1. Introduction
Antimicrobial resistance (AMR) is a global threat to human health. AMR is a leading cause of death worldwide with an especially high burden in low-resource countries [
1]. According to the World Health Organization (WHO), bacterial antimicrobial resistance is one of the biggest threats to global health, food security, and development. Antibiotic resistance can affect anyone, of any age, in any country, and leads to longer hospital stays, higher medical costs, and increased mortality [
2]. Most recent estimates have revealed that, by 2050, antibiotic resistance will have reduced the world’s population by as much as 444 million [
3,
4]. According to the Antimicrobial Resistance Collaborators [
5], AMR is a health problem whose dimensions are at least comparable to major diseases such as HIV and malaria. Furthermore, the Antimicrobial Resistance Collaborators identified six leading pathogens for deaths attributed to resistance, 1.
Escherichia coli, followed by 2.
Staphylococcus aureus, 3.
Klebsiella pneumoniae, 4.
Streptococcus pneumoniae, 5.
Acinetobacter baumannii, and 6.
Pseudomonas aeruginosa.Antibiotic resistance is of particular relevance for intensive care medicine, as ICU physicians worldwide are facing more and more patients infected by bacteria for which limited or no adequate therapeutic options are available [
6,
7]. In intensive care units (ICUs), incidence rates for vancomycin-resistant enterococci (VRE) and Enterobacteriaceae resistant to third-generation cephalosporins and fluoroquinolones have been increasing [
8]. Of particular concern is the increase in carbapenem-resistant
K. pneumoniae [
9].
It has been shown that the use of antibiotics in ICUs, especially of carbapenems, is associated with the increasing development of antibiotic resistance [
10]. The WHO and multiple other groups and researchers agree that the increase in AMR requires global action and a strategic plan. A “One Health” approach is needed here; because AMR is such an essential global issue, to combat AMR, multiple sectors need to communicate and work together to achieve better public health outcomes [
4,
11,
12,
13,
14]. Therefore, as early as 2011, the WHO published a European strategic action plan on antibiotic resistance [
15]. This action plan has been implemented in Germany through the S3 Guideline “Strategies for ensuring rational antibiotic use”, which identifies the requirements and core strategies for antibiotic stewardship [
16,
17].
One of the core elements of antibiotic stewardship is the cooperation between ICU physicians and hospital pharmacists with special qualifications in infectious diseases. We aimed to further optimize antibiotic stewardship (ABS) and integrate ABS into intensive care interprofessional grand rounds with attending ICU physicians, hospital pharmacists, and ICU nursing staff. We aimed to facilitate and initiate interprofessional ABS, integrating and empowering physicians, hospital pharmacists, and nurses. Furthermore, we added ABS education to the specific in-house curricula for students of medicine, pharmacy, and nursing [
18]. To adequately educate healthcare professionals and students for a global society, we suggest expanding and optimizing interprofessional ABS [
19,
20,
21,
22].
Our MICU at a German university medical center is specialized in the treatment of gastrointestinal and liver diseases. Particularly noteworthy is the MICU’s focus on liver transplantation, which is reflected in the high number of patients on the waiting list for liver transplantation with acute decompensation of disease and acute-on-chronic liver failure. Infections in patients with cirrhosis represent an increasing health and economic burden [
23,
24]. Patients with liver cirrhosis are prone to acute decompensation, acute-on-chronic liver failure (ACLF), and hospitalization, including the application of broad-spectrum antibiotics due to infections. Infections constitute the most frequent etiology of ACLF which is defined by the presence of organ failure [
25,
26,
27]. Therefore, infections have been proposed as the fourth major complication of liver cirrhosis in addition to ascites, hepatic encephalopathy, and gastrointestinal hemorrhage [
28]. Among bacterial infections, spontaneous bacterial peritonitis, sepsis, and pneumonia were more frequently associated with ACLF than other infections in the CANONIC study [
27].
Of major concern is the increasing prevalence of infections with multidrug-resistant organisms in patients with chronic liver disease [
29,
30,
31,
32]. Thus, patients on the waiting list for liver transplantation constitute a particularly vulnerable patient group, and implementation of infection control measures and optimized ABS programs are essential in transplantation centers.
Here, we have addressed the following topics among the broad spectrum of ABS: (i) indication and selection of therapy, (ii) optimization of dosing, (iii) drug interactions, (iv) side effects, and (v) pharmacokinetic, pharmacodynamic, and pharmacoeconomic issues. To combat AMR, a “One Health” strategy is needed. This strategy relies on interprofessional learning and education to develop healthcare professionals who have learned to cooperate across and beyond disciplines to continuously improve the quality of care. Here, we show our results after the implementation of an interprofessional approach to ABS. Taken together, the knowledge and skills of healthcare professionals in prescribing antimicrobial drugs improved. Furthermore, carbapenem consumption decreased as an important result of our tailored and interprofessional approach to ABS. In contrast to the increasing numbers of critically ill patients and the augmenting severity of diseases, expenditure, and cost of antibiotics on our medical intensive care unit (MICU) declined. This highlights the importance and the benefit of continuous monitoring of antibiotic consumption in (M) ICUs.
2. Results
In this analysis, the medical and economic results of an interprofessional approach to ABS through comprehensive collaboration between ICU physicians, pharmacists, and nursing staff at a MICU were studied over a 10-year period from 2012 until 2021. The control period comprised the years 2012–2014. In 2015 we started our interprofessional ABS intervention including interprofessional training of intensive care physicians, hospital pharmacists, medical students, pharmacology students, nurses, and clinical nurse assistants. Our aim was to improve knowledge about antibiotics and to strengthen antimicrobial optimization in the ICU.
Our MICU at a German university medical center has a specific focus on gastroenterology, hepatology, infectious diseases, and endocrinology. Particularly noteworthy is the MICU’s focus on transplant medicine, which is reflected in the high number of immunosuppressed patients. The unit is comprised of 14 full-service intensive care beds under the guidance of a gastroenterologist and a multidisciplinary critical care team composed of physicians, nurses, respiratory therapists, occupational therapists, speech therapists, pharmacists, case coordinators, and medical and pharmacology students. Patients were often transferred from other hospitals for liver transplantation or interventional endoscopy.
As the patient population on our MICU changed from 2020 due to the COVID-19 pandemic, medical and economic results for 2020 and 2021 are presented as separate items.
2.1. Establishment of a Close Interprofessional Cooperation between MICU Physicians, Nursing Staff, and Pharmacists
From 2015, i.e., the beginning of the ABS intervention period, we have put a special focus on the close cooperation between the MICU physicians and the pharmacists of the on-site hospital pharmacy. Several pharmacists of the on-site hospital pharmacy are specialized in antimicrobial stewardship. This central axis of cooperation between MICU physicians and pharmacists has continuously been expanded over the years. Furthermore, colleagues from microbiology, virology, and hospital hygiene are involved in ABS in accordance with the national guidelines [
16].
There is a very close daily exchange and consultation between the MICU physicians and pharmacists. The pharmacists can assess the medication of all the patients at the MICU at any time by accessing an electronic chart. Thus, the attending MICU physician can consult with the pharmacist at any time. A hospital pharmacist is permanently assigned to the intensive care unit so that there is continuity, and the pharmacist knows the patients in the intensive care unit. Every week, there is a grand round with intensive care physicians, hospital pharmacists, nurses, students, and staff doctors from hospital hygiene, virology, and microbiology. These antibiotic stewardship rounds in our MICU include the rapid identification and optimal treatment of bacterial infections in our critically ill patients, based on pharmacokinetic and pharmacodynamic characteristics, and shortening the duration of antibiotic administration. Furthermore, we aimed to prepare future pharmacists, medical doctors, and nurses for a more comprehensive and interprofessional ABS. We believe that every effort should be made to incorporate interprofessional collaboration into ABS education. In addition, we encouraged the participation of inpatient staff nurses as antimicrobial stewards. Guidelines on antimicrobial stewardship emphasize the importance of an interdisciplinary team, but current practice focuses primarily on defining the role of infectious disease physicians and pharmacists. Therefore, we believe that the role of inpatient staff nurses as antimicrobial stewards should be explored.
2.2. Documentation and Implementation of Intervention Proposals
To document the results of an interprofessional approach to ABS and the close interprofessional cooperation between MICU physicians and pharmacists, in March 2018 a joint platform for documenting the corresponding measures was created in the patient data management system (Metavision® System; iMDsoft®).
Based on 742 medication reviews, analyses of the documentation showed that the preparation time of the pharmacists per MICU patient was 19.7 minutes on average. Per medication check, 0.85 intervention proposals were developed by the pharmacist.
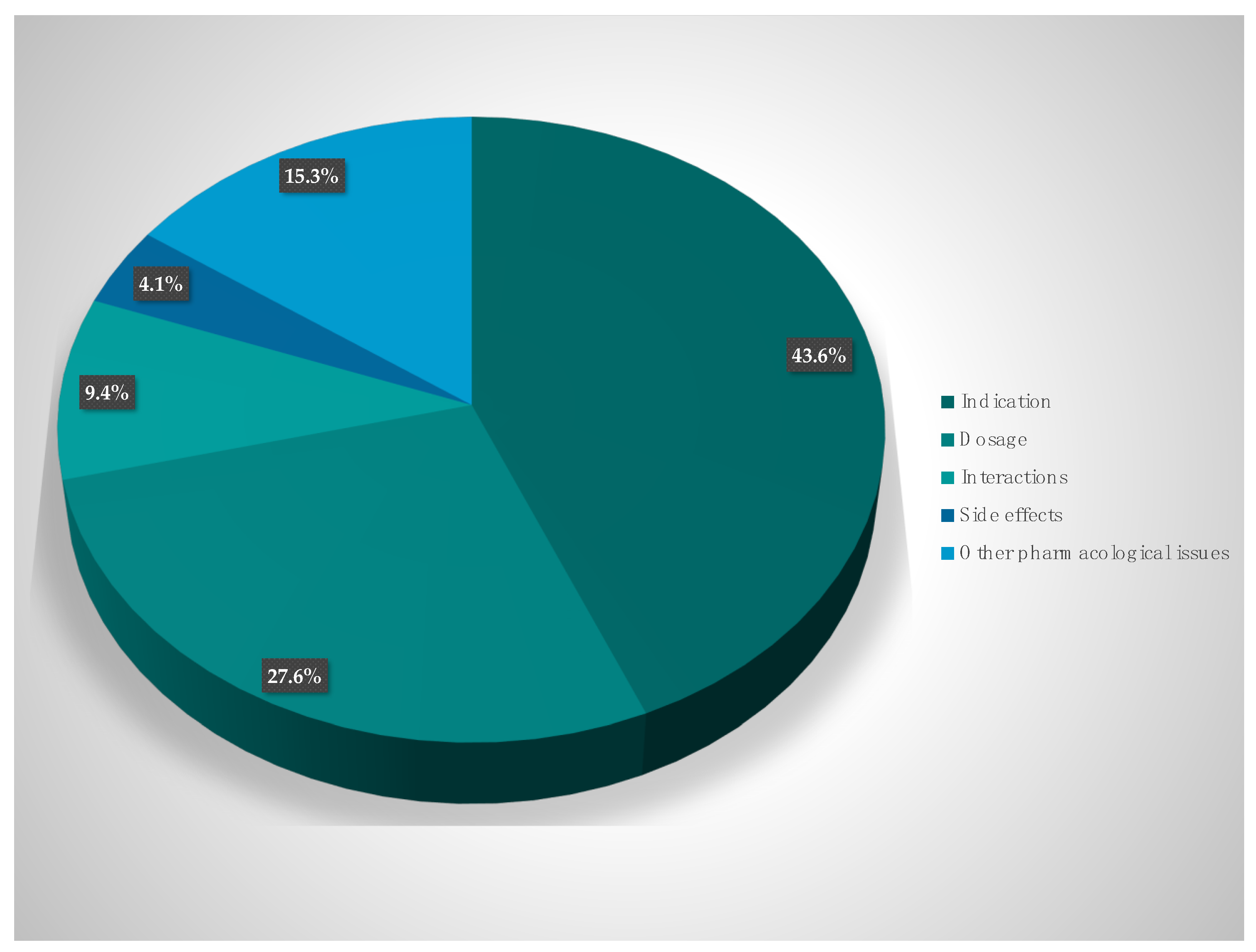
The suggestions of the hospital pharmacists for optimization can be divided into the following categories (i) indication and selection of therapy (43.6%), (ii) optimization of dosing (27.6%), (iii) drug interactions (9.4%), (iv) side effects (4.1%), and (v) other pharmacokinetic and pharmacodynamic issues (15.3%). The suggestions of the pharmacists were discussed among the interprofessional team, and 86.1% were consequently implemented and the prescription of antibiotics was changed (
Figure 1).
2.3. Development of Antibiotic Consumption
Our interprofessional approach to ABS has significantly reduced the use of antibiotics at the MICU, which is shown by a decrease in the use density of antibiotics. Application density is calculated in recommended daily doses (RDD) per 100 patient days (PD), which is an established measurement of hospital antibiotic use [
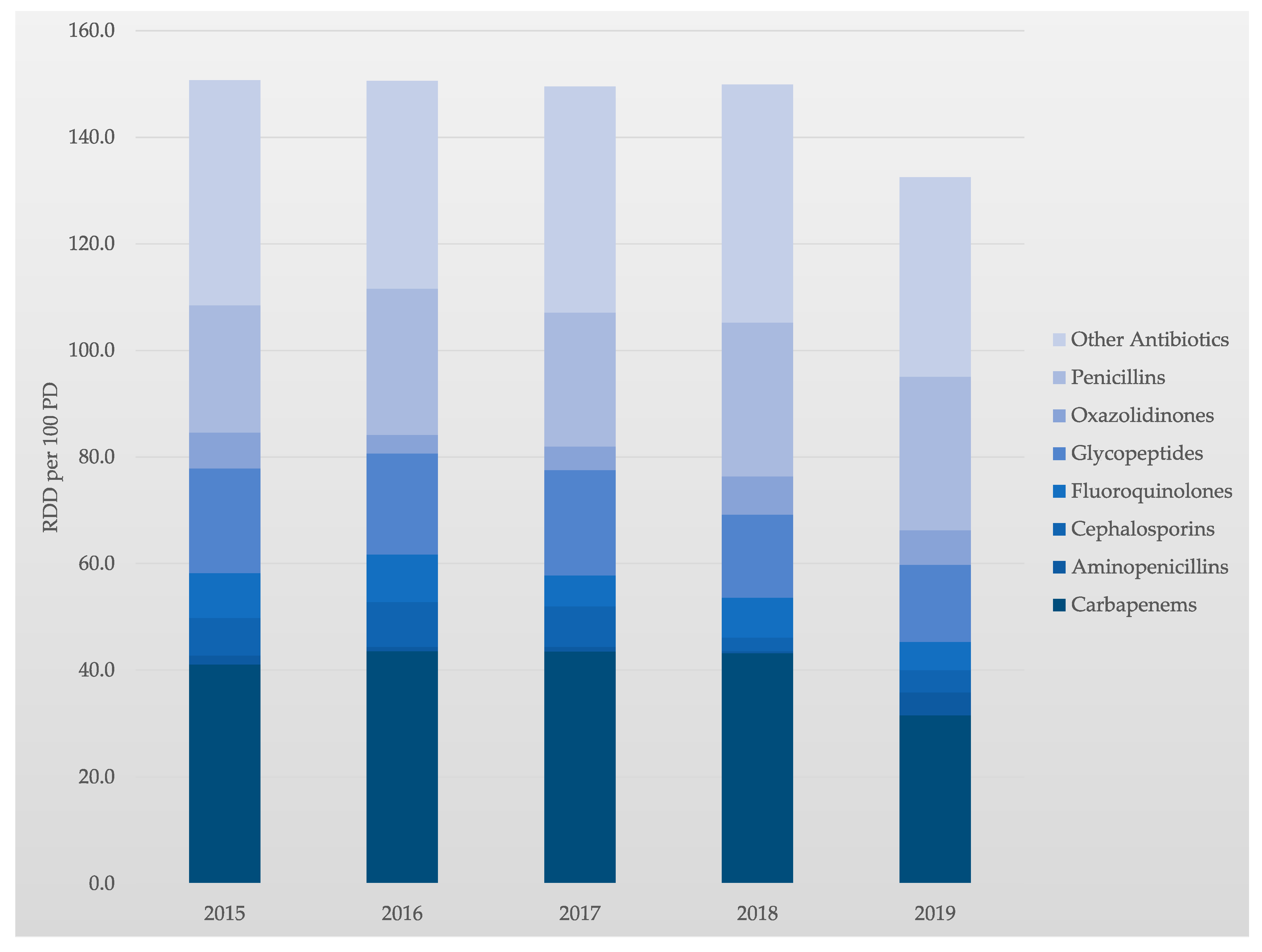
33]. Comparing the years 2015 and 2019, the application density of antibiotics was reduced by 12.2% from 150.9 RDD/100 PD to 132.5 RDD/100 PD.
A particular aim was to review the available data on carbapenem use in our MICU. Carbapenem is a broad-spectrum antibiotic family that keeps an excellent activity to extended-spectrum β-lactamases. It becomes a drug of choice for empirical therapy of suspected sepsis in known or presumably known Extended-spectrum beta-lactamase (ESBL) carriers. However, emerging carbapenem resistance has been related to the increase of carbapenem consumption in ICUs. In contrast, and as a result of our interprofessional ABS, the application density of carbapenems was reduced by 23.4% from 41.1 RDD/100 PD to 31.5 RDD/100 PD (2015 vs. 2019). Comparing 2015 and 2019, the consumption of cephalosporins, fluoroquinolones, glycopeptides, and linezolid were also reduced by 40.0%, 36.9%, 26.4%, and 3.0%, respectively.
In parallel, the use of penicillins and aminopenicillins in our MICU increased, by 20.5% and 152.9%, respectively (2015 vs. 2019). This development is due to a change in the antibiotic class away from carbapenems towards penicillins and aminopenicillins. Thus, tailored and interprofessional stewardship programs are essential to better control carbapenem use and subsequent antimicrobial resistance. The development of the application density of antibiotics over time is shown in
Table 1 and
Figure 2.
2.4. Development of Expenditure on Antibiotics
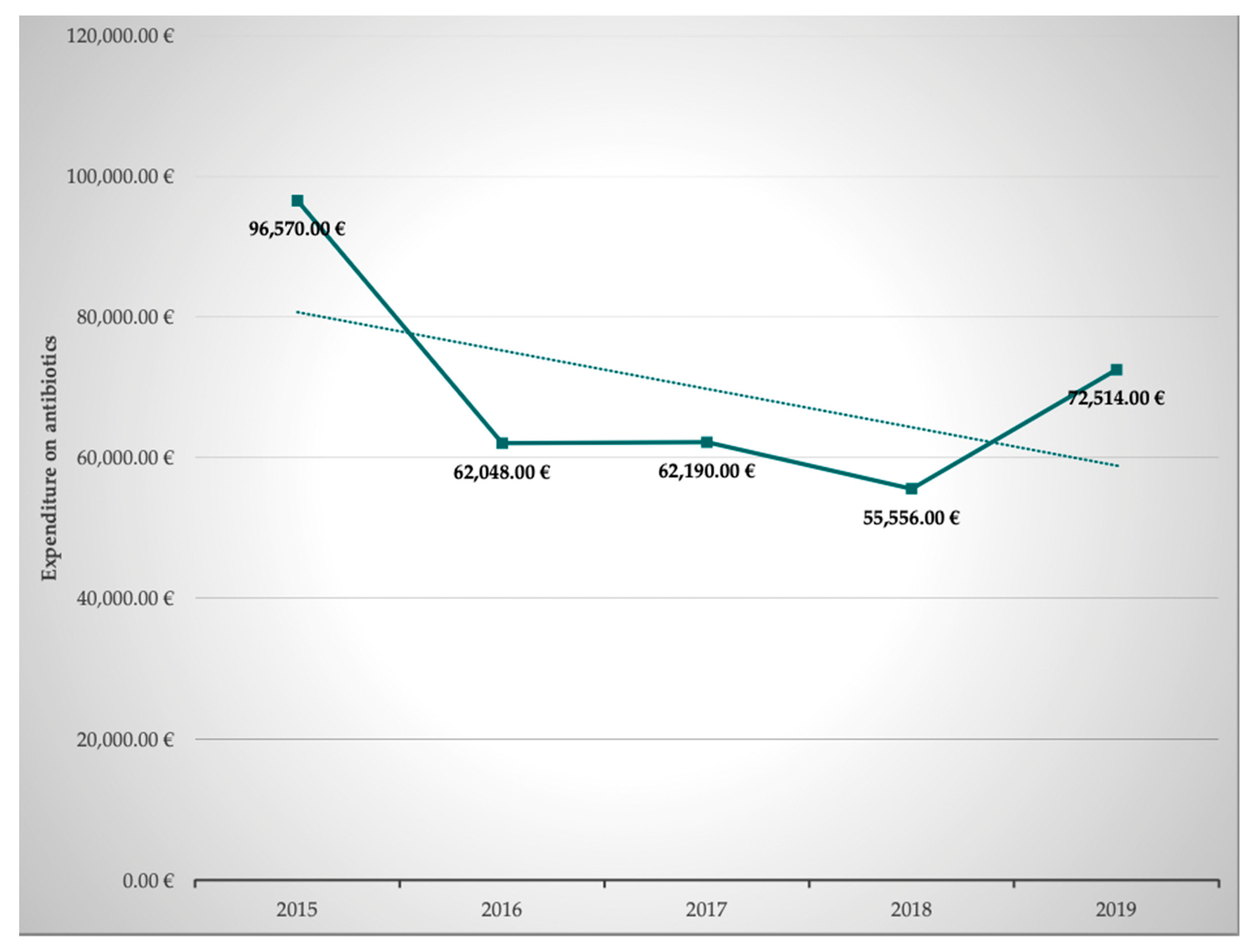
In addition to the optimization of prescription, dosing, and pharmacokinetic and pharmacodynamic aspects, we set a special focus on pharmacoeconomics. Pharmacoeconomics is a subdiscipline of health economics and evaluates the cost and effects of pharmaceutical drugs or drug therapies. We reasoned that these are important aspects for the curricula of medical doctors, pharmacists, and nursing staff as well. Both inappropriate use of antibiotics and lack of access to antibiotics are threats to global public health and interprofessional education should raise awareness towards socioeconomic as well as sociodemographic data and challenges. The corresponding data of our MICU were obtained from our hospital pharmacists. The total overall expenditure on antibiotics per year clearly decreased by 24.9% from EUR 96,570.75 to EUR 72,514.54 in the observed 5-year period between 2015 and 2019. An overview of the development of the expenditure on antibiotics is given in
Table 2 and
Figure 3.
2.5. Development of Clinical Performance
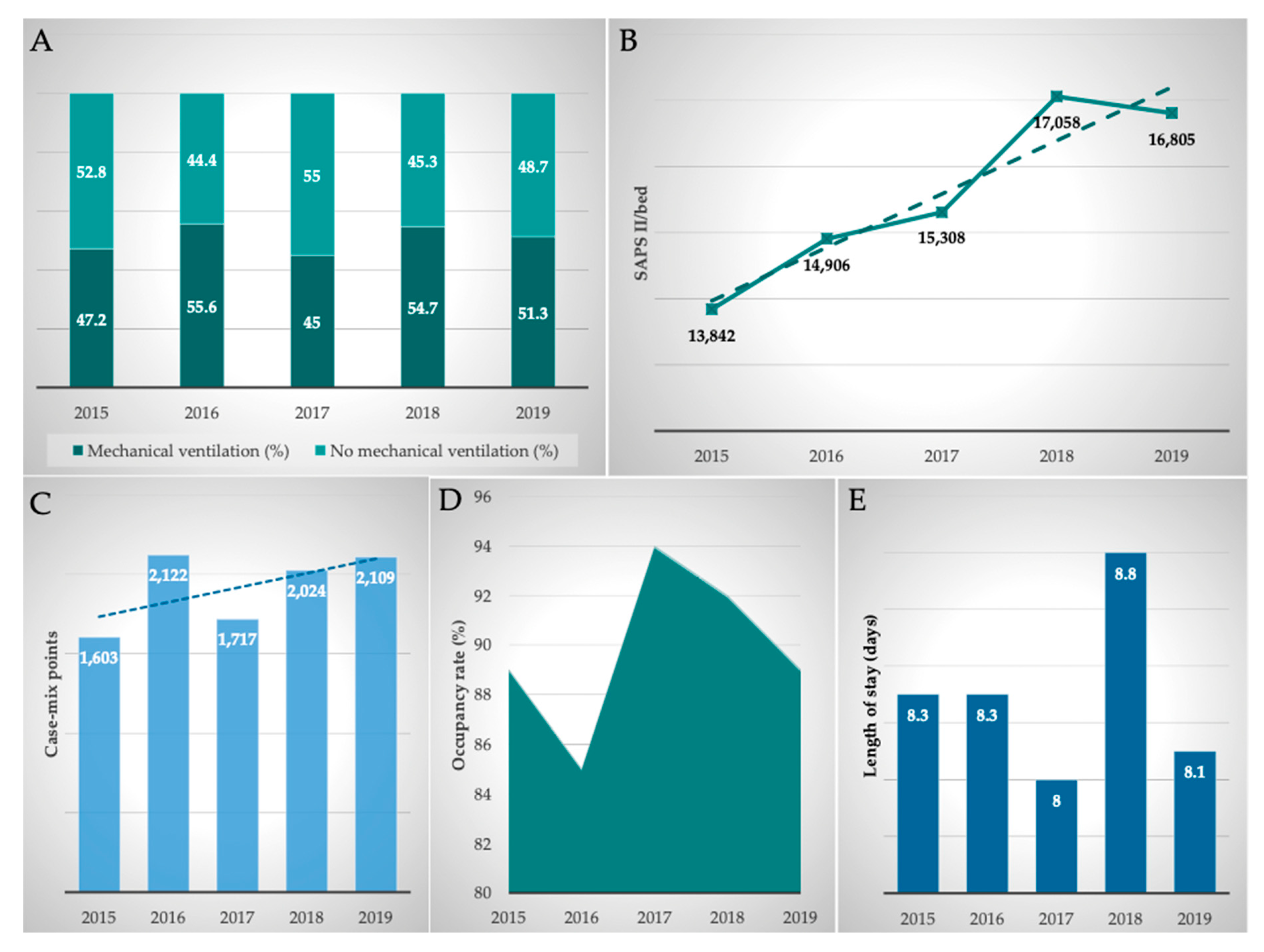
The clinical performance indicators of the MICU were comprehensively analyzed. Therefore, occupancy rate (in%), length of stay (in days), mechanical ventilation (in%), and Simplified Acute Physiological Score II (SAPS II)/bed and case mix points (total, per year, German-Diagnosis Related Groups (G-DRGs)) were included in our analyzes.
The occupancy rate was consistently at a high level, averaging 89.9%, reflecting the permanently high demand for beds at this specific (liver and infectious diseases and liver transplant) MICU. Due to the high severity of their disease, the length of stay of the patients was 8.3 days on average. Overall, there was a slight decrease in the length of stay between 2015 and 2019. On average, 50.8% of patients were mechanically ventilated. The ventilation rate increased by 8.7% to 51.3% in the analyzed period.
The severity of the disease is particularly well represented by the SAPS II, which was automatically calculated and documented daily at midnight by the patient data management system (Metavision® System; iMDsoft®). On average, 15,548 SAPS II points per bed and per year were achieved in the MICU. Comparing the years 2015 and 2019, the SAPS score increased by 21.4%.
By analyzing the intensive care G-DRGs obtained in the department, the case mix points generated in the ICU were calculated. On average, 1915 ± 239 case mix points were generated at the MICU per year. Comparing the years 2015 and 2019, case mix points increased by 31.6% from 1603 to 2109. Furthermore, it must be taken into consideration that additional interventional procedures have been performed in the MICU which also triggered non-intensive care G-DRGs and were included in the department’s total G-DRG reimbursement. Thus, the case mix points achieved in the MICU are higher than those shown in
Table 3.
In summary, all parameters examined showed a good development of clinical performance, with the rise in SAPS II points (+21.4%) and case mix points (+31.6%) being particularly noteworthy. An overview of clinical performance parameters is given in
Figure 4.
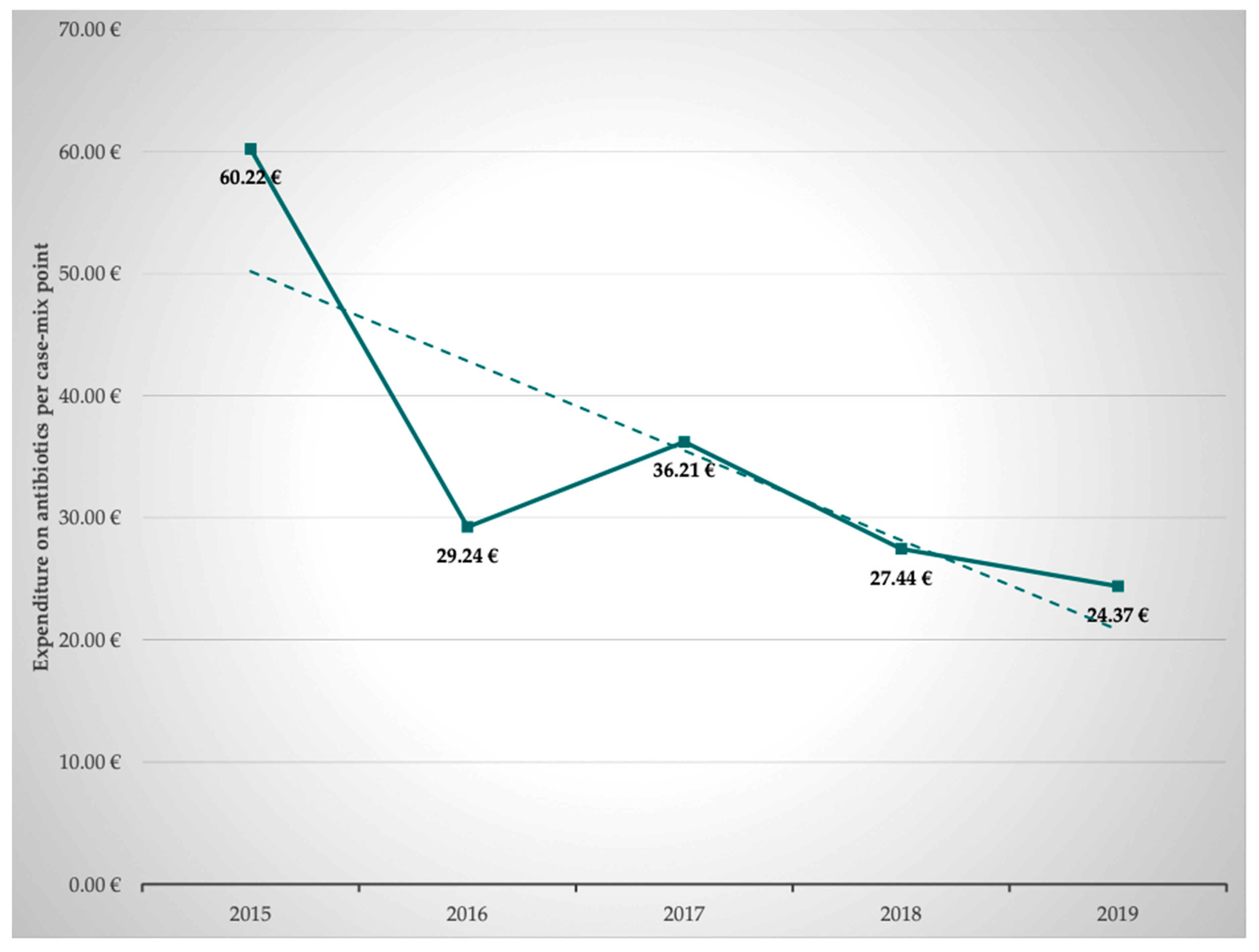
2.6. Calculation of the Expenditure on Antibiotics per Case Mix Point–G-DRG
Subsequently, the annual development of expenditure on antibiotics per case mix point was calculated based on the costs for antibiotics and the achieved case mix points. This revealed that the expenditure on antibiotics per case mix point averaged 37.50 ± 13.20 €, decreasing from 60.22 € in 2015 to 34.37 € in 2019. This represents savings of 41.9%. In parallel and as shown above, application density (RDD/100 PD) of antibiotics decreased. Thus, the decline in expenditure on antibiotics is clearly an effect of interprofessional ABS and due to less antibiotic use, not due to lower prices of antibiotics (
Table 1,
Figure 2).
The development of the expenditure on antibiotics per case mix point is shown in
Table 4 and
Figure 5. Based on the respective G-DRG state basal rates, we could show that 1.85% (2015) and 0.97% (2019) of the revenue generated by the G-DRGs was spent on antibiotics. This calculation shows a 52% reduction in expenditure on antibiotics.
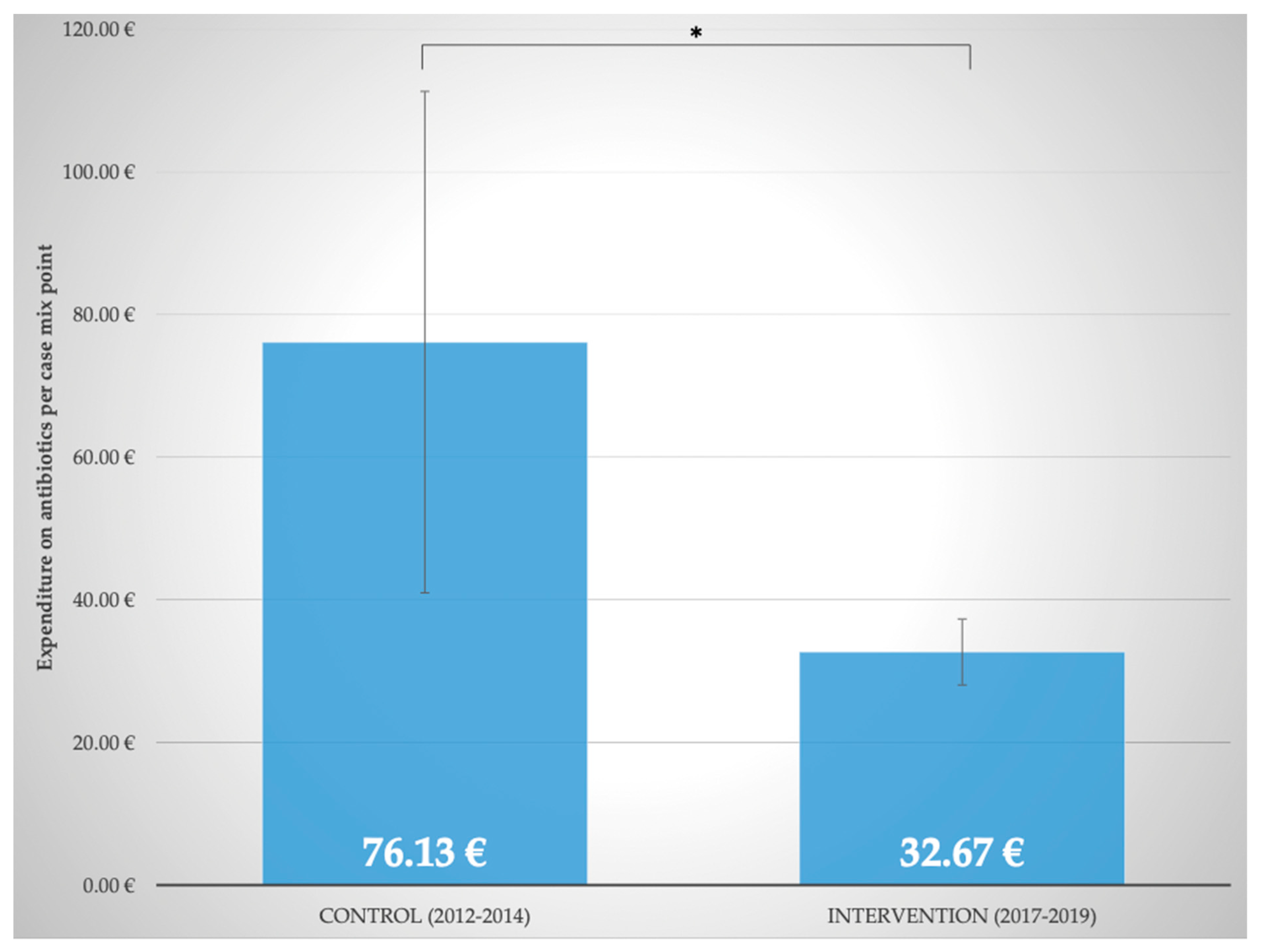
2.7. Comparison with Control Period (2012–2014) before Implementation of Interprofessional ABS
To further analyze the benefits of the close interprofessional cooperation between physicians, pharmacists, and nurses in ABS, a comparison with a control period from 2012–2014 was performed. As displayed in
Figure 6, expenditure on antibiotics per case mix point significantly decreased (
p ≤ 0.05) from 76.13 ± 35.19 EUR (2012–2014, control period) to 32.67 ± 4.62 EUR (2017–2019, last three years of intervention period). Moreover, despite an increase in disease severity, isolations of patients due to multidrug-resistant pathogens were significantly (
p ≤ 0.05) reduced from 40.81 ± 3.84% to 27.00 ± 2.78% (2012–2014, control period vs. 2017–2019, last three years of intervention period). This is an indication that infectious complications decreased.
2.8. Developments during the SARS-CoV-2 Pandemic
Due to structures that had been well established for years, the close interprofessional collaboration between MICU physicians and pharmacists was continued and intensified during the SARS-CoV-2 pandemic. This was an important support in the care of the often complex and seriously ill COVID-19 patients.
During the SARS-CoV-2 pandemic in 2020, there was a surge in the consumption density of antibiotics to 155.4 RDD/100 PD and an increase in total expenditure on antibiotics to 76,764 EUR. This increase can be explained by the numbers of severely ill COVID-19 patients treated in the ward, who often required long and comprehensive antibiotic therapy due to bacterial superinfections. Furthermore, the SARS-CoV-2 pandemic also led to price increases for some antibiotics. However, in 2021, despite continued treatment of COVID-19 patients, consumption density reduced to 147.8 RDD/100 PD and the expenditures declined to 75,292 EUR.
3. Discussion
Here, we suggest extending the concept of ABS beyond the technical aspects of antibiotic resistance to a health systems approach to preserve antibiotic effectiveness as a “One Health” strategy. The focus of our study was to implement and evaluate the impact of interprofessional education, learning, and collaboration of hospital pharmacists, attending ICU physicians, and ICU nursing staff in a comprehensive approach to ABS on the application density of antibiotics, quality of care, and expenditure in a MICU of a university center of tertiary care.
Our special focus was on the interprofessional ABS education of health care professionals and students to encourage cooperation across disciplines and professions.
Our results show that over a 7-year intervention period, despite increasing case severity of our patients, fewer antibiotics, and especially fewer broad-spectrum antibiotics, e.g., carbapenems, were used. In addition, overall expenditure on antibiotics decreased. From our point of view, the interprofessional collaboration led to an enhanced understanding of the “silent pandemic” [
34] of antibiotic resistance and how it can be addressed from a health systems perspective beyond disciplines and professions. The value of the hospital pharmacist in antibiotic stewardship has been highlighted before [
19,
35,
36,
37]. Evidence on the formal inclusion of nurses in ABS remains limited. Formal inclusion of nurses in ABS activities has been associated with improved nurse knowledge, nurse confidence, and improved clinical outcomes for patients [
38]. However, the formal inclusion of nurses in ABS does not yet represent actual clinical practice. Our department has a strong focus on interprofessional learning and education. Consequently, we have implemented ABS on the MICU, integrating staff nurses and nurses at the bedside. We think that there is an urgent need for interprofessional cooperation to support a health systems approach to contain antimicrobial resistance. Therefore, and in addition to the MICU rounds, we have created a new online platform that enables not only documentation but also communication and sustainability.
The first step in the development of any interprofessional antibiotic stewardship (ABS) program is to build an interprofessional and multidisciplinary team encompassing the necessary expertise in the complex management of infections and interprofessional learning. Interprofessional education is an approach recommended for improving the prescribing practice of antibiotics [
21,
39,
40]. We cannot exclude that—in addition to the implementation of our interprofessional ABS program—other practice changes, for example, better use of VAP prevention or implementation of new ICU guidelines contributed to the observed positive effects on antibiotic application density, expenditure, and quality of care. However, there was no change neither in the key individuals of the MICU team nor in the structure of our MICU during the observation period that may have confounded the outcome of our study.
The benefit of interprofessional ABS outweighs the time invested by the health professionals. The pharmacist’s weekly preparation time averaged 19.7 min per patient. In the current literature, 30 min per patient has been suggested for preparation [
37]. The difference between our data and the literature may be explained by the fact that a specific pharmacist is permanently assigned to our MICU and thus knows the patients and their diseases and clinical course very well.
The suggestions by the interprofessional team can be grouped into the following categories: (i) indication and selection of therapy (43.6%), (ii) optimization of dosing (27.6%), (iii) drug interactions (9.4%), (iv) side effects (4.1%), and (v) pharmacokinetic, pharmacodynamic and pharmacoeconomic issues (15.3%). This is in accordance with the current literature which reports on dose adjustments and specific therapeutic indications as the most frequent recommendations [
41] resulting from ABS. A total of 86.1% of the recommendations of the hospital pharmacist were fully implemented. This very good implementation rate is most likely due to the interprofessional communication between ICU doctors, ICU nurses, and pharmacists. Previous studies reported implementation rates of only 50% when the ABS consultation was restricted to a written note only [
42,
43].
In our study, the overall consumption of antibiotics was reduced by 12.2% from 150.9 RDD/100 PD to 132.5 RDD/100 PD in the years 2015 to 2019. At first glance, this appears to be less than in comparable studies, which reported a reduction in the consumption density of antibiotics of approximately 20% [
8,
44]. When interpreting this data, it must be taken into consideration that the clinical performance of our MICU in this period increased significantly. This is reflected by the increase in SAPS II/bed by 21.4% and the increase in case mix points by 31.6%. In addition, the expenditure on antibiotics was analyzed, showing a decrease of 24.9%. This observation is in line with the literature [
41]. Finally, the expenditure on antibiotics was set in relation to the case mix points. This revealed a marked decrease in the antibiotic expenditure of 42.9% per case mix point. From our point of view, this ratio best reflects the savings achieved through the interprofessional collaboration between ICU doctors, nurses, and pharmacists while treating patients with increasing severity of their disease over the years as a tertiary referral center.
Not only did we succeed in reducing the overall consumption density and expenditure of antibiotics, but we also paid special attention to reducing broad-spectrum antibiotics, e.g., carbapenems. In the observation period, the application density of carbapenems reduced 23.4% from 41.1 RDD/100 PD to 31.5 RDD/100 PD (2015 vs. 2019). The reduction of the consumption density of carbapenems is considered a generally accepted and “read out” goal of ABS and is clearly substantiated in the corresponding guidelines. Of clinical importance, not only is the reduction of the consumption of carbapenems a declared goal of ABS but also a reduction in the consumption of other broad-spectrum antibiotic groups should be achieved [
16]. We accomplished a decline in the consumption of cephalosporins, fluoroquinolones, glycopeptides, and linezolid by 40.0%, 36.9%, 26.4%, and 3.0%, respectively, following the implementation of interprofessional ABS on the MICU.
Since penicillins and aminopenicillins are considered ideal in the context of antimicrobial resistance, it is a declared goal to switch from other antibiotic classes to aminopenicillins if possible. Overall, the use of penicillins and aminopenicillins has increased in German intensive care units in recent years. In our analysis, the use of penicillins and aminopenicillins clearly increased, by 20.5% and 152.9%, respectively (2015 vs. 2019).
In summary, it can be shown that comprehensive interprofessional cooperation between ICU physicians, ICU nurses, and hospital pharmacists in a comprehensive approach to ABS can achieve a clear reduction in the consumption density of antibiotics. Furthermore, a switch towards penicillins and aminopenicillins can clearly be achieved in severely ill MICU patients. The benefits for patient care come with economic benefits and thus represents multiple wins.
Based on our data, we suggest interprofessional cooperation among ICU physicians, ICU nurses, and hospital pharmacists as an innovative and sustainable approach to optimize future ABS programs and to educate health care professionals for a global health systems approach.
4. Materials and Methods
The present study is a retrospective analysis conducted at the MICU of the Department of Internal Medicine I at the University Hospital Regensburg. The MICU is specialized in gastroenterology, hepatology, infectious diseases, endocrinology, rheumatology, and liver transplantation. On average, 14 beds have been operated during the study period. The ICU’s catchment area includes 2.0 million people from the south of Germany; it provides tertiary clinical care and tertiary referral-center care functions.
Primary data were obtained from the SAP® (Systemanalyse Programmentwicklung, Walldorf, Germany) hospital system and the Metavision® patient data management system. In addition, pharmacoeconomic data were provided by the hospital pharmacists and financial reports from the hospital administration. Statistical analyses were performed with the help of SPSS® (Statistical Package for Social Sciences, IBM, Armonk, New York, United States). A one-tailed t-test was performed; p-values less than or equal to 0.05 were considered statistically significant. This study was granted approval by the ethics committee of the University Hospital Regensburg, Regensburg, Germany (21-2520-104, 14 July 2021).
In this study, a 10-year period between 2012 and 2021 was analyzed. The control period comprised the years 2012–2014 and the intervention period was from 2015 to 2021. The years 2020 and 2021 were examined separately due to the extraordinary situation caused by the COVID-19 pandemic and the resulting change in the patient collective on the MICU.
Of clinical importance, a joint platform in the patient data management system (Metavision®, iMDsoft®, Düsseldorf, Germany) was created for the systematic documentation of the ABS-related therapy adjustments which were categorized in antibiotic use before and after the interprofessional grand round and included specific ABS strategies like de-escalation, duration of treatment, and administration optimization. A specific focus was set on the reduction in the prescribing of broad-spectrum antibiotics.
Of clinical relevance, critical illness is associated with changes in pharmacokinetics and pharmacodynamics, which challenge dose-finding and optimization. This is particularly relevant for hydrophilic drugs, e.g., beta-lactam antibiotics. Therefore, special attention was paid to exploring these challenges influencing optimal antibiotic application in the intensive care setting.
Furthermore, the engagement of bedside nurses in antimicrobial stewardship and infection prevention activities was encouraged. We believe that novel strategies to integrate bedside nurses in antimicrobial stewardship are needed and that the respective curricula will have to be adapted accordingly. In addition, we are dedicated to improving interprofessional education on antimicrobial stewardship of all the professions represented in our grand rounds and the application of antibiotics in ICUs.
The application density of antibiotics is presented in recommended daily dose per 100 patient days (RDD/100 PD), which is a reliable benchmark for assessing trends in prescriptions. In our analysis, RDD was used as a scale for the use of antibiotics as it shows smaller deviations from actual prescriptions compared with the WHO-DDD (Defined Daily Dose) system, which may lead to misclassifications in benchmark analyses [
33].
5. Conclusions
Through interprofessional collaboration between physicians, hospital pharmacists, and nurses, we could broaden the concept of ABS and integrate interprofessional education and interprofessional learning to better understand antibiotic resistance and to optimize the use of antibiotics in the ICU. In our study, an interprofessional approach to ABS led to a decrease in overall antibiotic consumption, a marked decline in the prescription of broad-spectrum antibiotics, and achieved better economic results.
Thus, the responsible use of resources and high-performance medicine are not contradictory. In our view, close interprofessional and interdisciplinary collaboration will be of outstanding importance in the future for the implementation of global strategies to contain antimicrobial resistance and to initiate a health systems approach to contain antibiotic resistance.
Author Contributions
Conceptualization, S.S. (Stephan Schmid), M.M., and A.K.; methodology, S.S. (Stephan Schmid), M.M., and A.K.; investigation, S.S. (Stephan Schmid), M.M., and A.K.; writing—original draft preparation, S.S. (Stephan Schmid), M.M., K.G., and S.S. (Sophie Schlosser); writing—review and editing, S.S. (Stephan Schmid) S.S. (Sophie Schlosser), V.P., K.G., M.M., and A.K.; visualization, S.S. (Stephan Schmid) and S.S. (Sophie Schlosser); supervision, M.M. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of University Hospital Regensburg (protocol code 21-2520-104, 14 July 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
The authors thank the patients and acknowledge the support of the interprofessional team of the Department of Internal Medicine I and the hospital pharmacy at the University Hospital Regensburg, Regensburg, Germany.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmad, M.; Khan, A.U. Global economic impact of antibiotic resistance: A review. J. Glob. Antimicrob. Resist. 2019, 19, 313–316. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 1 February 2022).
- RAND Cooperation, Estimating the Economic Costs of Antimicrobial Resistance. Available online: https://www.rand.org/randeurope/research/projects/antimicrobial-resistance-costs.html (accessed on 1 February 2022).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Hopkins, S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators, Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [CrossRef]
- Kollef, M.H.; Bassetti, M.; Francois, B.; Burnham, J.; Dimopoulos, G.; Garnacho-Montero, J.; Wunderink, R.G.; Postma, M.J.; Torres, A.; Welte, T.; et al. The intensive care medicine research agenda on multidrug-resistant bacteria, antibiotics, and stewardship. Intensive Care Med. 2017, 43, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Lepape, A.; Jean, A.; De Waele, J.; Friggeri, A.; Savey, A.; Vanhems, P.; Kohlenberg, A.; Gustin, M.P.; Monnet, D.L.; Garnacho-Montero, J. European intensive care physicians’ experience of infections due to antibiotic-resistant bacteria. Antimicrob. Resist. Infect. Control 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Schröder, S.; Klein, M.K.; Heising, B.; Lemmen, S.W. Sustainable implementation of antibiotic stewardship on a surgical intensive care unit evaluated over a 10-year period. Infection 2020, 48, 117–124. [Google Scholar] [CrossRef]
- Remschmidt, C.; Schneider, S.; Meyer, E.; Schroeren-Boersch, B.; Gastmeier, P.; Schwab, F. Surveillance der Antibiotika-Anwendung und Resistenzentwicklung auf Intensivstationen (SARI). Dtsch. Arztebl. Int. 2017, 114, 858–865. [Google Scholar]
- Zhang, Y.-Z. Antibiotic stewardship programmes in intensive care units: Why, how, and where are they leading us. World J. Crit. Care Med. 2015, 4, 13–28. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Robotham, J.V.; Knight, G.M. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control 2018, 7, 1–17. [Google Scholar] [CrossRef]
- Lim, C.; Takahashi, E.; Hongsuwan, M.; Wuthiekanun, V.; Thamlikitkul, V.; Hinjoy, S.; Limmathurotsakul, D.; Day, N.P.J.; Peacock, S.J. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. Elife 2016, 5, e18082. [Google Scholar] [CrossRef]
- Temkin, E.; Fallach, N.; Almagor, J.; Gladstone, B.P.; Tacconelli, E.; Carmeli, Y. Estimating the number of infections caused by antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in 2014: A modelling study. Lancet Glob. Heal. 2018, 6, e969–e979. [Google Scholar] [CrossRef]
- World Health Organization, European Strategic Action Plan on Antibiotic Resistance. Available online: https://www.euro.who.int/__data/assets/pdf_file/0008/147734/wd14E_AntibioticResistance_111380.pdf (accessed on 1 February 2022).
- De With, K. S3-Leitlinie Strategien zur Sicherung Rationaler Antibiotika-Anwendung im Krankenhaus AWMF-Registernummer 092/001–Update 2018. Available online: https://www.awmf.org/leitlinien/detail/ll/092-001.html (accessed on 1 February 2022).
- Pollack, L.A.; Srinivasan, A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014, 59, S97–S100. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.H.; Tay, E.; Heng, S.T.; Guo, H.; Kwa, A.L.H.; Ng, T.M.; Chow, A.; Somani, J.; Lye, D.C.B. Hospital Pharmacists and Antimicrobial Stewardship: A Qualitative Analysis. Antibiotics 2021, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Gotterson, F.; Buising, K.; Manias, E. Nurse role and contribution to antimicrobial stewardship: An integrative review. Int. J. Nurs. Stud. 2021, 117, 103787. [Google Scholar] [CrossRef]
- Bailey, L.C.; Mager, N.A.D. Global health education in doctor of pharmacy programs. Am. J. Pharm. Educ. 2016, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guilding, C.; Hardisty, J.; Randles, E.; Statham, L.; Green, A.; Bhudia, R.; Matthan, J.; Teodorczuk, A.; Scott, L. Designing and evaluating an interprofessional education conference approach to antimicrobial education. BMC Med. Educ. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Padigos, J.; Reid, S.; Kirby, E.; Broom, J. Knowledge, perceptions and experiences of nurses in antimicrobial optimization or stewardship in the intensive care unit. J. Hosp. Infect. 2021, 109, 10–28. [Google Scholar] [CrossRef]
- Schultze, T.G.; Ferstl, P.G.; Villinger, D.; Hogardt, M.; Bechstein, W.O.; Göttig, S.; Kempf, V.A.; Welker, W.-M.; Waidmann, O.; Trebicka, J.; et al. Molecular Surveillance of Carbapenem-Resistant Gram-Negative Bacteria in Liver Transplant Candidates. Front. Microbiol. 2021, 12, 791574. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kamath, P.S.; Reddy, K.R. The Evolving Challenge of Infections in Cirrhosis. N. Engl. J. Med. 2021, 384, 2317–2330. [Google Scholar] [CrossRef]
- Fernández, J.; Acevedo, J.; Wiest, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martínez, J.; Saliba, F.; Jalan, R.; et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2017, 67, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Engelmann, C.; Reiberger, T.; Acevedo, J.; Gatti, P.; et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 2020, 73, 842–854. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Arroyo, V.; Laleman, W.; Zeuzem, S.; Trebicka, J.; et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 44, 1426–1437. [Google Scholar] [CrossRef]
- Arroyo, V.; Angeli, P.; Moreau, R.; Jalan, R.; Clària, J.; Trebicka, J.; Fernández, J.; Gustot, T.; Caraceni, P.; Bernardi, M.; et al. The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J. Hepatol. 2021, 74, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Acevedo, J.; Castro, M.; Garcia, O.; Rodriguez de Lope, C.; Roca, D.; Arroyo, V.; Corradi, F.; Mensa, J.; Ginès, P.; et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology 2012, 55, 1551–1561. [Google Scholar] [CrossRef]
- Fernández, J.; Prado, V.; Trebicka, J.; Amoros, A.; Gustot, T.; Wiest, R.; Arroyo, V.; Bañares, R.; Piano, S.; Janicko, M.; et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J. Hepatol. 2019, 70, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Salerno, F.; Borzio, M.; Pedicino, C.; Simonetti, R.; Rossini, A.; Boccia, S.; Grossi, P.; Cacciola, I.; Burroughs, A.K.; Manini, M.A.; et al. The impact of infection by multidrug-resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int. 2017, 37, 71–79. [Google Scholar] [CrossRef]
- Piano, S.; Bartoletti, M.; Tonon, M.; Baldassarre, M.; Chies, G.; Romano, A.; Angeli, P.; Brocca, A.; Bernardi, M.; Caraceni, P.; et al. Assessment of Sepsis-3 criteria and quick SOFA in patients with cirrhosis and bacterial infections. Gut 2018, 67, 1892–1899. [Google Scholar] [CrossRef]
- Först, G.; de With, K.; Weber, N.; Borde, J.; Querbach, C.; Kleideiter, J.; Schröder, P.; Steib-Bauert, M.; Kern, W.V.; ABS-QI Study Group; et al. Validation of adapted daily dose definitions for hospital antibacterial drug use evaluation: A multicentre study. J. Antimicrob. Chemother. 2017, 72, 2931–2937. [Google Scholar] [CrossRef]
- Cars, O.; Chandy, S.J.; Mpundu, M.; Peralta, A.Q.; Zorzet, A.; So, A.D. Resetting the agenda for antibiotic resistance through a health systems perspective. Lancet Glob. Health 2021, 9, e1022–e1027. [Google Scholar] [CrossRef]
- Dunn, K.; O’Reilly, A.; Silke, B.; Rogers, T.; Bergin, C. Implementing a pharmacist-led sequential antimicrobial therapy strategy: A controlled before-and-after study. Int. J. Clin. Pharm. 2011, 33, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Magedanz, L.; Silliprandi, E.M.; Santos, R.P.D. Impact of the pharmacist on a multidisciplinary team in an antimicrobial stewardship program: A quasi-experimental study. Int. J. Clin. Pharm. 2012, 34, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Lesprit, P.; Landelle, C.; Brun-Buisson, C. Clinical impact of unsolicited post-prescription antibiotic review in surgical and medical wards: A randomized controlled trial. Clin. Microbiol. Infect. 2013, 19, E91–E97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gursoy, G.; Omrum, U.Z.U.N.; Metan, G.; Yildirim, M.; Bahap, M.; Demirkan, S.K.; Serhat, U.N.A.L.; Berker, M.; Hazirolan, G.; Akova, M.; et al. Do antimicrobial stewardship programs improve the quality of care in ICU patients diagnosed with infectious diseases following consultation? Experience in a tertiary care hospital. Int. J. Infect. Dis. 2022, 115, 201–207. [Google Scholar] [CrossRef]
- Dornan, T.; Ashcroft, D.; Heathfield, H.; Lewis, P.; Miles, J.; Taylor, D. An in depth investigation into causes of prescribing errors by foundation trainees in relation to their medical education: EQUIP study. In Final Report for the GMC; General Medical Council: London, UK, 2009. [Google Scholar]
- Davey, P.; Garner, S. Professional education on antimicrobial prescribing: A report from the Specialist Advisory Committee on Antimicrobial Resistance (SACAR) Professional Education Subgroup. J. Antimicrob. Chemother. 2007, 60 (Suppl. S1), i27–i32. [Google Scholar] [CrossRef]
- Nault, V.; Pepin, J.; Beaudoin, M.; Perron, J.; Moutquin, J.M.; Valiquette, L. Sustained impact of a computer-assisted antimicrobial stewardship intervention on antimicrobial use and length of stay. J. Antimicrob. Chemother. 2017, 72, 933–940. [Google Scholar] [CrossRef]
- Masiá, M.; Matoses, C.; Padilla, S.; Murcia, A.; Sánchez, V.; Romero, I.; Navarro, A.; Hernández, I.; Gutiérrez, F. Limited efficacy of a nonrestricted intervention on antimicrobial prescription of commonly used antibiotics in the hospital setting: Results of a randomized controlled trial. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 597–605. [Google Scholar] [CrossRef]
- Fariñas, M.C.; Saravia, G.; Calvo-Montes, J.; Benito, N.; Martínez-Garde, J.J.; Fariñas-Alvarez, C.; Gómez-Fleitas, M.; Agüero, R.; Amado, J.-A.; Martínez-Martínez, L.; et al. Adherence to recommendations by infectious disease consultants and its influence on outcomes of intravenous antibiotic-treated hospitalized patients. BMC Infect. Dis. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Elligsen, M.; Walker, S.A.N.; Pinto, R.; Simor, A.; Mubareka, S.; Rachlis, A.; Allen, V.; Daneman, N. Audit and Feedback to Reduce Broad-Spectrum Antibiotic Use among Intensive Care Unit Patients A Controlled Interrupted Time Series Analysis. Infect. Control Hosp. Epidemiol. 2012, 33, 354–361. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).