Multidrug-Resistant Methicillin-Resistant Coagulase-Negative Staphylococci in Healthy Poultry Slaughtered for Human Consumption
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Bacterial Isolates
4.2. Phenotypic Antibiotic Resistance Testing
4.3. DNA Extraction
4.4. Antimicrobial-Resistant Genes
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Parlet, C.P.; Brown, M.M.; Horswill, A.R. Commensal staphylococci influence Staphylococcus aureus skin colonization and disease. Trends Microbiol. 2019, 27, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, G.; Di Bonaventura, G.; Savini, V. Chapter 1—Staphylococcal Taxonomy. In Pet-to-Man Travelling Staphylococci; Academic Press: Cambridge, MA, USA, 2018; pp. 1–10. ISBN 978-0-12-813547-1. [Google Scholar]
- Smith, J.T.; Andam, C.P. Extensive horizontal gene transfer within and between species of coagulase-negative Staphylococcus. Genome Biol. Evol. 2021, 13, evab206. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Ziebuhr, W.; Becker, K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019, 25, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [Green Version]
- Michalik, M.; Samet, A.; Podbielska-Kubera, A.; Savini, V.; Międzobrodzki, J.; Kosecka-Strojek, M. Coagulase-negative staphylococci (CoNS) as a significant etiological factor of laryngological infections: A review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Noshak, M.A.; Rezaee, M.A.; Hasani, A.; Mirzaii, M. The role of the coagulase-negative staphylococci (CoNS) in infective endocarditis; a narrative review from 2000 to 2020. Curr. Pharm. Biotechnol. 2020, 21, 1140–1153. [Google Scholar] [CrossRef]
- MacFadyen, A.C.; Harrison, E.M.; Drigo, I.; Parkhill, J.; Holmes, M.A.; Paterson, G.K. A mecC allotype, mecC3, in the CoNS Staphylococcus caeli, encoded within a variant SCCmecC. J. Antimicrob. Chemother. 2019, 74, 547–552. [Google Scholar] [CrossRef]
- Loncaric, I.; Kübber-Heiss, A.; Posautz, A.; Ruppitsch, W.; Lepuschitz, S.; Schauer, B.; Feßler, A.T.; Krametter-Frötscher, R.; Harrison, E.M.; Holmes, M.A.; et al. Characterization of mecC gene-carrying coagulase-negative Staphylococcus spp. isolated from various animals. Vet. Microbiol. 2019, 230, 138–144. [Google Scholar] [CrossRef]
- Zong, Z.; Peng, C.; Lü, X. Diversity of SCCmec Elements in Methicillin-Resistant Coagulase-Negative Staphylococci Clinical Isolates. PLoS ONE 2011, 6, e20191. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Nalepa, B.; Sierpińska, M.; Łaniewska-Trokenheim, Ł. Coagulase-negative staphylococci (CoNS) isolated from ready-to-eat food of animal origin—Phenotypic and genotypic antibiotic resistance. Food Microbiol. 2015, 46, 222–226. [Google Scholar] [CrossRef]
- Argemi, X.; Hansmann, Y.; Prola, K.; Prévost, G. Coagulase-negative staphylococci pathogenomics. Int. J. Mol. Sci. 2019, 20, 1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, V.; Pereira, J.E.; Maltez, L.; Ferreira, E.; Manageiro, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Diversity of methicillin-resistant staphylococci among wild Lepus granatensis: First detection of mecA-MRSA in hares. FEMS Microbiol. Ecol. 2019, 96, fiz204. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Garland-Lewis, G.; Trufan, S.; Meschke, S.J.; Fowler, H.; Shean, R.C.; Greninger, A.L.; Rabinowitz, P.M. Distribution of Staphylococcus species in dairy cows, workers and shared farm environments. FEMS Microbiol. Lett. 2018, 365, fny146. [Google Scholar] [CrossRef] [PubMed]
- Suepaul, S.; Georges, K.; Unakal, C.; Boyen, F.; Sookhoo, J.; Ashraph, K.; Yusuf, A.; Butaye, P. Determination of the frequency, species distribution and antimicrobial resistance of staphylococci isolated from dogs and their owners in Trinidad. PLoS ONE 2021, 16, e0254048. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kim, S.D.; Park, J.H.; Yang, S.-J. Species Distribution, Antimicrobial Resistance, and Enterotoxigenicity of Non-aureus Staphylococci in Retail Chicken Meat. Antibiotics 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.; Carnaccini, S.; Driggers, T.; Shivaprasad, H.L. Ulcerative Dermatitis and Valvular Endocarditis Associated with Staphylococcus aureus in a Hyacinth Macaw (Anadorhynchus hyacinthinus). Avian Dis. 2014, 58, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, R.L.; de Melo, D.A.; Bronzato, G.F.; Souza, V.R.d.S.; Holmström, T.C.N.; de Mattos de Oliveira Coelho, S.; de Silva Coelho, I.; de Souza, M.M.S. Characterization of Staphylococcus spp. isolates and β-lactam resistance in broiler chicken production. Braz. J. Vet. Med. 2021, 43, e00720. [Google Scholar] [CrossRef]
- Bhargava, K.; Zhang, Y. Characterization of methicillin-resistant coagulase-negative staphylococci (MRCoNS) in retail meat. Food Microbiol. 2014, 42, 56–60. [Google Scholar] [CrossRef]
- Silva, V.; Vieira-Pinto, M.; Saraiva, C.; Manageiro, V.; Reis, L.; Ferreira, E.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Prevalence and Characteristics of Multidrug-Resistant Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) CC398 Isolated from Quails (Coturnix Coturnix Japonica) Slaughtered for Human Consumption. Animals 2021, 11, 2038. [Google Scholar] [CrossRef]
- Bernier-Lachance, J.; Arsenault, J.; Usongo, V.; Parent, É.; Labrie, J.; Jacques, M.; Malouin, F.; Archambault, M. Prevalence and characteristics of Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) isolated from chicken meat in the province of Quebec, Canada. PLoS ONE 2020, 15, e0227183. [Google Scholar] [CrossRef]
- Tang, Y.; Larsen, J.; Kjeldgaard, J.; Andersen, P.S.; Skov, R.; Ingmer, H. Methicillin-resistant and -susceptible Staphylococcus aureus from retail meat in Denmark. Int. J. Food Microbiol. 2017, 249, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Okorie-Kanu, O.J.; Anyanwu, M.U.; Ezenduka, E.V.; Mgbeahuruike, A.C.; Thapaliya, D.; Gerbig, G.; Ugwuijem, E.E.; Okorie-Kanu, C.O.; Agbowo, P.; Olorunleke, S.; et al. Molecular epidemiology, genetic diversity and antimicrobial resistance of Staphylococcus aureus isolated from chicken and pig carcasses, and carcass handlers. PLoS ONE 2020, 15, e0232913. [Google Scholar] [CrossRef] [PubMed]
- Bala, H.K.; Igwe, J.C.; Olayinka, B.O.; Olonitola, O.S.; Onaolapo, J.A.; Okafo, C.N. Antibiotic susceptibility profile of Staphylococcus aureus isolated from healthy chickens in poultry farms in Kano state, Nigeria. Sky J. Microbiol. Res. 2016, 4, 42–46. [Google Scholar]
- European Medicines Agency European Surveillance of Veterinary Antimicrobial Consumption. ‘Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020′ EMA/58183/2021; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Marek, A.; Pyzik, E.; Stępień-Pyśniak, D.; Dec, M.; Jarosz, Ł.S.; Nowaczek, A.; Sulikowska, M. Biofilm-Formation Ability and the Presence of Adhesion Genes in Coagulase-Negative Staphylococci Isolates from Chicken Broilers. Animals 2021, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Younis, W.; Sabra, M.; Sayed, H.H. Occurrence and characterization of coagulase positive and negative Staphylococci isolated from Japanese quails and broiler chickens at Qena Governorate, Egypt. SVU-Int. J. Vet. Sci. 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Moawad, A.A.; Hotzel, H.; Awad, O.; Roesler, U.; Hafez, H.M.; Tomaso, H.; Neubauer, H.; El-Adawy, H. Evolution of Antibiotic Resistance of Coagulase-Negative Staphylococci Isolated from Healthy Turkeys in Egypt: First Report of Linezolid Resistance. Microorganisms 2019, 7, 476. [Google Scholar] [CrossRef] [Green Version]
- Pyzik, E.; Marek, A.; Stępień-Pyśniak, D.; Urban-Chmiel, R.; Jarosz, Ł.S.; Jagiełło-Podębska, I. Detection of antibiotic resistance and classical enterotoxin genes in coagulase-negative staphylococci isolated from poultry in Poland. J. Vet. Res. 2019, 63, 183. [Google Scholar] [CrossRef] [Green Version]
- Saha, O.; Rakhi, N.N.; Istiaq, A.; Islam, I.; Sultana, M.; Hossain, M.A.; Rahaman, M.M. Evaluation of Commercial Disinfectants against Staphylococcus lentus and Micrococcus spp. of Poultry Origin. Vet. Med. Int. 2020, 2020, 8811540. [Google Scholar] [CrossRef]
- Boamah, V.E.; Agyare, C.; Odoi, H.; Adu, F.; Gbedema, S.Y.; Dalsgaard, A. Prevalence and antibiotic resistance of coagulase-negative Staphylococci isolated from poultry farms in three regions of Ghana. Infect. Drug Resist. 2017, 10, 175–183. [Google Scholar] [CrossRef] [Green Version]
- El-Nagar, S.; Abd El-Azeem, M.W.; Nasef, S.A.; Sultan, S. Prevalence of toxigenic and methicillin resistant staphylococci in poultry chain production. J. Adv. Vet. Res. 2017, 7, 33–38. [Google Scholar]
- Pyzik, E.; Marek, A. Characterization of bacteria of the genus Staphylococcus isolated from the eggs of Japanese quail (Coturnix japonica). Pol. J. Vet. Sci. 2012, 15, 791–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzauskas, M.; Couto, N.; Kerziene, S.; Siugzdiniene, R.; Klimiene, I.; Virgailis, M.; Pomba, C. Prevalence, species distribution and antimicrobial resistance patterns of methicillin-resistant staphylococci in Lithuanian pet animals. Acta Vet. Scand. 2015, 57, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhaouadi, S.; Soufi, L.; Campanile, F.; Dhaouadi, F.; Sociale, M.; Lazzaro, L.; Cherif, A.; Stefani, S.; Elandoulsi, R.B. Prevalence of meticillin-resistant and -susceptible coagulase-negative staphylococci with the first detection of the mecC gene among cows, humans and manure in Tunisia. Int. J. Antimicrob. Agents 2020, 55, 105826. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, X.; Liang, J.; Li, Q.; Lin, H.; Lin, C.; Liu, H.; Zhou, D.; Lu, W.; Sun, Z.; et al. Characterization of florfenicol resistance genes in the coagulase-negative Staphylococcus (CoNS) isolates and genomic features of a multidrug-resistant Staphylococcus lentus strain H29. Antimicrob. Resist. Infect. Control 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Seni, J.; Mshana, S.E.; Msigwa, F.; Iddi, S.; Mazigo, H.; Parkhill, J.; Holmes, M.A.; Paterson, G.K. Draft genome sequence of a multidrug-resistant caprine isolate of Staphylococcus cohnii subsp. urealyticus from Tanzania encoding ermB, tet(K), dfrG, fusF and fosD. J. Glob. Antimicrob. Resist. 2019, 18, 163–165. [Google Scholar] [CrossRef]
- Lienen, T.; Schnitt, A.; Hammerl, J.A.; Marino, S.F.; Maurischat, S.; Tenhagen, B.-A. Multidrug-resistant Staphylococcus cohnii and Staphylococcus urealyticus isolates from German dairy farms exhibit resistance to beta-lactam antibiotics and divergent penicillin-binding proteins. Sci. Rep. 2021, 11, 6075. [Google Scholar] [CrossRef]
- Nemeghaire, S.; Argudín, M.A.; Haesebrouck, F.; Butaye, P. Molecular epidemiology of methicillin-resistant Staphylococcus sciuri in healthy chickens. Vet. Microbiol. 2014, 171, 357–363. [Google Scholar] [CrossRef]
- Agyare, C.; Boamah, V.E.; Zumbi, C.N.; Osei, F.B. Antibiotic use in poultry production and its effects on bacterial resistance. In Antimicrobial Resistance—A Global Threat; IntechOpen: London, UK, 2018; pp. 33–50. [Google Scholar]
- Schwarz, S.; Werckenthin, C.; Kehrenberg, C. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob. Agents Chemother. 2000, 44, 2530–2533. [Google Scholar] [CrossRef] [Green Version]
- Wendlandt, S.; Shen, J.; Kadlec, K.; Wang, Y.; Li, B.; Zhang, W.-J.; Feßler, A.T.; Wu, C.; Schwarz, S. Multidrug resistance genes in staphylococci from animals that confer resistance to critically and highly important antimicrobial agents in human medicine. Trends Microbiol. 2015, 23, 44–54. [Google Scholar] [CrossRef]
- Schoenfelder, S.M.K.; Dong, Y.; Feßler, A.T.; Schwarz, S.; Schoen, C.; Köck, R.; Ziebuhr, W. Antibiotic resistance profiles of coagulase-negative staphylococci in livestock environments. Vet. Microbiol. 2017, 200, 79–87. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Schwarz, S.; Zhao, Q.; Shen, Z.; Wu, C.; Shen, J. Multidrug resistance gene cfr in methicillin-resistant coagulase-negative staphylococci from chickens, ducks, and pigs in China. Int. J. Med. Microbiol. 2013, 303, 84–87. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, Y.; Schwarz, S.; Zhao, Q.; Shen, J.; Wu, C. Genetic environment of the multi-resistance gene cfr in methicillin-resistant coagulase-negative staphylococci from chickens, ducks, and pigs in China. Int. J. Med. Microbiol. 2014, 304, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Petinaki, E. Resistance of Staphylococci to Macrolides-Lincosamides-Streptogramins B (MLSB): Epidemiology and Mechanisms of Resistance. In Staphylococcus Aureus; Hemeg, H., Ozbak, H., Afrin, F., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Syed, M.A.; Ullah, H.; Tabassum, S.; Fatima, B.; Woodley, T.A.; Ramadan, H.; Jackson, C.R. Staphylococci in poultry intestines: A comparison between farmed and household chickens1. Poult. Sci. 2020, 99, 4549–4557. [Google Scholar] [CrossRef] [PubMed]
- Hamed, E.A.; Abdelaty, M.F.; Sorour, H.K.; Roshdy, H.; AbdelRahman, M.A.A.; Magdy, O.; Ibrahim, W.A.; Sayed, A.; Mohamed, H.; Youssef, M.I.; et al. Monitoring of Antimicrobial Susceptibility of Bacteria Isolated from Poultry Farms from 2014 to 2018. Vet. Med. Int. 2021, 2021, 6739220. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicro. EFSA J. 2021, 19, e06712. [Google Scholar]
- Chen, H.-J.; Hung, W.-C.; Lin, Y.-T.; Tsai, J.-C.; Chiu, H.-C.; Hsueh, P.-R.; Teng, L.-J. A novel fusidic acid resistance determinant, fusF, in Staphylococcus cohnii. J. Antimicrob. Chemother. 2015, 70, 416–419. [Google Scholar] [CrossRef] [Green Version]
- Gardete, S.; Aires-De-Sousa, M.; Faustino, A.; Ludovice, A.M.; de Lencastre, H. Identification of the First Vancomycin Intermediate-Resistant Staphylococcus aureus (VISA) Isolate from a Hospital in Portugal. Microb. Drug Resist. 2008, 14, 1–6. [Google Scholar] [CrossRef]
- Ali, Y.; Islam, M.A.; Muzahid, N.H.; Sikder, M.O.F.; Hossain, M.A.; Marzan, L.W. Characterization, prevalence and antibiogram study of Staphylococcus aureus in poultry. Asian Pac. J. Trop. Biomed. 2017, 7, 253–256. [Google Scholar] [CrossRef]
- Bortolaia, V.; Espinosa-Gongora, C.; Guardabassi, L. Human health risks associated with antimicrobial-resistant enterococci and Staphylococcus aureus on poultry meat. Clin. Microbiol. Infect. 2016, 22, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Damien, D.; David, L.; Paul, C.J.; Markus, K.; Olivier, S.P.; Régine, T.; Richard, B.; Julien, D. Identification of a Variety of Staphylococcus Species by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2010, 48, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Almeida, F.; Carvalho, J.A.; Castro, A.P.; Ferreira, E.; Manageiro, V.; Tejedor-Junco, M.T.; Caniça, M.; Igrejas, G.; Poeta, P. Emergence of community-acquired methicillin-resistant Staphylococcus aureus EMRSA-15 clone as the predominant cause of diabetic foot ulcer infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sparling, J.; Chow, B.L.; Elsayed, S.; Hussain, Z.; Church, D.L.; Gregson, D.B.; Louie, T.; Conly, J.M. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J. Clin. Microbiol. 2004, 42, 4947–4955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnellmann, C.; Gerber, V.; Rossano, A.; Jaquier, V.; Panchaud, Y.; Doherr, M.G.; Thomann, A.; Straub, R.; Perreten, V. Presence of new mecA and mph(C) variants conferring antibiotic resistance in Staphylococcus spp. isolated from the skin of horses before and after clinic admission. J. Clin. Microbiol. 2006, 44, 4444–4454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutcliffe, J.; Grebe, T.; Tait-Kamradt, A.; Wondrack, L. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 1996, 40, 2562–2566. [Google Scholar] [CrossRef] [Green Version]
- Shopsin, B.; Mathema, B.; Alcabes, P.; Said-Salim, B.; Lina, G.; Matsuka, A.; Martinez, J.; Kreiswirth, B.N. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians. J. Clin. Microbiol. 2003, 41, 456–459. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Sanz, E.; Torres, C.; Lozano, C.; Fernandez-Perez, R.; Aspiroz, C.; Ruiz-Larrea, F.; Zarazaga, M. Detection, molecular characterization, and clonal diversity of methicillin-resistant Staphylococcus aureus CC398 and CC97 in Spanish slaughter pigs of different age groups. Foodborne Pathog. Dis. 2010, 7, 1269–1277. [Google Scholar] [CrossRef]
- Wondrack, L.; Massa, M.; Yang, B.V.; Sutcliffe, J. Clinical strain of Staphylococcus aureus inactivates and causes efflux of macrolides. Antimicrob. Agents Chemother. 1996, 40, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Lina, G.; Quaglia, A.; Reverdy, M.E.; Leclercq, R.; Vandenesch, F.; Etienne, J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 1999, 43, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Bozdogan, B.; Berrezouga, L.; Kou, M.S.; Yurek, D.A.; Farley, K.A.; Stockman, B.J.; Leclercq, R. A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob. Agents Chemother. 1999, 43, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Lozano, C.; Aspiroz, C.; Rezusta, A.; Gómez-Sanz, E.; Simon, C.; Gómez, P.; Ortega, C.; Revillo, M.J.; Zarazaga, M.; Torres, C. Identification of novel vga(A)-carrying plasmids and a Tn5406-like transposon in meticillin-resistant Staphylococcus aureus and Staphylococcus epidermidis of human and animal origin. Int. J. Antimicrob. Agents 2012, 40, 306–312. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Jensen, L.B.; Aarestrup, F.M. Detection of the satA gene and transferability of virginiamycin resistance in Enterococcus faecium from food- animals. FEMS Microbiol. Lett. 1998, 168, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M.; Agers, L.Y.; Ahrens, P.; JŁrgensen, J.L.; Madsen, M.; Jensen, L.B. Antimicrobial susceptibility and presence of resistance genes in staphylococci from poultry. Vet. Microbiol. 2020, 74, 353–364. [Google Scholar] [CrossRef]
- Van de Klundert, J.A.M.; Vliegenthart, J.S. PCR detection of genes coding for aminoglycoside-modifying enzymes. In Diagnostic Molecular Microbiology: Principles and Applications; Persing, D.H., Smith, T.F., Tenover, F.C., White, T.J., Eds.; American Society for Microbiology: Washington, DC, USA, 1993; pp. 547–552. [Google Scholar]
- Kehrenberg, C.; Schwarz, S. Distribution of Florfenicol Resistance Genes fexA and cfr among Chloramphenicol-Resistant Staphylococcus Isolates. Antimicrob. Agents Chemother. 2006, 50, 1156–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, Y.; Wu, C.; Schwarz, S.; Shen, Z.; Jeon, B.; Ding, S.; Zhang, Q.; Shen, J. A novel phenicol exporter gene, fexB, found in enterococci of animal origin. J. Antimicrob. Chemother. 2012, 67, 322–325. [Google Scholar] [CrossRef] [Green Version]
- Mclaws, F.; Chopra, I.; O’Neill, A.J. High prevalence of resistance to fusidic acid in clinical isolates of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2008, 61, 1040–1043. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Hung, W.C.; Tseng, S.P.; Tsai, J.C.; Hsueh, P.R.; Teng, L.J. Fusidic acid resistance determinants in Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 2010, 54, 4985–4991. [Google Scholar] [CrossRef] [Green Version]
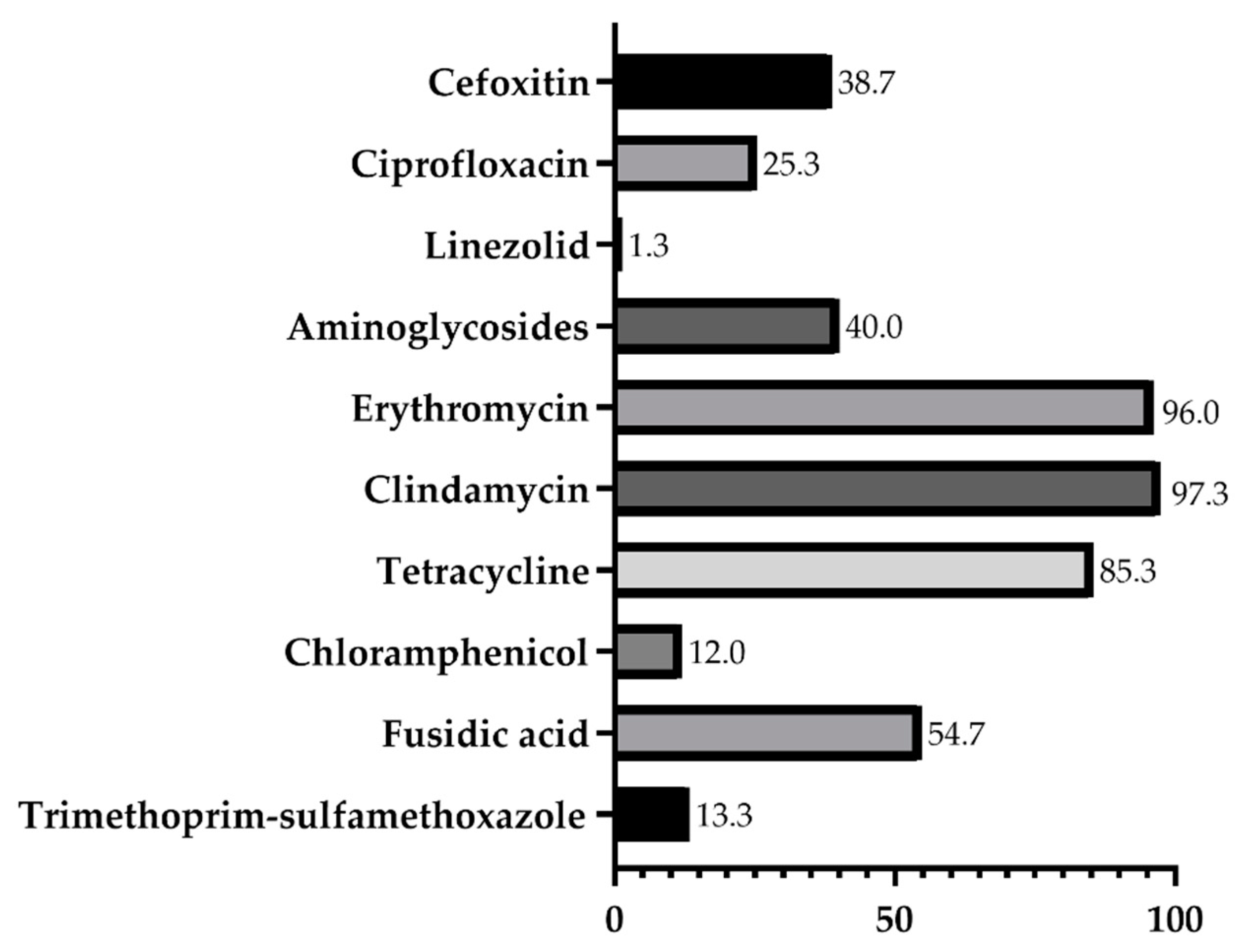
| Animal | Number of Animals Sampled | Number of CoNS Carriers (%) | Isolates Recovered | S. lentus | S. urealyticus | S. sciuri | S. haemolyticus |
|---|---|---|---|---|---|---|---|
| Quails | 100 | 47 (47) | 51 | 15 | 19 | 14 | 3 |
| Commercial chickens | 50 | 13 (26) | 13 | 11 | 2 | - | - |
| Homebred chickens | 70 | 11 (15.7) | 11 | 10 | - | 1 | - |
| Total | 220 | 71 (32.3) | 75 | 36 | 21 | 15 | 3 |
| Species | Number of Isolates | Antimicrobial Resistance | |
|---|---|---|---|
| Phenotype | Genotype | ||
| S. lentus | 36 | PEN11, FOX4, CIP11, CN2, TOB14, KAN9, ERY35, CD36, TET25, C4, FD12, SXT6 | mecA36, ermA8, ermB8, ermC28, mphC29, aph(3′)-IIIa9, ant(4′)-Ia12, str2, tetL19, tetK14, tetO1, tetM2, catp1941, dfrK6, dfrD2 |
| S. urealyticus | 21 | PEN21, FOX18, CIP3, CN4, TOB6, KAN5, ERY21, CD21, TET21, C3, FD17 | mecA21, ermA1, ermB7, ermC19, mphC16, aph(3′)-IIIa5, ant(4′)-Ia2, str2, tetL17, tetK18, tetO13, tetM4 |
| S. sciuri | 15 | PEN14, FOX6, LNZ1, CIP3, TOB8, KAN4, ERY14, CD14, TET15, C2, FD10, SXT2 | mecA15, cfr1, ermB9, ermC7, mphC9, aph(3′)-IIIa3, ant(4′)-Ia7, str1, tetL11, tetK12, tetO2, tetM3, dfrK1 |
| S. haemolyticus | 3 | PEN3, FOX1, CIP2, TOB2, KAN1, ERY2, CD2, TET3, FD2, SXT2 | mecA3, ermB1, ermC2, mphC2, aph(3′)-IIIa2, ant(4′)-Ia1, str1, tetL3, tetK1, dfrK1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Caniça, M.; Ferreira, E.; Vieira-Pinto, M.; Saraiva, C.; Pereira, J.E.; Capelo, J.L.; Igrejas, G.; Poeta, P. Multidrug-Resistant Methicillin-Resistant Coagulase-Negative Staphylococci in Healthy Poultry Slaughtered for Human Consumption. Antibiotics 2022, 11, 365. https://doi.org/10.3390/antibiotics11030365
Silva V, Caniça M, Ferreira E, Vieira-Pinto M, Saraiva C, Pereira JE, Capelo JL, Igrejas G, Poeta P. Multidrug-Resistant Methicillin-Resistant Coagulase-Negative Staphylococci in Healthy Poultry Slaughtered for Human Consumption. Antibiotics. 2022; 11(3):365. https://doi.org/10.3390/antibiotics11030365
Chicago/Turabian StyleSilva, Vanessa, Manuela Caniça, Eugénia Ferreira, Madalena Vieira-Pinto, Cândido Saraiva, José Eduardo Pereira, José Luis Capelo, Gilberto Igrejas, and Patrícia Poeta. 2022. "Multidrug-Resistant Methicillin-Resistant Coagulase-Negative Staphylococci in Healthy Poultry Slaughtered for Human Consumption" Antibiotics 11, no. 3: 365. https://doi.org/10.3390/antibiotics11030365
APA StyleSilva, V., Caniça, M., Ferreira, E., Vieira-Pinto, M., Saraiva, C., Pereira, J. E., Capelo, J. L., Igrejas, G., & Poeta, P. (2022). Multidrug-Resistant Methicillin-Resistant Coagulase-Negative Staphylococci in Healthy Poultry Slaughtered for Human Consumption. Antibiotics, 11(3), 365. https://doi.org/10.3390/antibiotics11030365










