Microbial Spectra and Clinical Outcomes from Endoscopically Drained Pancreatic Fluid Collections: A Descriptive Cohort Study
Abstract
1. Introduction
2. Results
2.1. Epidemiological and Clinical Characteristics of the Study Population
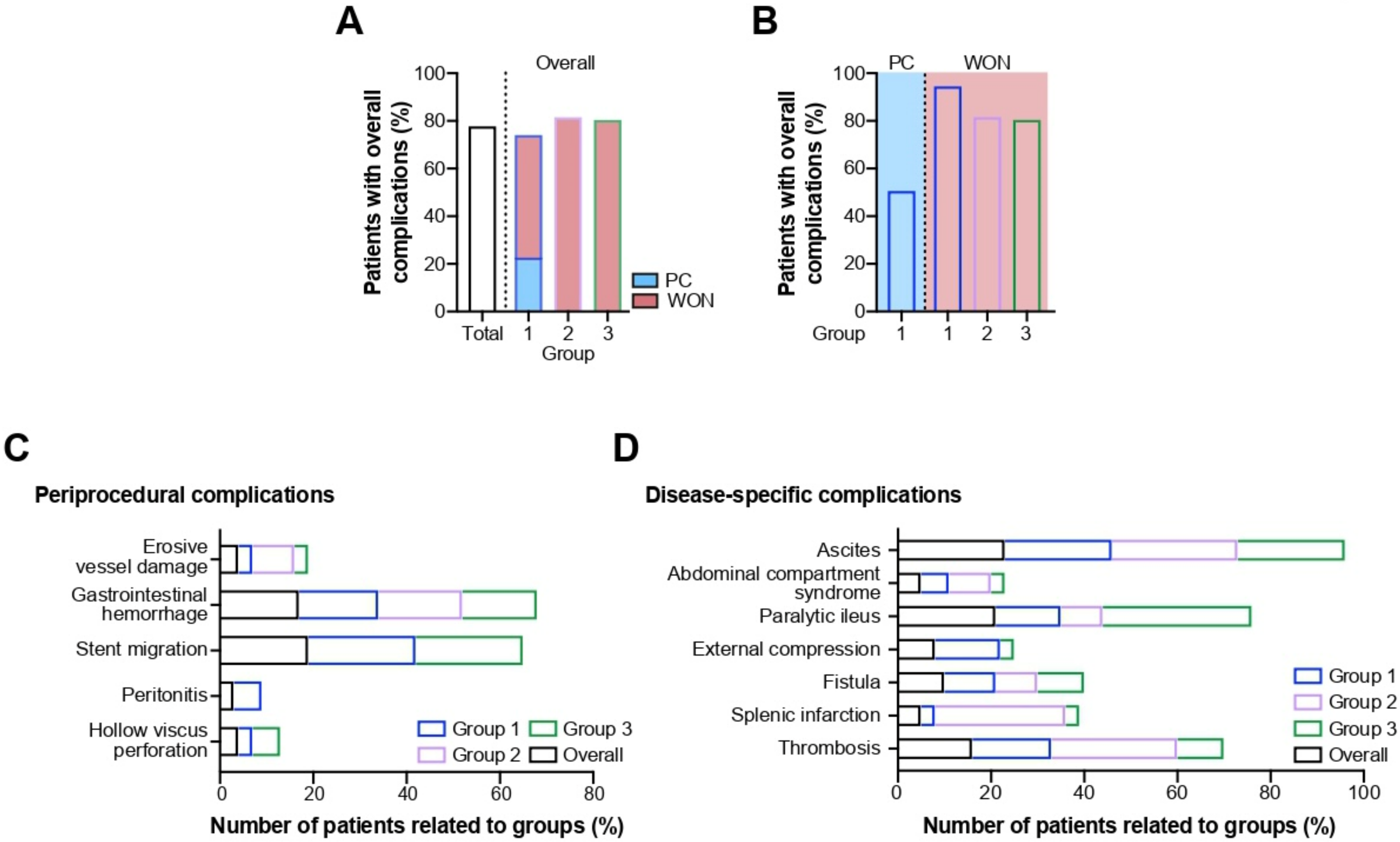
2.2. Procedure- and Disease-Related Complications
2.2.1. Periprocedural Complications
2.2.2. Disease-Specific Complications
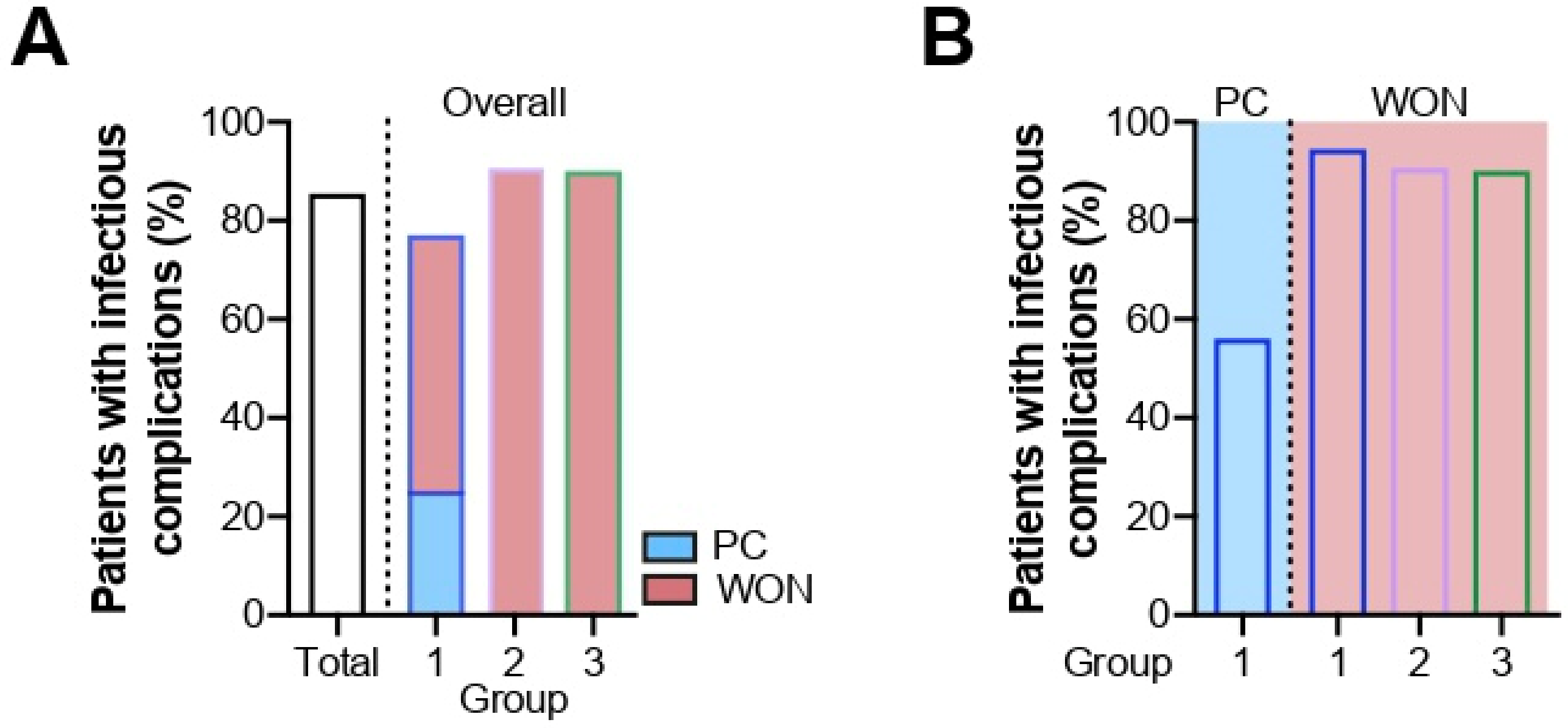
2.3. Infectious Complications
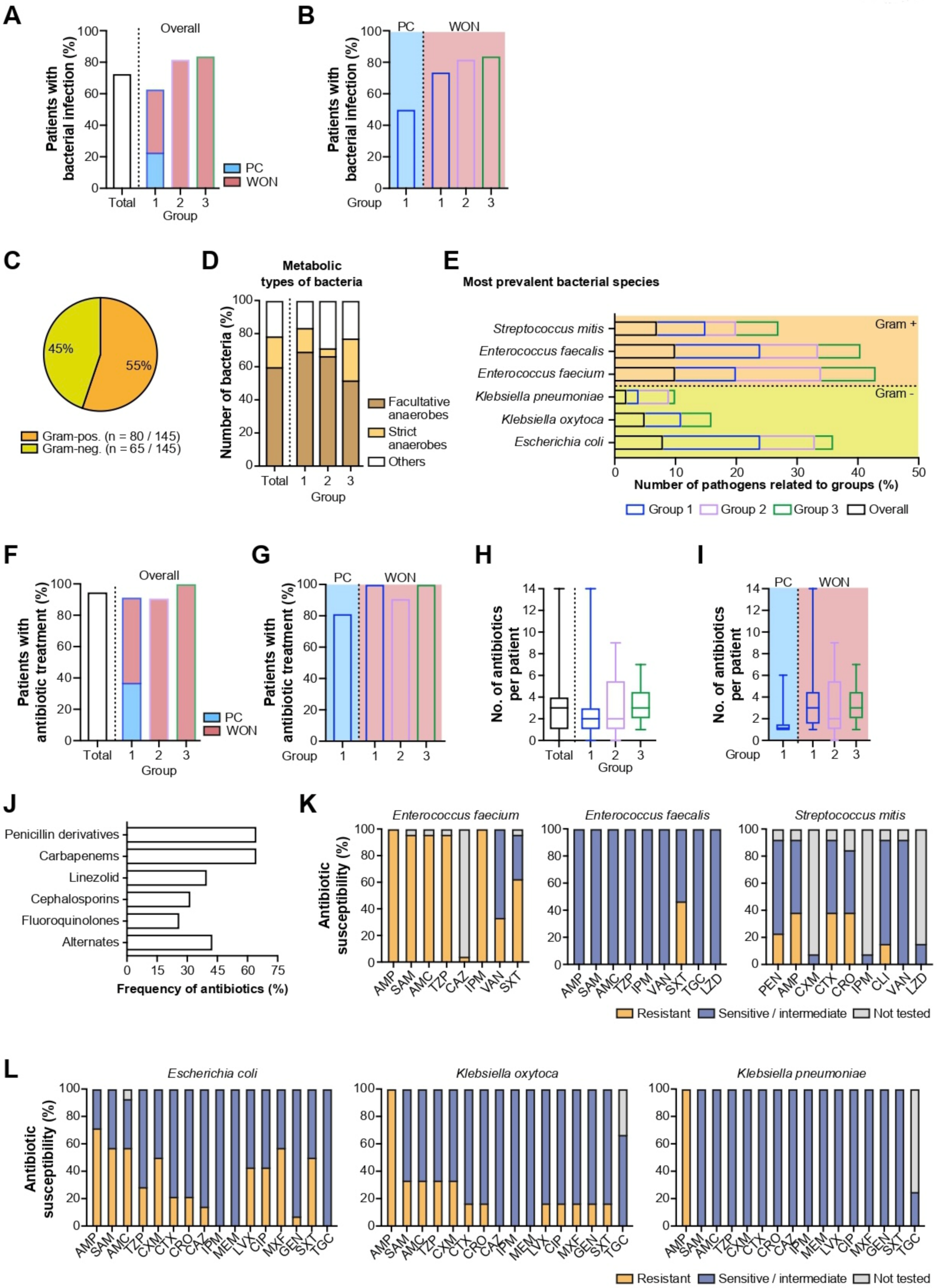
2.3.1. Bacterial Infections
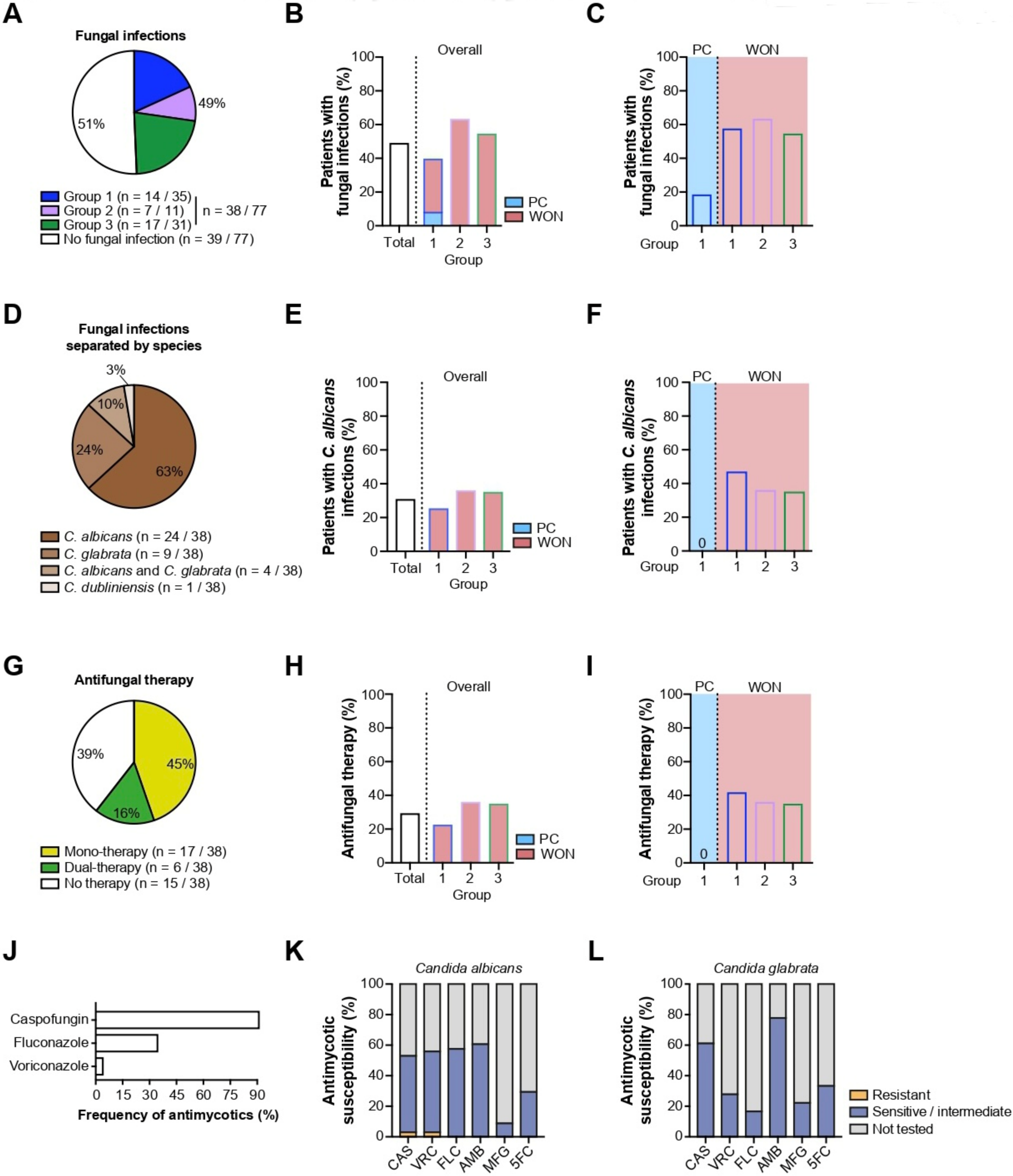
2.3.2. Fungal Infections
3. Discussion
4. Materials and Methods
4.1. Study Design and Data Acquisition
4.2. Stent Devices and Description of Procedure
4.3. Rationale for Anti-Infective Management
4.4. Outcome Measures
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popa, C.C.; Badiu, D.C.; Rusu, O.C.; Grigorean, V.T.; Neagu, S.I.; Strugaru, C.R. Mortality prognostic factors in acute pancreatitis. J. Med. Life 2016, 9, 413–418. [Google Scholar]
- Shi, N.; Liu, T.; de la Iglesia-Garcia, D.; Deng, L.; Jin, T.; Lan, L.; Zhu, P.; Hu, W.; Zhou, Z.; Singh, V.; et al. Duration of organ failure impacts mortality in acute pancreatitis. Gut 2020, 69, 604–605. [Google Scholar] [CrossRef]
- Ouyang, G.; Pan, G.; Liu, Q.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Zhou, Z.; Wen, Y. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. BMC Med. 2020, 18, 388. [Google Scholar] [CrossRef]
- Dumnicka, P.; Maduzia, D.; Ceranowicz, P.; Olszanecki, R.; Drożdż, R.; Kuśnierz-Cabala, B. The Interplay between Inflammation, Coagulation and Endothelial Injury in the Early Phase of Acute Pancreatitis: Clinical Implications. Int. J. Mol. Sci. 2017, 18, 354. [Google Scholar] [CrossRef]
- Komara, N.L.; Paragomi, P.; Greer, P.J.; Wilson, A.S.; Breze, C.; Papachristou, G.I.; Whitcomb, D.C. Severe acute pancreatitis: Capillary permeability model linking systemic inflammation to multiorgan failure. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G573–G583. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Grimm, H.; Binmoeller, K.F.; Soehendra, N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest. Endosc. 1992, 38, 170–171. [Google Scholar] [CrossRef]
- Wiersema, M.J. Endosonography-guided cystoduodenostomy with a therapeutic ultrasound endoscope. Gastrointest. Endosc. 1996, 44, 614–617. [Google Scholar] [CrossRef]
- Kawakami, H.; Itoi, T.; Sakamoto, N. Endoscopic ultrasound-guided transluminal drainage for peripancreatic fluid collections: Where are we now? Gut Liver 2014, 8, 341–355. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zhou, A.; Zhao, G.; Li, P. Comparative outcomes of endoscopic ultrasound-guided lumen-apposing mental stents drainage for pancreatic pseudocysts and walled-off necrosis: Case series and meta-analysis. Chronic Dis. Transl. Med. 2021, 7, 157–168. [Google Scholar] [CrossRef]
- Stecher, S.S.; Simon, P.; Friesecke, S.; Glitsch, A.; Kühn, J.P.; Lerch, M.M.; Mayerle, J. Delayed severe bleeding complications after treatment of pancreatic fluid collections with lumen-apposing metal stents. Gut 2017, 66, 1871–1872. [Google Scholar] [CrossRef]
- Beger, H.G.; Bittner, R.; Block, S.; Büchler, M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology 1986, 91, 433–438. [Google Scholar] [CrossRef]
- Gerzof, S.G.; Banks, P.A.; Robbins, A.H.; Johnson, W.C.; Spechler, S.J.; Wetzner, S.M.; Snider, J.M.; Langevin, R.E.; Jay, M.E. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology 1987, 93, 1315–1320. [Google Scholar] [CrossRef]
- Beger, H.G.; Büchler, M.; Bittner, R.; Block, S.; Nevalainen, T.; Roscher, R. Necrosectomy and postoperative local lavage in necrotizing pancreatitis. Br. J. Surg. 1988, 75, 207–212. [Google Scholar] [CrossRef]
- Delcenserie, R.; Yzet, T.; Ducroix, J.P. Prophylactic antibiotics in treatment of severe acute alcoholic pancreatitis. Pancreas 1996, 13, 198–201. [Google Scholar]
- Ignatavicius, P.; Vitkauskiene, A.; Pundzius, J.; Dambrauskas, Z.; Barauskas, G. Effects of prophylactic antibiotics in acute pancreatitis. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2012, 14, 396–402. [Google Scholar] [CrossRef][Green Version]
- Pederzoli, P.; Bassi, C.; Vesentini, S.; Campedelli, A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg. Gynecol. Obstet. 1993, 176, 480–483. [Google Scholar]
- Schwarz, M.; Isenmann, R.; Meyer, H.; Beger, H.G. Antibiotic use in necrotizing pancreatitis. Results of a controlled study. Dtsch. Med. Wochenschr. 1997, 122, 356–361. [Google Scholar] [CrossRef]
- Villatoro, E.; Mulla, M.; Larvin, M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst. Rev. 2010, 2010, Cd002941. [Google Scholar] [CrossRef]
- IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP 2013, 13, E1–E15. [CrossRef]
- Beyer, G.H.; Michl, P.; Gress, T.M.; Algül, H.; Neesse, A.; Meining, A.; Seufferlein, T.W.; Rosendahl, J.; Kahl, S.; Keller, J.; et al. S3-Leitlinie Pankreatitis. Leitlinie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS). Z. Gastroenterol. 2021, 59, 691–776. [Google Scholar]
- Singh, R.R.; Mitchell, W.; David, Y.; Cheesman, A.; Dixon, R.E.; Nagula, S.; DiMaio, C.J.; Greenwald, D.A.; Kumta, N.A. Pancreatic Fungal Infection in Patients With Necrotizing Pancreatitis: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2021, 55, 218–226. [Google Scholar] [CrossRef]
- Trikudanathan, G.; Navaneethan, U.; Vege, S.S. Intra-abdominal fungal infections complicating acute pancreatitis: A review. Am. J. Gastroenterol. 2011, 106, 1188–1192. [Google Scholar] [CrossRef]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Karsenti, D.; Bourlier, P.; Dorval, E.; Scotto, B.; Giraudeau, B.; Lanotte, R.; de Calan, L.; Mesny, J.; Lagarrigue, F.; Metman, E. Morbidity and mortality of acute pancreatitis. Prospective study in a French university hospital. Presse Med. 2002, 31, 727–734. [Google Scholar]
- Yadav, J.; Yadav, S.K.; Kumar, S.; Baxla, R.G.; Sinha, D.K.; Bodra, P.; Besra, R.C.; Baski, B.M.; Prakash, O.; Anand, A. Predicting morbidity and mortality in acute pancreatitis in an Indian population: A comparative study of the BISAP score, Ranson’s score and CT severity index. Gastroenterol. Rep. 2016, 4, 216–220. [Google Scholar] [CrossRef]
- Mohan, B.P.; Jayaraj, M.; Asokkumar, R.; Shakhatreh, M.; Pahal, P.; Ponnada, S.; Navaneethan, U.; Adler, D.G. Lumen apposing metal stents in drainage of pancreatic walled-off necrosis, are they any better than plastic stents? A systematic review and meta-analysis of studies published since the revised Atlanta classification of pancreatic fluid collections. Endosc. Ultrasound 2019, 8, 82–90. [Google Scholar] [CrossRef]
- Shah, R.J.; Shah, J.N.; Waxman, I.; Kowalski, T.E.; Sanchez-Yague, A.; Nieto, J.; Brauer, B.C.; Gaidhane, M.; Kahaleh, M. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2015, 13, 747–752. [Google Scholar] [CrossRef]
- Walter, D.; Will, U.; Sanchez-Yague, A.; Brenke, D.; Hampe, J.; Wollny, H.; López-Jamar, J.M.; Jechart, G.; Vilmann, P.; Gornals, J.B.; et al. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: A prospective cohort study. Endoscopy 2015, 47, 63–67. [Google Scholar] [CrossRef]
- Yoon, S.B.; Lee, I.S.; Choi, M.G. Metal versus plastic stents for drainage of pancreatic fluid collection: A meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 729–738. [Google Scholar] [CrossRef]
- Tan, S.; Zhong, C.; Ren, Y.; Luo, X.; Xu, J.; Peng, Y.; Fu, X.; Tang, X. Are Lumen-Apposing Metal Stents More Effective Than Plastic Stents for the Management of Pancreatic Fluid Collections: An Updated Systematic Review and Meta-analysis. Gastroenterol. Res. Pract. 2020, 2020, 4952721. [Google Scholar] [CrossRef]
- Bazerbachi, F.; Sawas, T.; Vargas, E.J.; Prokop, L.J.; Chari, S.T.; Gleeson, F.C.; Levy, M.J.; Martin, J.; Petersen, B.T.; Pearson, R.K.; et al. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: A systematic review and meta-analysis. Gastrointest. Endosc. 2018, 87, 30–42e15. [Google Scholar] [CrossRef]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.K.; Sutton, B.; Hawes, R.; Varadarajulu, S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut 2019, 68, 1200–1209. [Google Scholar] [CrossRef]
- Ge, P.S.; Young, J.Y.; Jirapinyo, P.; Dong, W.; Ryou, M.; Thompson, C.C. Comparative Study Evaluating Lumen Apposing Metal Stents Versus Double Pigtail Plastic Stents for Treatment of Walled-Off Necrosis. Pancreas 2020, 49, 236–241. [Google Scholar] [CrossRef]
- Chandrasekhara, V.; Barthet, M.; Devière, J.; Bazerbachi, F.; Lakhtakia, S.; Easler, J.J.; Peetermans, J.A.; McMullen, E.; Gjata, O.; Gourlay, M.L.; et al. Safety and efficacy of lumen-apposing metal stents versus plastic stents to treat walled-off pancreatic necrosis: Systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E1639–E1653. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Kowalski, T.E.; Loren, D.E.; Khalid, A.; Soomro, A.; Mazhar, S.M.; Isby, L.; Kahaleh, M.; Karia, K.; Yoo, J.; et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: Clinical outcomes and success. Gastrointest. Endosc. 2017, 85, 758–765. [Google Scholar] [CrossRef]
- Boxhoorn, L.; Fockens, P.; Besselink, M.G.; Bruno, M.J.; van Hooft, J.E.; Verdonk, R.C.; Voermans, R.P. Endoscopic Management of Infected Necrotizing Pancreatitis: An Evidence-Based Approach. Curr. Treat. Options Gastroenterol. 2018, 16, 333–344. [Google Scholar] [CrossRef]
- Chantarojanasiri, T.; Ratanachu-Ek, T.; Isayama, H. When Should We Perform Endoscopic Drainage and Necrosectomy for Walled-Off Necrosis? J. Clin. Med. 2020, 9, 4072. [Google Scholar] [CrossRef]
- Chen, Y.I.; Yang, J.; Friedland, S.; Holmes, I.; Law, R.; Hosmer, A.; Stevens, T.; Franco, M.C.; Jang, S.; Pawa, R.; et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: A large international multicenter study. Endosc. Int. Open 2019, 7, E347–E354. [Google Scholar] [CrossRef]
- Schmidt, P.N.; Roug, S.; Hansen, E.F.; Knudsen, J.D.; Novovic, S. Spectrum of microorganisms in infected walled-off pancreatic necrosis—Impact on organ failure and mortality. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP 2014, 14, 444–449. [Google Scholar] [CrossRef]
- Kochhar, R.; Noor, M.T.; Wig, J. Fungal infections in severe acute pancreatitis. J. Gastroenterol. Hepatol. 2011, 26, 952–959. [Google Scholar] [CrossRef]
- Hoerauf, A.; Hammer, S.; Müller-Myhsok, B.; Rupprecht, H. Intra-abdominal Candida infection during acute necrotizing pancreatitis has a high prevalence and is associated with increased mortality. Crit. Care Med. 1998, 26, 2010–2015. [Google Scholar] [CrossRef]
- King, N.K.; Siriwardana, H.P.; Wood, B.; Siriwardena, A.K. Trends in fungal colonization of pancreatic necrosis in patients undergoing necrosectomy for acute pancreatitis. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2005, 7, 120–123. [Google Scholar] [CrossRef]
- Werge, M.; Roug, S.; Novovic, S.; Schmidt, P.N.; Hansen, E.F.; Knudsen, J.D. Fungal Infections in Patients With Walled-off Pancreatic Necrosis. Pancreas 2016, 45, 1447–1451. [Google Scholar] [CrossRef]
- De Waele, J.J.; Vogelaers, D.; Blot, S.; Colardyn, F. Fungal infections in patients with severe acute pancreatitis and the use of prophylactic therapy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003, 37, 208–213. [Google Scholar] [CrossRef]
- Kylänpää, M.L.; Repo, H.; Puolakkainen, P.A. Inflammation and immunosuppression in severe acute pancreatitis. World J. Gastroenterol. 2010, 16, 2867–2872. [Google Scholar] [CrossRef]
- Albers, D.; Meining, A.; Hann, A.; Ayoub, Y.K.; Schumacher, B. Direct endoscopic necrosectomy in infected pancreatic necrosis using lumen-apposing metal stents: Early intervention does not compromise outcome. Endosc. Int. Open 2021, 9, E490–E495. [Google Scholar] [CrossRef]
- Ryan, B.M.; Venkatachalapathy, S.V.; Huggett, M.T. Safety of lumen-apposing metal stents (LAMS) for pancreatic fluid collection drainage. Gut 2017, 66, 1530–1531. [Google Scholar] [CrossRef]
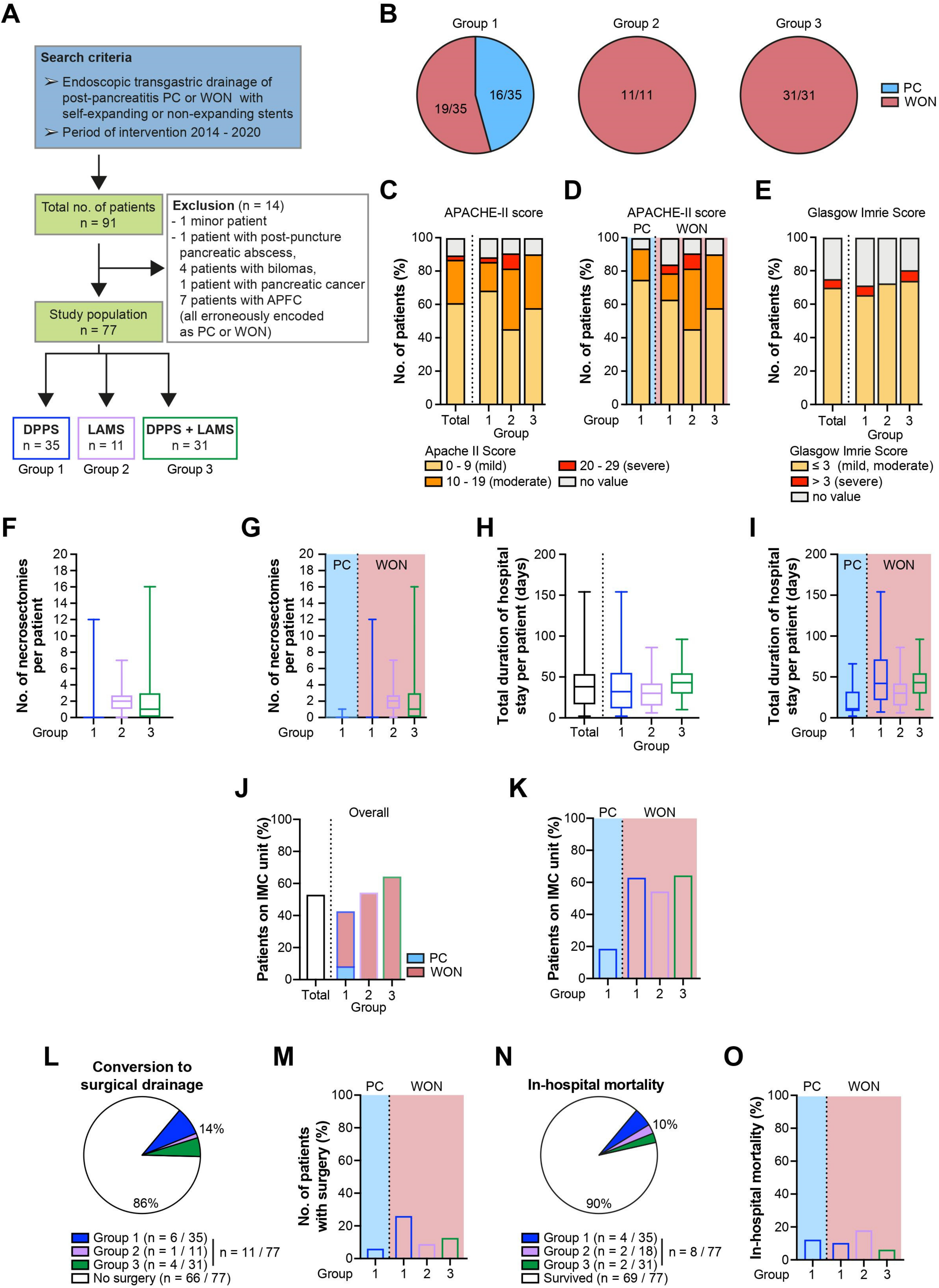
| Overall | Group 1 | PC | WON | Group 2 | Group 3 | |
|---|---|---|---|---|---|---|
| Number of Patients | 77 | 35 | 16 | 19 | 11 | 31 |
| Female, n (%) | 23 (30) | 10 (29) | 3 (19) | 7 (37) | 4 (36) | 9 (29) |
| Male, n (%) | 54 (70) | 25 (71) | 13 (81) | 12 (63) | 7 (64) | 22 (71) |
| Age (years) (mean ± standard deviation) | 55 ± 17 | 55 ± 14 | 49 ± 14 | 59 ± 12 | 56 ± 17 | 55 ± 19 |
| Apache-II score | ||||||
| 0–9, n (%) | 47 (61) | 24 (69) | 12 (75) | 12 (63) | 5 (45) | 18 (58) |
| 10–19, n (%) | 20 (26) | 6 (17) | 3 (19) | 3 (16) | 4 (36) | 10 (32) |
| 20–29, n (%) | 2 (3) | 1 (3) | 0 (0) | 1 (5) | 1 (9) | 0 (0) |
| >30, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Glasgow Imrie score | ||||||
| ≤3, n (%) | 54 (70) | 23 (66) | 9 (56) | 14 (74) | 8 (73) | 8 (73) |
| >3, n (%) | 4 (5) | 2 (6) | 1 (6) | 1 (5) | 0 (0) | 0 (0) |
| not recorded, n (%) | 19 (25) | 10 (29) | 6 (38) | 4 (21) | 3 (27) | 3 (27) |
| Total duration of hospital stay per patient (days) (median, 25%; 75% quartile) | 38 (16; 54) | 32 (11; 56) | 11 (7.75; 33) | 42 (21; 72) | 30 (14.5; 43) | 43 (29; 55) |
| Number of endoscopic necrosectomy sessions (median, 25%; 75% quartile) | 0 (0; 2) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | 2 (1; 2.75) | 1 (0; 3) |
| Conversion to surgery, n (%; 95% CI) | 11 (14; 8–24%) | 6 (17; 8–33%) | 1 (6; 0–30%) | 5 (26; 11–49%) | 1 (9; 0–40%) | 4 (13; 5–29%) |
| Transfer to IMC, n (%; CI) | 41 (53, 42–64%) | 15 (43; 28–59%) | 3 (19; 6–44%) | 12 (63; 41–81%) | 6 (55; 28–79%) | 20 (65; 47–79%) |
| Mortality, n (%, 95% CI) | 8 (10; 5–19%) | 4 (11; 4–27%) | 2 (13; 2–37%) | 2 (11; 2–33%) | 2 (18; 4–49%) | 2 (6; 7–22%) |
| Overall | Group 1 | PC | WON | Group 2 | Group 3 | |
|---|---|---|---|---|---|---|
| Overall complications | 60 (78; 67–86%) | 26 (74; 58–86%) | 8 (50; 28–72%) | 18 (95; 74–100%) | 9 (82; 51–96%) | 25 (81; 63–91%) |
| Periprocedural adverse events | ||||||
| Gastrointestinal hemorrhage, n (%; 95% CI) | 13 (17; 10–27%) | 6 (17; 8–33%) | 2 (13; 2–37%) | 4 (21; 8–44%) | 2 (18; 4–49%) | 5 (16; 7–33%) |
| Perforation, n (%; 95% CI) | 3 (4, 1–11%) | 1 (3; 0–16%) | 0 (0; 0–23%) | 1 (5; 0–26%) | 0 (0; 0–30%) | 2 (6; 1–22%) |
| Stent migration, n (%; 95% CI) | 15 (19, 12–30%) | 8 (23; 12–39%) | 2 (13; 2–37%) | 6 (32; 15–54%) | 0 (0; 0–30%) | 7 (23; 11–40%) |
| Erosive vessel damage, n (%) | 3 (4) | 1 (3) | 0 (0) | 1 (5) | 1 (9) | 1 (3) |
| Cardiovascular complications | ||||||
| Hemodynamic instability, n (%) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 1 (3) |
| Thrombosis, n (%) | 12 (16) | 6 (17) | 3 (19) | 3 (16) | 3 (27) | 3 (10) |
| Splenic infarction, n (%) | 4 (5) | 1 (3) | 1 (6) | 0 (0) | 2 (28) | 1 (3) |
| Respiratory complications | ||||||
| Pneumonia, n (%) | 17 (22) | 7 (20) | 3 (19) | 4 (21) | 5 (45) | 5 (16) |
| Respiratory failure, n (%) | 5 (6) | 2 (6) | 1 (6) | 1 (5) | 1 (9) | 2 (6) |
| Need for mechanical ventilation, n (%) | 13 (17) | 5 (14) | 2 (13) | 3 (16) | 4 (36) | 4 (13) |
| Pleural effusion, n (%) | 32 (42) | 14 (40) | 1 (6) | 13 (68) | 5 (45) | 13 (42) |
| Gastrointestinal complications | ||||||
| Fistula, n (%) | 8 (10) | 4 (11) | 1 (6) | 3 (16) | 1 (9) | 3 (10) |
| Ascites, n (%) | 18 (23) | 8 (23) | 3 (19) | 5 (26) | 3 (27) | 7 (23) |
| Ileus, n (%) | 16 (21) | 5 (14) | 1 (6) | 4 (21) | 1 (9) | 10 (32) |
| Abdominal compartment syndrome, n (%) | 4 (5) | 2 (6) | 0 (0) | 2 (11) | 1 (9) | 1 (3) |
| Gastric/duodenal outlet syndrome, n (%) | 6 (8) | 5 (14) | 3 (19) | 2 (11) | 0 (0) | 1 (3) |
| Peritonitis, n (%; CI) | 2 (3, 0–10%) | 2 (6; 1–20%) | 0 (0; 0–23%) | 2 (11; 2–33%) | 0 (0; 0–30%) | 0 (0; 0–13%) |
| Biliary complications, n (%) | 6 (8) | 4 (11) | 2 (13) | 2 (11) | 1 (9) | 1 (3) |
| Renal complications | ||||||
| Acute renal failure, n (%) | 18 (23) | 5 (14) | 2 (13) | 3 (16) | 5 (45) | 8 (26) |
| Overall | Group 1 | PC | WON | Group 2 | Group 3 | |
|---|---|---|---|---|---|---|
| Overall infection, (%; 95% CI) | 66 (86, 76–92%) | 28 (80; 64–90%) | 9 (56; 33–77%) | 18 (95; 74–100%) | 10 (91; 60–100%) | 28 (90; 74–97%) |
| Bacterial infection, n (%; 95% CI) | 56 (73; 62–81%) | 22 (63; 46–77%) | 8 (50; 28–72%) | 14 (74; 51–89%) | 9 (82; 51–96%) | 26 (84; 67–93%) |
| Bacterial pathogens, n (%) | 145 (100) | 49 (100) | 11 (100) | 38 (100) | 21 (100) | 75 (100) |
| Gram-positive, n (%; CI) | 80 (55; 47–63%) | 26 (53; 39–66%) | 6 (55; 28–79%) | 20 (53; 37–68%) | 11 (52; 32–72%) | 43 (57; 46–68%) |
| Gram-negative, n (%; CI) | 65 (45; 37–53%) | 23 (47; 34–61%) | 5 (45; 21–72%) | 18 (47; 32–63%) | 10 (48; 28–68%) | 32 (43; 32–54%) |
| Strict anaerobes, n (%) | 27 (19) | 7 (14) | 4 (36) | 5 (8) | 1 (5) | 19 (25) |
| Facultative anaerobes, n (%) | 87 (60) | 34 (69) | 6 (55) | 28 (74) | 14 (67) | 39 (52) |
| Use of antibiotics, n (%; CI) | 73 (95, 87–98%) | 32 (91; 77–98%) | 13 (81; 56–94%) | 19 (100; 80–100%) | 10 (91; 60–100%) | 31 (100; 87–100%) |
| Number of antibiotics per patient (median, 25%; 75% quartile) | 3 (1; 4) | 2 (1; 3) | 1 (1; 1.5) | 3 (1.5; 4.5) | 2 (1; 5.5) | 3 (2; 4.5) |
| Fluoroquinolones | 19 | |||||
| Cephalosporines | 23 | |||||
| Penicillins and β-lactamase inhibitors | 47 | |||||
| Carbapenems | 47 | |||||
| Linezolide | 29 | |||||
| Others | 31 | |||||
| Fungal infection, n (%; 95% CI) | 38 (49; 38–60%) | 14 (40; 26–56%) | 3 (19; 6–44%) | 11 (58; 36–77%) | 7 (64; 35–85%) | 17 (55; 38–71%) |
| Candida albicans, n (%, 95% CI) | 24 (31; 22–42%) | 9 (26; 14–42%) | 0 (0; 0–23%) | 9 (47; 27–68%) | 4 (36; 15–65%) | 11 (35; 21–53%) |
| Candida glabrata, n (%, 95% CI) | 9 (12; 6–21) | 4 (11; 4–27%) | 2 (13; 2–37%) | 2 (11; 2–33%) | 2 (18; 4–49%) | 3 (10; 3–26%) |
| Candida dubliniensis, n (%, 95% CI) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (9; 0–40%) | 0 (0) |
| Candida tropicalis + albicans + glabrata, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Use of antimycotis, n (%; 95% CI) | 23 (31) | 8 (23;12–39%) | 0 (0; 0–23%) | 8 (42; 23–63%) | 4 (36; 15–65%) | 11 (36; 19–55%) |
| 0, n (%) | 54 (70) | 27 (77) | 16 (100) | 11 (58) | 7 (64) | 20 (65) |
| 1, n (%) | 17 (22) | 5 (14) | 0 (0) | 5 (26) | 2 (18) | 10 (32) |
| 2, n (%) | 6 (8) | 3 (9) | 0 (0) | 3 (16) | 2 (18) | 1 (3) |
| Voriconazole | 1 | |||||
| Fluconazole | 8 | |||||
| Caspofungin | 21 |
| Overall | Group 1 | Group 2 | Group 3 | |
|---|---|---|---|---|
| Gram-positive bacteria | 80 | 26 | 11 | 43 |
| Facultative anaerobes | 54 | 19 | 9 | 26 |
| Enterococcus faecium | 15 | 5 | 3 | 7 |
| Enterococcus faecalis | 14 | 7 | 2 | 5 |
| Streptococcus mitis | 10 | 4 | 1 | 5 |
| Staphylococcus haemolyticus | 5 | 1 | 1 | 3 |
| Staphylococcus hominis | 1 | 0 | 0 | 1 |
| Staphylococcus aureus | 2 | 1 | 1 | 0 |
| Staphylococcus epidermidis | 4 | 1 | 0 | 3 |
| Streptococcus oralis | 1 | 0 | 0 | 1 |
| Streptococcus salivarius | 1 | 0 | 0 | 1 |
| Streptococcus sanguinis | 1 | 0 | 1 | 0 |
| Strict Anaerobes | 7 | 3 | 0 | 4 |
| Lactobacillus species | 3 | 2 | 0 | 1 |
| Actinomyces species | 1 | 0 | 0 | 1 |
| Bifidobacterium species | 1 | 0 | 0 | 1 |
| Peptoniphilus saccharolyticus | 1 | 0 | 0 | 1 |
| Propionibacterium acnes | 1 | 1 | 0 | 0 |
| Others | 19 | 4 | 2 | 13 |
| Streptococcus anginosus | 9 | 1 | 1 | 7 |
| Corynebacterium species | 1 | 0 | 0 | 1 |
| Streptococcus species | 4 | 2 | 0 | 2 |
| Other Gram-positive species | 5 | 1 | 1 | 3 |
| Gram-negative bacteria | 65 | 23 | 10 | 32 |
| Facultative Anaerobes | 33 | 15 | 5 | 13 |
| Escherichia coli | 12 | 8 | 2 | 2 |
| Klebsiella oxytoca | 7 | 3 | 0 | 4 |
| Klebsiella pneumoniae | 3 | 1 | 1 | 1 |
| Enterobacter cloacae | 4 | 0 | 1 | 3 |
| Citrobacter freundii | 2 | 0 | 1 | 1 |
| Proteus vulgaris | 1 | 0 | 0 | 1 |
| Aeromonas caviae | 1 | 1 | 0 | 0 |
| Eikenella species | 1 | 0 | 0 | 1 |
| Hafnia alvei | 1 | 1 | 0 | 0 |
| Morganella morganii | 1 | 1 | 0 | 0 |
| Strict Anaerobes | 20 | 4 | 1 | 15 |
| Bacteroides fragilis | 3 | 1 | 1 | 1 |
| Prevotella buccae | 3 | 0 | 0 | 3 |
| Veillonella species | 3 | 1 | 0 | 2 |
| Prevotella denticola | 2 | 0 | 0 | 2 |
| Prevotella oralis | 2 | 0 | 0 | 2 |
| Prevotella disiens | 1 | 0 | 0 | 1 |
| Prevotella melaninogenica | 1 | 1 | 0 | 0 |
| Prevotella species | 3 | 1 | 0 | 2 |
| Megasphaera species | 1 | 0 | 0 | 1 |
| Fusobacterium species | 1 | 0 | 0 | 1 |
| Others | 12 | 4 | 4 | 4 |
| Pseudomonas aeruginosa | 3 | 1 | 1 | 1 |
| Acinetobacter Iwolfii | 1 | 0 | 0 | 1 |
| Stenotrophomonas maltophilia | 2 | 1 | 1 | 0 |
| Other Gram-negative species | 6 | 2 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hentschel, V.; Walter, B.; Harder, N.; Arnold, F.; Seufferlein, T.; Wagner, M.; Müller, M.; Kleger, A. Microbial Spectra and Clinical Outcomes from Endoscopically Drained Pancreatic Fluid Collections: A Descriptive Cohort Study. Antibiotics 2022, 11, 420. https://doi.org/10.3390/antibiotics11030420
Hentschel V, Walter B, Harder N, Arnold F, Seufferlein T, Wagner M, Müller M, Kleger A. Microbial Spectra and Clinical Outcomes from Endoscopically Drained Pancreatic Fluid Collections: A Descriptive Cohort Study. Antibiotics. 2022; 11(3):420. https://doi.org/10.3390/antibiotics11030420
Chicago/Turabian StyleHentschel, Viktoria, Benjamin Walter, Noemi Harder, Frank Arnold, Thomas Seufferlein, Martin Wagner, Martin Müller, and Alexander Kleger. 2022. "Microbial Spectra and Clinical Outcomes from Endoscopically Drained Pancreatic Fluid Collections: A Descriptive Cohort Study" Antibiotics 11, no. 3: 420. https://doi.org/10.3390/antibiotics11030420
APA StyleHentschel, V., Walter, B., Harder, N., Arnold, F., Seufferlein, T., Wagner, M., Müller, M., & Kleger, A. (2022). Microbial Spectra and Clinical Outcomes from Endoscopically Drained Pancreatic Fluid Collections: A Descriptive Cohort Study. Antibiotics, 11(3), 420. https://doi.org/10.3390/antibiotics11030420







