± 10.6 | 50.8 ± 10.9 | 49.8 ± 0.2 | 0.27 | 50.9 ± 10.9 | 49.8 ± 10.2 | 0.27 |
| Non-smoker | 62 (87.3) | 38 (88.4) | 24 (85.7) | 0.74 | 39 (88.6) | 23 (85.2) | 0.67 |
| Underlying disease | 8 (18.2) | 4 (14.8) | 0.71 | ||||
| Previous pulmonary tuberculosis | 12 (16.9) | 8 (18.6) | 4 (14.3) | 0.63 | 5 (11.4) | 1 (3.7) | 0.26 |
| DM | 6 (8.5) | 5 (11.6) | 1 (3.6) | 0.23 | 1 (2.3) | 1 (3.7) | 0.69 |
| ESRD | 2 (2.8) | 1 (2.3) | 1 (3.6) | 0.75 | 7 (15.9) | 4 (14.8) | 0.5 |
| Malignancy | 11 (15.5) | 7 (16.3) | 4 (14.3) | 0.82 | 4 (9.1) | 4 (14.8) | 0.95 |
| Rheumatic disorder | 8 (11.3) | 6 (14) | 2 (7.1) | 0.37 | 3 (6.8) | 2 (7.4) | 0.92 |
| Asthma | 5 (7.0) | 3 (7) | 2 (7.1) | 0.97 | 8 (18.2) | 5 (18.5) | 0.56 |
| COPD | 13 (18.3) | 7 (16.3) | 6 (21.4) | 0.63 | 36 (81.8) | 23 (85.2) | 0.29 |
| Sputum acid fast smear, positive | 59 (83.1) | 36 (83.7) | 23 (82.1) | 0.86 | |||
| Radiographic pattern | 13 (29.5) | 5 (18.5) | 0.30 | ||||
| Fibrocavitation | 18 (25.4) | 13 (30.2) | 5 (17.9) | 0.24 | 36 (81.8) | 23 (85.2) | 0.35 |
| Nodular bronchiectasis | 59 (83.1) | 35 (81.4) | 24 (85.7) | 0.63 | 64.1 ± 13.6 | 62.9 ± 12.4 | 0.69 |
| Radiographic score | 5.94 ± 3.11 | 6.86 ± 3.42 | 4.46 ± 2.14 | 0.001 | 7.21 ± 3.22 | 4.64 ± 2.41 | <0.001 |
| Surgical resection | 6 (8.5) | 3 (7) | 3 (10.7) | 0.58 | 3 (6.8) | 3(11.1) | 0.86 |
| Mycobacterium species | |||||||
| M. abscessus subsp., unclassified | 26 (36.6) | 17 (39.5) | 9 (32.1) | 0.66 | 18 (40.9) | 8 (29.6) | 0.78 |
| M. abscessus subsp. massiliense | 7 (9.9) | 5 (11.6) | 2 (7.1) | 0.53 | 5 (11.3) | 2 (7.4) | 0.23 |
| M. abscessus subsp. abscessus | 38 (53.5) | 21 (48.8) | 17 (60.7) | 0.59 | 21 (47.7) | 17 (62.9) | 0.65 |
| MIC * (µg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | Range (µg/mL) | |||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | ||||
| TMP-SMX | ≤2 | ≥4 | ||||
| 17 (50) | 0 | 17(50) | 4 | 16 | 0.25–16 | |
| Ciprofloxacin | ≤1 | 2 | ≥4 | |||
| 4 (11.8) | 4 (11.8) | 26 (76.5) | 4 | 8 | 0.25–8 | |
| Moxifloxacin | ≤1 | 2 | ≥4 | |||
| 3 (8.82) | 3 (8.82) | 28 (82.4) | 8 | 16 | 0.5–16 | |
| Cefoxitin | ≤16 | 32 | ≥64 | |||
| 4 (11.8) | 14 (41.2) | 16 (47.1) | 32 | 128 | 4–256 | |
| Amikacin | ≤16 | 32 | ≥64 | |||
| 32 (94.1) | 1 (2.9) | 1 (2.9) | 16 | 16 | 4–64 | |
| Doxycycline | ≤1 | 2 | ≥4 | |||
| 1 (2.9) | 3 (8.8) | 30 (88.2) | 16 | 32 | 0.5–32 | |
| Clarithromycin ERT ** | ≤4 | 8 | ≥16 | |||
| 31 (91.2) | 0 | 3 (8.8) | 0.25 | 1 | 0.125–32 | |
| Clarithromycin LRT*** | ≤4 | 8 | ≥16 | |||
| 12 (35.3) | 0 | 22 (64.7) | 16 | 32 | 0.125–32 | |
| Imipenem | ≤8 | 16 | ≥32 | |||
| 9 (26.5) | 16 (47) | 9 (26.5) | 16 | 32 | 8–128 | |
| Minocycline | ≤4 | ≥8 | ||||
| 4 (11.8) | 0 | 30 (88.2) | 8 | 16 | 1–16 | |
| Linezolid | ≤8 | 16 | ≥32 | |||
| 6 (17.6) | 7 (20.6) | 21 (61.8) | 32 | 64 | 2–64 | |
| Tigecycline | ≤1 | ≥2 | ||||
| 33 (97) | 0 | 1 (3) | 0.25 | 0.5 | 0.125–2 | |
| Treatment Modality | Total (N = 71) | Microbiology Failure (N = 43) | Microbiology Success (N = 28) | p Value | Treatment Failure (N = 44) | Treatment Success (N = 27) | p Value |
|---|---|---|---|---|---|---|---|
| Macrolide use | |||||||
| Clarithromycin | 38 | 24 (63.2) | 14 (36.8) | 0.63 | 25 (65.8) | 13 (34.2) | 0.47 |
| Azithromycin | 30 | 17 (56.7) | 13 (43.3) | 0.56 | 17 (56.6) | 13 (43.3) | 0.43 |
| Non-macrolide use | 11 | 7 (63.6) | 4 (36.4) | 0.82 | 7 (63.6) | 4 (36.4) | 0.92 |
| Resistance | |||||||
| Delayed macrolide resistance * | 22 | 13 (59.1) | 9 (40.1) | 0.91 | 9 (40.1) | 13 (59.1) | 0.91 |
| Macrolide susceptible | 12 | 7(58.3) | 5(41.7) | 0.86 | 7 (58.3) | 5 (41.7) | 0.71 |
| Parenteral drug | |||||||
| Parenteral drug use <4 weeks | 57 | 37 (64.9) | 20 (35.1) | 0.13 | 38 (66.7) | 19 (33.3) | 0.37 |
| Parenteral drug use ≥4 weeks | 14 | 6 (42.9) | 8 (57.1) | 0.13 | 6 (42.9) | 8 (57.1) | 0.37 |
| Amikacin | 18 | 8 (44.4) | 10 (55.6) | 0.1 | 8 (44.4) | 10 (55.6) | 0.37 |
| Imipenem | 8 | 7 (87.5) | 1 (12.5) | 0.09 | 7 (87.5) | 1 (12.5) | 0.04 |
| Fluoroquinolone | 32 | 21 (65.6) | 11 (34.4) | 0.43 | 21 (65.6) | 11 (34.3) | 0.95 |
| Imipenem and amikacin | 2 | 2 (100) | 0 | 0.52 | 2 (100) | 0 | 0.49 |
| Fluoroquinolone and amikacin | 5 | 4(80) | 1(20) | 0.64 | 4 (80) | 1(20) | 0.36 |
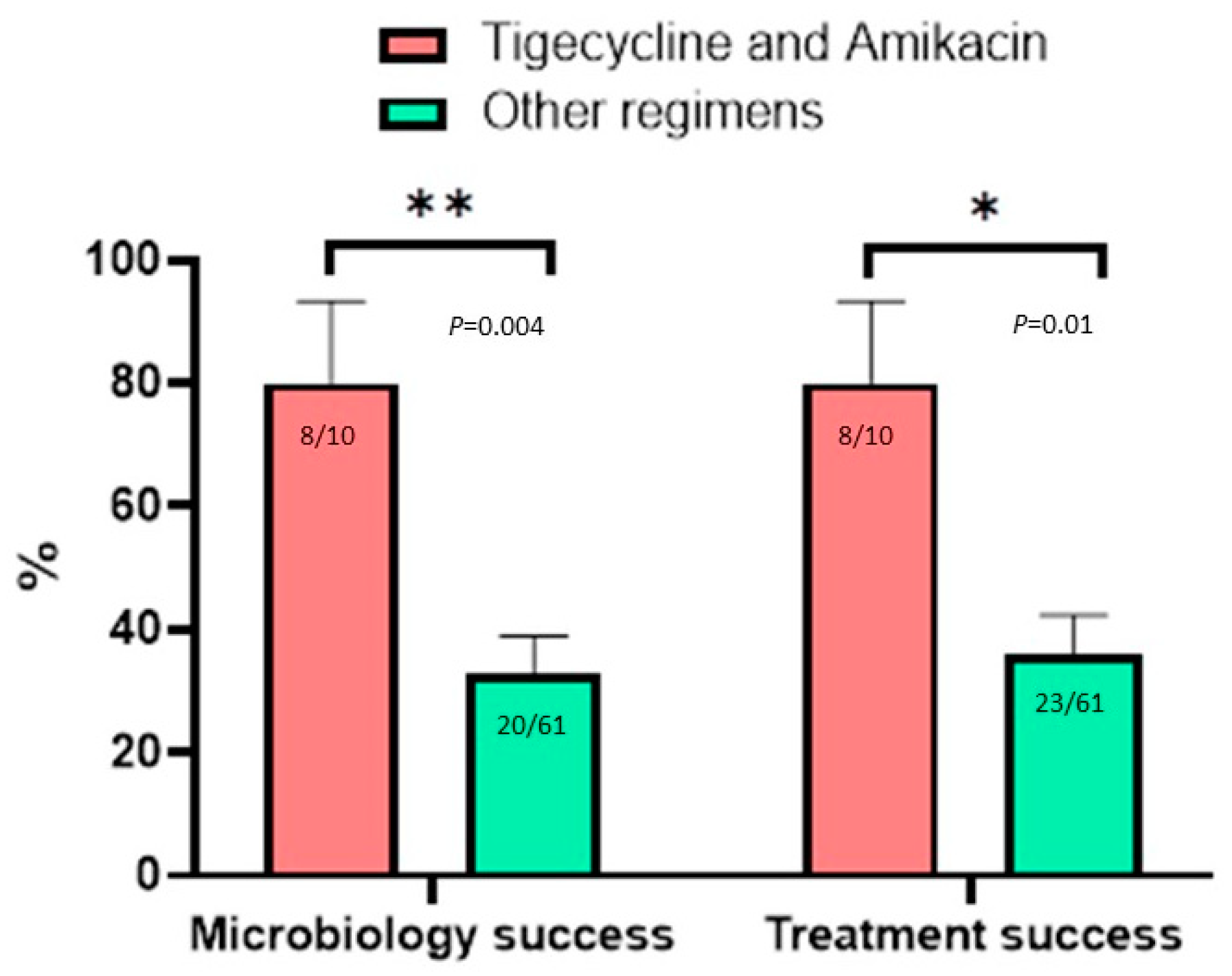
| Tigecycline and amikacin | 10 | 2 (20) | 8 (80) | 0.005 | 2 (20) | 8 (80) | 0.02 |
| Antibiotics | Microbiology Success | Treatment Success | ||||
|---|---|---|---|---|---|---|
| Adjusted OR | 95% CI | p-Value | Adjusted OR | 95% CI | p-Value | |
| Macrolide | 1.097 | 0.227–5.292 | 0.9 | 0.719 | 0.151–3.425 | 0.67 |
| Amikacin | 0.771 | 0.105–5.672 | 0.79 | 0.656 | 0.812–4.831 | 0.67 |
| Imipenem | 0.193 | 0.019–2.017 | 0.17 | 0.169 | 0.330–1.808 | 0.14 |
| Fluoroquinolone | 1.688 | 0.463–6.155 | 0.14 | 2.487 | 0.698–8.850 | 0.16 |
| Tigecycline and amikacin | 17.724 | 1.227–267.206 | 0.03 | 14.085 | 1.103–166.667 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-H.; Wang, P.-H.; Pan, S.-W.; Wei, Y.-F.; Chen, C.-Y.; Lee, H.-S.; Shu, C.-C.; Wu, T.-S. Treatment Outcome in Patients with Mycobacterium abscessus Complex Lung Disease: The Impact of Tigecycline and Amikacin. Antibiotics 2022, 11, 571. https://doi.org/10.3390/antibiotics11050571
Yang J-H, Wang P-H, Pan S-W, Wei Y-F, Chen C-Y, Lee H-S, Shu C-C, Wu T-S. Treatment Outcome in Patients with Mycobacterium abscessus Complex Lung Disease: The Impact of Tigecycline and Amikacin. Antibiotics. 2022; 11(5):571. https://doi.org/10.3390/antibiotics11050571
Chicago/Turabian StyleYang, Jeng-How, Ping-Huai Wang, Sheng-Wei Pan, Yu-Feng Wei, Chung-Yu Chen, Ho-Sheng Lee, Chin-Chung Shu, and Ting-Shu Wu. 2022. "Treatment Outcome in Patients with Mycobacterium abscessus Complex Lung Disease: The Impact of Tigecycline and Amikacin" Antibiotics 11, no. 5: 571. https://doi.org/10.3390/antibiotics11050571
APA StyleYang, J.-H., Wang, P.-H., Pan, S.-W., Wei, Y.-F., Chen, C.-Y., Lee, H.-S., Shu, C.-C., & Wu, T.-S. (2022). Treatment Outcome in Patients with Mycobacterium abscessus Complex Lung Disease: The Impact of Tigecycline and Amikacin. Antibiotics, 11(5), 571. https://doi.org/10.3390/antibiotics11050571






