Citrus bergamia: Kinetics of Antimicrobial Activity on Clinical Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Citrus Bergamia Risso et Poiteau
2.2. Preparation of Distilled Extract of Bergamot
2.3. Chemical Characterization of the Distilled Extract of Bergamot
2.4. Microorganisms Tested
2.5. Broth Microdilution Assay
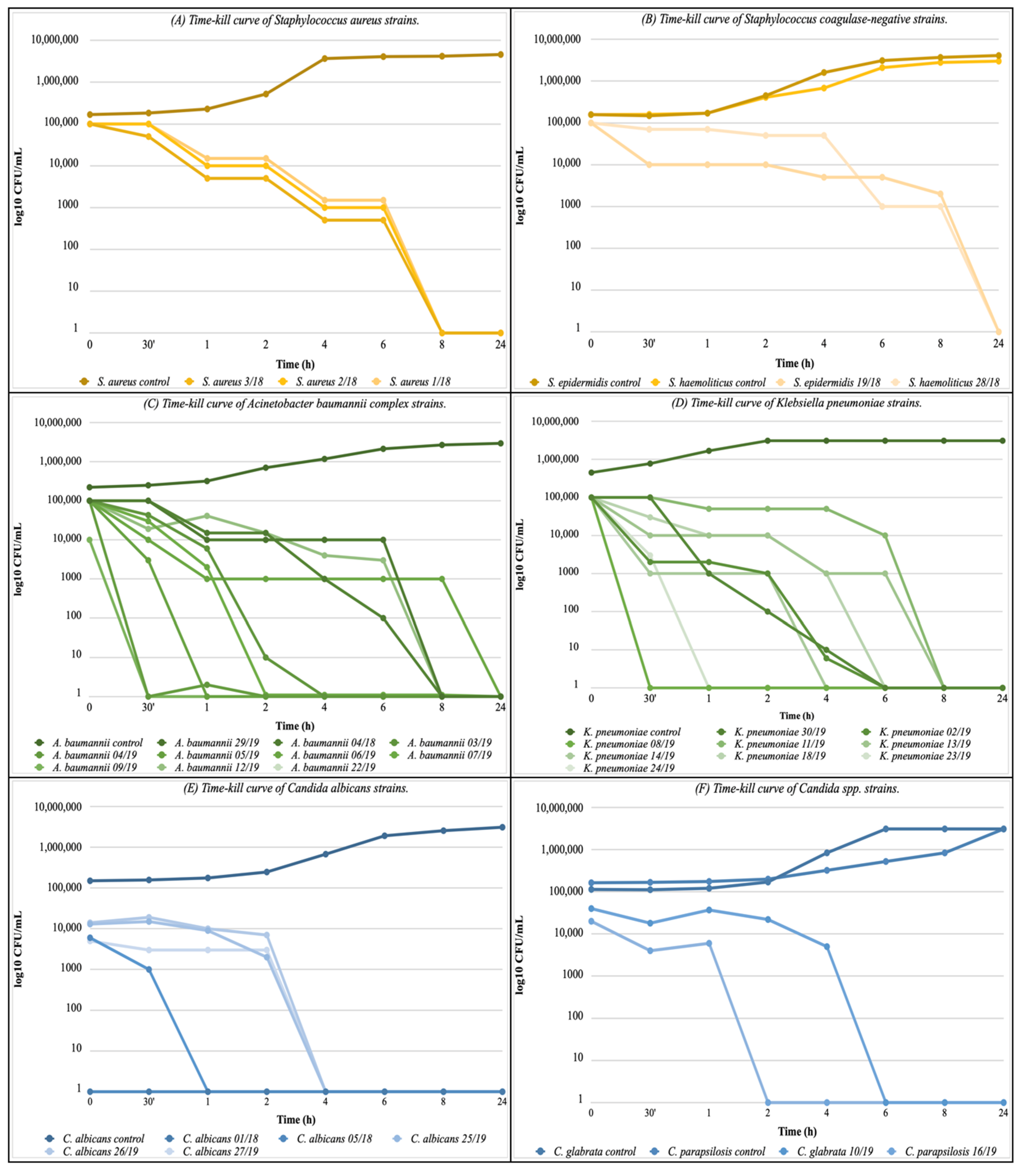
2.6. Time-Kill Assay
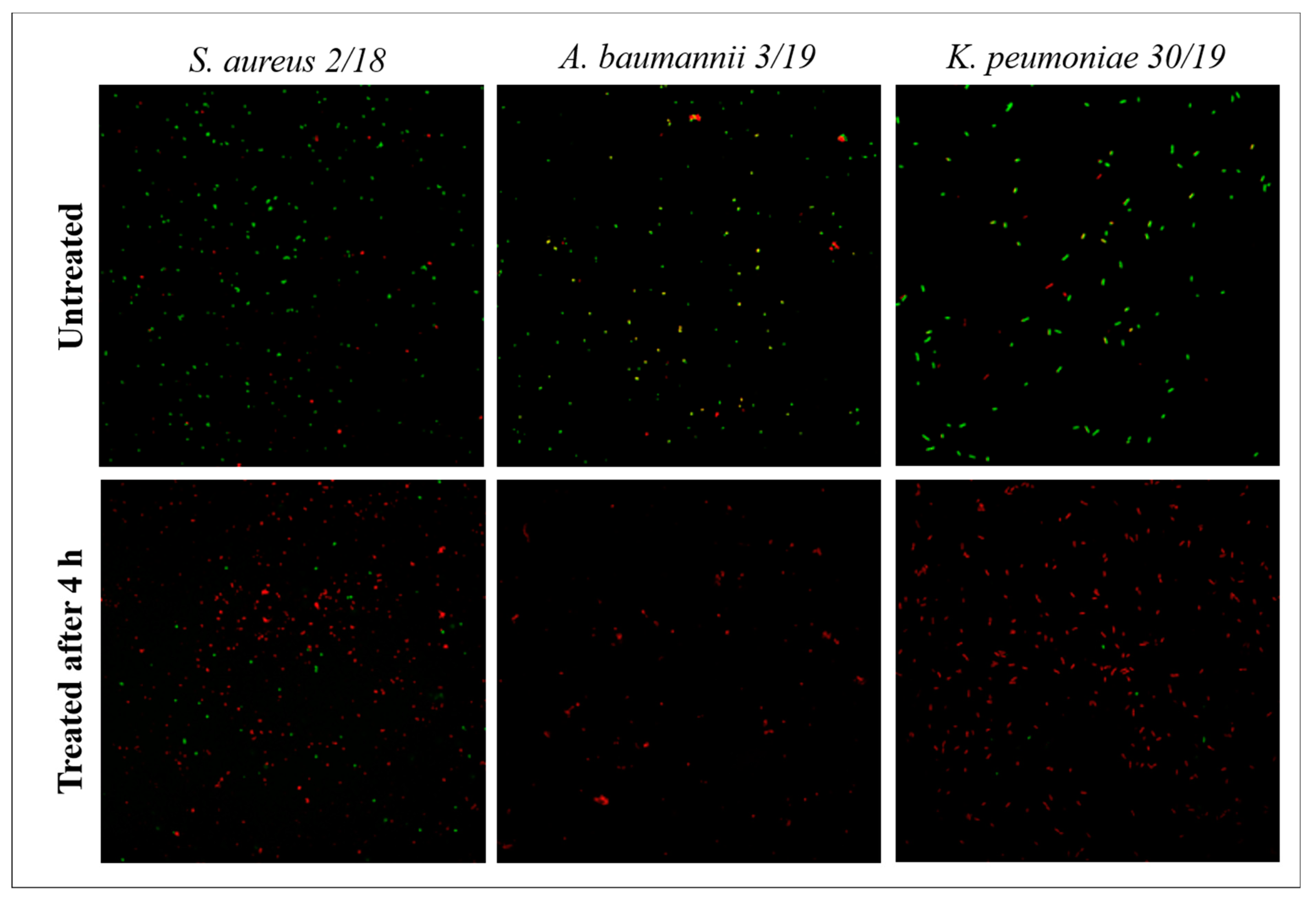
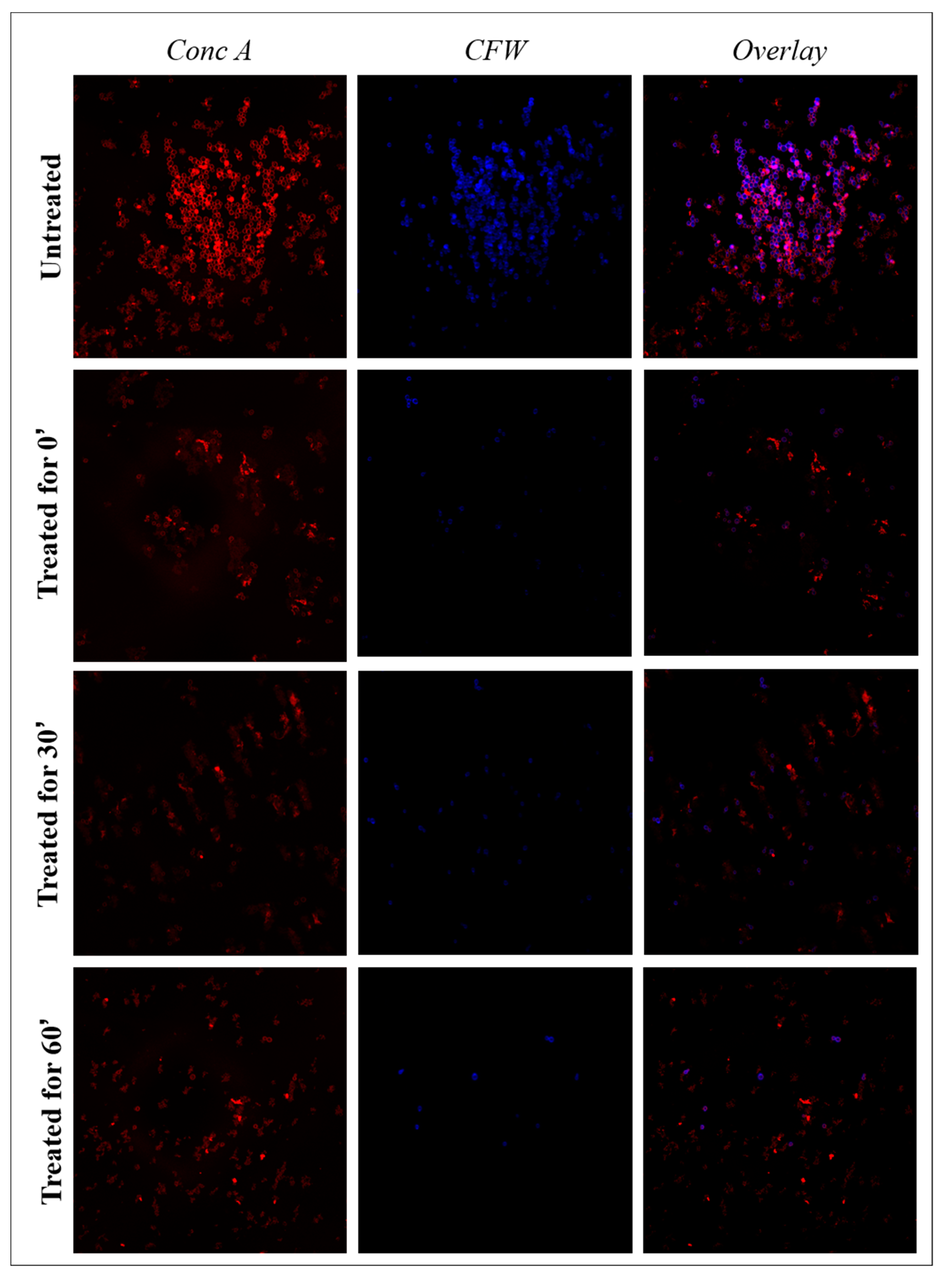
2.7. Confocal Microscopy
3. Results
3.1. Antimicrobial Activity
3.2. Time-Kill Assay
3.3. Confocal Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glass Whole-Genome Sequencing for Surveillance of Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2020.
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 21 February 2022).
- Hamad, M.; Al-Marzooq, F.; Srinivasulu, V.; Omar, H.A.; Sulaiman, A.; Zaher, D.M.; Orive, G.; Al-Tel, T.H. Antibacterial Activity of Small Molecules Which Eradicate Methicillin-Resistant Staphylococcus aureus Persisters. Front. Microbiol. 2022, 13, 823394. [Google Scholar] [CrossRef] [PubMed]
- Bartal, C.; Rolston, K.V.I.; Nesher, L. Carbapenem-resistant Acinetobacter baumannii: Colonization, Infection and Current Treatment Options. Infect Dis. Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, Y.; Yue, H.; Huang, X.; Zhou, G. Risk factors for and clinical outcomes of ceftazidime-avibactam-resistant carbapenem-resistant Klebsiella pneumoniae nosocomial infections: A single-center retrospective study. Infection 2022. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, D.E.; Faraag, A.H.I.; Abu El-Wafa, W.M. In vitro study on the potential fungicidal effects of atorvastatin in combination with some azole drugs against multidrug resistant Candida albicans. World J. Microbiol. Biotechnol. 2021, 37, 191. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Bogavac, M.; Radovanović, B.; Sudji, J.; Tesanović, K.; Janjusević, L. Origanum vulgare essential oil affects pathogens causing vaginal infections. J. Appl. Microbiol. 2017, 122, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Catalfamo, M.; Laganà, P.; Rappazzo, A.C.; Raymo, V.; Zampino, D.; Zaccone, R. Screening of antimicrobial activity of citrus essential oils against pathogenic bacteria and Candida strains. Flavour. Fragr. J. 2019, 34, 187–200. [Google Scholar] [CrossRef]
- Tanhaeian, A.; Sekhavati, M.H.; Moghaddam, M. Antimicrobial activity of some plant essential oils and an antimicrobial-peptide against some clinically isolated pathogens. Chem. Biol. Technol. Agric. 2020, 7, 13. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2015, 72, 165–172. [Google Scholar] [CrossRef]
- Quirino, A.; Morelli, P.; Marano, V.; Cortese, G.; Barreca, G.S.; Liberto, M.C.; Focà, A. In vitro antimicrobial activity of natural essence and distilled extract of bergamot against Drug Resistance clinical isolates. Med. Aromat. Plants 2016, S3, 1–8. [Google Scholar] [CrossRef]
- Quirino, A.; Morelli, P.; Capua, G.; Arena, G.; Matera, G.; Liberto, M.C.; Focà, A. Synergistic and antagonistic effects of Citrus bergamia distilled extract and its major components on drug resistant clinical isolates. Nat. Prod. Res. 2018, 34, 1626–1629. [Google Scholar] [CrossRef]
- Wikler, M.A.; Clinical and Laboratory Standards Institute. Development of In Vitro Susceptibility Testing Criteria and Quality Control Parameters. Approved Giudeline, 5th ed.; CLSI Document M23; 2018; Volume 38, Available online: https://clsi.org/standards/products/microbiology/documents/m23s/ (accessed on 21 February 2022).
- Xing, X.; Liao, Z.; Tan, F.; Zhu, Z.; Jiang, Y.; Cao, Y. Effect of nicotinamide against Candida albicans. Front. Microbiol. 2019, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Barreca, D.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Distribution of flavonoids and furocoumarins in juices from cultivars of Citrus bergamia Risso. J. Agric. Food Chem. 2007, 55, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Nalin, F.; Simonetti, P. Evaluation of flavonoids and furanocoumarins from Citrus bergamia (Bergamot) juice and identification of new compounds. Molecules 2008, 13, 2220–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salerno, R.; Casale, F.; Calandruccio, C.; Procopio, A. Characterization of flavonoids in Citrus bergamia (Bergamot) polyphenolic fraction by liquid chromatography-high resolution mass spectrometry (LC/HRMS). Pharma Nutr. 2016, 4, S1–S7. [Google Scholar] [CrossRef]
- Bok, S.H.; Lee, S.H.; Park, Y.B.; Bae, K.H.; Son, K.H.; Jeong, T.S. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: Cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J. Nutr. 1999, 129, 1182–1185. [Google Scholar] [CrossRef]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and ckitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Dugo, L.; Stancanelli, R.; Dugo, G. LCMS for the identification of oxygen heterocyclic compounds in citrus essential oils. J. Pharm. Biomed. Anal. 2000, 24, 147–154. [Google Scholar] [CrossRef]
- Dugo, P.; Presti, M.L.; Ohman, M.; Fazio, A.; Dugo, G.; Mondello, L. Determination of flavonoids in citrus juices by micro HPLC-ESI/MS. J. Sep. Sci. 2005, 28, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.Y.; Son, K.H.; Know, C.S.; Kang, S.S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Shingu-Vazquez, M.; Traven, A. Mitochondria and fungal pathogenesis: Drug tolerance, virulence, and potential for antifungal therapy. Eukaryot. Cell 2011, 10, 1376–1383. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Wongsariya, K.; Phanthong, P.; Bunyapraphatsara, N.; Srisukh, V.; Chomnawang, M.T. Synergistic interaction and mode of action of Citrus hystrix essential oil against bacteria causing periodontal diseases. Pharm. Biol. 2014, 52, 273–280. [Google Scholar] [CrossRef]
- Sakkas, H.; Economou, V.; Gousia, P.; Bozidis, P.; Sakkas, V.A.; Petsios, S.; Mpekoulis, G.; Ilia, A.; Papadopoulou, C. Antibacterial efficacy of commercially available essential oils tested against Drug-Resistant Gram-positive pathogens. Appl. Sci. 2018, 8, 2201. [Google Scholar] [CrossRef] [Green Version]
- Boonyanugomol, W.; Kraisriwattana, K.; Rukseree, K.; Boonsam, K.; Narachai, P. In vitro synergistic antibacterial activity of the essential oil from Zingiber cassumunar Roxb against extensively drug-resistant Acinetobacter baumannii strains. J. Infect. Public Health 2017, 10, 586–592. [Google Scholar] [CrossRef]
- Habsah, M.; Amran, M.; Mackeen, M.M.; Lajis, N.H.; Kikuzaki, H.; Nakatani, N.; Rahman, A.A.; Ghafar; Ali, A.M. Screening of Zingiberaceae extracts forantimicrobial and antioxidant activities. J. Ethnopharmacol. 2000, 72, 403–410. [Google Scholar] [CrossRef]
- Nakamura, S.; Iwami, J.; Matsuda, H.; Wakayama, H.; Pongpiriyadacha, Y.; Yoshikawa, M. Structures of new phenylbutanoids and nitricoxide production inhibitors from the rhizomes of Zingiber cassumunar. Chem. Pharm. Bull. 2009, 57, 1267–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, N.G.; Vaz, M.S.M.; Radai, J.A.S.; Kassuya, A.L.K.; Formagio, A.S.N.; Graciani, F.S.; Leal, M.L.; Rodrigo Juliano Oliveira, R.J.; da Silva, K.E.; Croda, J.; et al. Antimicrobial activity of plant extracts against carbapenem-producing Klebsiella pneumoniae and in vivo toxicological assessment. J. Toxicol. Environ. Health Part A 2020, 83, 719–729. [Google Scholar] [CrossRef] [PubMed]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanguinetti, M.; Posteraro, B.; Romano, L.; Battaglia, F.; Lopizzo, T.; De Carolis, E.; Fadda, G. In vitro activity of Citrus bergamia (bergamot) oil against clinical isolates of dermatophytes. J. Antimicrob. Chemother. 2007, 59, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Freire, J.C.P.; de Júnior, J.K.O.; de Silva, D.F.; de Sousa, J.P.; Guerra, F.Q.S.; de Oliveira Lima, E. Antifungal activity of essential oils against Candida albicans strains isolated from users of dental prostheses. Evid.-Based Complement. Altern. Med. 2017, 2017, 7158756. [Google Scholar] [CrossRef] [Green Version]
- Shahina, Z.; El-Ganiny, A.M.; Minion, J.; Whiteway, M.; Sultana, T.; Dahms, T.E.S. Cinnamomum zeylanicum bark essential oil induces cell wall remodelling and spindle defects in Candida albicans. Fungal Biol. Biotechnol. 2018, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.H.; Graham, L.; Adukwu, E.C. In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911–8924. [Google Scholar] [CrossRef]


| Compounds | tR (min.) | Distillate (w/w%) | Bp (°C) |
|---|---|---|---|
| Cyclic monoterpenes | |||
| α-pinene | 4.24 | 1.03 | 155 |
| β-pinene | 5.99 | 6.56 | 167–167 |
| α-phellandrene | 6.89 | 0.04 | 171–172 |
| α-terpinene | 7.18 | 0.16 | 173–175 |
| Limonene | 7.48 | 30.2 | 176 |
| p-cimene | 7.89 | 0.18 | 177 |
| ɣ-terpinene | 8.37 | 11.95 | 182 |
| Terpinolene | 8.99 | 0.27 | 184–185 |
| Acyclic monoterpenes | |||
| Myrcene | 6.29 | 0.82 | 165 |
| Ocimene | 7.75 | 0.08 | 65–66 |
| Oxygenated acyclic monoterpenes | |||
| Linalool | 9.47 | 21.82 | 196–198 |
| Lynalyl acetate | 12.03 | 16.21 | 220 |
| Neral | 12.6 | 0.21 | 103 |
| Geranial | 13.09 | 0.11 | 229 |
| Neryl acetate | 13.91 | 0.28 | 134 (25 mmHg) |
| Oxygenated cyclic monoterpenes | |||
| Terpineol | 11.64 | 0.87 | 213–218 |
| Ester | |||
| Octyl acetate | 11.38 | 0.10 | 203–213 |
| Aldehydes | |||
| Decanal | 11.52 | Trace | 93–95 |
| Sesquiterpenes | |||
| Cariofillene | 14.38 | 0.14 | 128–129 |
| ID Stain | Isolation Site | Susceptible | Resistant |
|---|---|---|---|
| S. aureus 3/18 | MRSA nasal swab | FA, CM, E, GM, LZD, MUP, RA, TEC, TE, TGC, SXT, VA | P, LVX, OX |
| S. aureus 2/18 | MRSA nasal swab | FA, GM, LZD, MUP, TEC, TE, TGC, SXT, VA | CM, E, P, LVX, OX, RA |
| S. aureus 1/18 | MRSA nasal swab | FA, CM, E, GM, LZD, MUP, RA, TEC, TE, TGC, SXT, VA | P, LVX, OX |
| S. epidermidis 19/19 | Wound swab | LZD, RA, TE, TGC, VA | FA, CM, E, GM, LVX, OX, SXT |
| S. haemolyticus 28/19 | Wound swab | CM, LZD, RA, TGC, VA | FA, E, GM, LVX, OX, SXT |
| A. baumannii 29/19 | Throat swab | AMC, CS | CTX, MEM, GM, CIP, FOS, FT, SXT |
| A. baumannii 04/18 | Throat swab | CS | FOX, CTX, MEM, AM, GM, CIP, FOS, FT, CS, SXT |
| K. pneumoniae 30/19 | Rectal swab | GM | AMC, TZP, FOX, CTX, CAZ, FEP, ETP, MEM, AM, CIP, FOS, FT, CS, SXT |
| K. pneumoniae 02/19 | Rectal swab | AMC, TZP, ETP, MEM, AM, GM, FOS, CS | CTX, CAZ, FEP, CIP, SXT |
| A. baumannii 03/19 | Wound swab | CS | MEM, AM, GM, CIP, SXT |
| A. baumannii 04/19 | Rectal swab | AM, CS | MEM, GM, CIP, SXT |
| A. baumannii 05/19 | Throat swab | AM, CS | MEM, GM, CIP, SXT |
| A. baumannii 06/19 | Rectal swab | AM, CS | GM, CIP, SXT |
| A. baumannii 07/19 | Throat swab | AM, CS | MEM, GM, CIP, SXT |
| K. pneumoniae 08/19 | Throat swab | AM, FOS, CS | AMC, TZP, CTX, CAZ, FEP, ETP, MEM, GM, CIP, SXT |
| A. baumannii 09/19 | Bronchial aspirate | AM | MEM, GM, CIP, CS, SXT |
| K. pneumoniae 11/19 | Rectal swab | CS, SXT | AMC, TZP, CTX, CAZ, FEP, ETP, MEM, AM, GM, CIP, FOS |
| A. baumannii 12/19 | Throat swab | AM, CS | MEM, GM, CIP, SXT |
| K. pneumoniae 13/19 | Rectal swab | AM, FOS, CS | AMC, TZP, CTX, CAZ, FEP, ETP, MEM, GM, CIP, SXT |
| K. pneumoniae 14/19 | Urinary catheter | FOS, CS | AMC, TZP, CTX, CAZ, FEP, ETP, MEM, AM, GM, CIP, SXT |
| K. pneumoniae 18/19 | Rectal swab | MEM, AM, FOS, CS | AMC, TZP, CTX, CAZ, FEP, ETP, GM, CIP, TGC, SXT |
| A. baumannii 22/19 | Throat swab | CS | MEM, AM, GM, CIP, SXT |
| K. pneumoniae 23/19 | Throat swab | CS | AMC, TZP, CTX, CAZ, FEP, ETP, MEM, AM, GM, CIP, FOS, SXT |
| K. pneumoniae 24/19 | Rectal swab | CS | AMC, TZP, CTX, CAZ, FEP, ETP, MEM, AM, GM, CIP, FOS, SXT |
| C. albicans 01/18 | Vaginal swab | CAS, MYC, AMB | FLU, VOR |
| C. albicans 05/18 | Blood | FLU, VOR, CAS, MYC, AMB | |
| C. glabrata 10/19 | Bronchial aspirate | CAS, MYC, AMB | |
| C. parapsilosis 16/19 | Urinary catheter | FLU, VOR, CAS, MYC, AMB | |
| C. albicans 25/19 | Foot skin swab | FLU, VOR, CAS, MYC, AMB | |
| C. albicans 26/19 | Nasal discharge | FLU, VOR, CAS, MYC, AMB | |
| C. albicans 27/19 | Wound swab | FLU, VOR, CAS, MYC, AMB |
| ID Strain | MBC | ID Strain | MBC |
|---|---|---|---|
| S. aureus 3/18 | 2.5% v/v (1:40) | K. pneumoniae 11/19 | 1.25% v/v (1:80) |
| S. aureus 2/18 | 2.5% v/v (1:40) | A. baumannii 12/19 | 1.25% v/v (1:80) |
| S. aureus 1/18 | 5% v/v (1:20) | K. pneumoniae 13/19 | 5% v/v (1:20) |
| S. epidermidis 19/19 | 5 % v/v (1:20) | K. pneumoniae 14/19 | 1.25% v/v (1:80) |
| S. epidermidis 28/19 | 5% v/v (1:20) | K. pneumoniae 18/19 | 2.5% v/v (1:40) |
| A. baumannii 29/19 | 1.25% v/v (1:80) | A. baumannii 22/19 | 0.625% v/v (1:160) |
| A. baumannii 04/18 | 1.25% v/v (1:80) | K. pneumoniae 23/19 | 2.5% v/v (1:40) |
| K. pneumoniae 30/19 | 2.5% v/v (1:40) | K. pneumoniae 24/19 | 2.5% v/v (1:40) |
| K. pneumoniae 02/19 | 2.5% v/v (1:40) | C. albicans 01/18 | 2.5% v/v (1:40) |
| A. baumannii 03/19 | 0.625% v/v (1:160) | C. albicans 05/18 | 2.5% v/v (1:40) |
| A. baumannii 04/19 | 1.25% v/v (1:80) | C. glabrata 10/19 | 0.313% v/v (1:320) |
| A. baumannii 05/19 | 2.5% v/v (1:40) | C. parapsilosis 16/19 | 1.25% v/v (1:80) |
| A. baumannii 06/19 | 0.625% v/v (1:160) | C. albicans 25/19 | 1.25% v/v (1:80) |
| A. baumannii 07/19 | 1.25% v/v (1:80) | C. albicans 26/19 | 1.25% v/v (1:80) |
| K. pneumoniae 08/19 | 5% v/v (1:20) | C. albicans 27/19 | 1.25% v/v (1:80) |
| A. baumannii 09/19 | 2.5% v/v (1:40) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quirino, A.; Giorgi, V.; Palma, E.; Marascio, N.; Morelli, P.; Maletta, A.; Divenuto, F.; De Angelis, G.; Tancrè, V.; Nucera, S.; et al. Citrus bergamia: Kinetics of Antimicrobial Activity on Clinical Isolates. Antibiotics 2022, 11, 361. https://doi.org/10.3390/antibiotics11030361
Quirino A, Giorgi V, Palma E, Marascio N, Morelli P, Maletta A, Divenuto F, De Angelis G, Tancrè V, Nucera S, et al. Citrus bergamia: Kinetics of Antimicrobial Activity on Clinical Isolates. Antibiotics. 2022; 11(3):361. https://doi.org/10.3390/antibiotics11030361
Chicago/Turabian StyleQuirino, Angela, Valeria Giorgi, Ernesto Palma, Nadia Marascio, Paola Morelli, Angelo Maletta, Francesca Divenuto, Giuseppe De Angelis, Valentina Tancrè, Saverio Nucera, and et al. 2022. "Citrus bergamia: Kinetics of Antimicrobial Activity on Clinical Isolates" Antibiotics 11, no. 3: 361. https://doi.org/10.3390/antibiotics11030361
APA StyleQuirino, A., Giorgi, V., Palma, E., Marascio, N., Morelli, P., Maletta, A., Divenuto, F., De Angelis, G., Tancrè, V., Nucera, S., Gliozzi, M., Musolino, V., Carresi, C., Mollace, V., Liberto, M. C., & Matera, G. (2022). Citrus bergamia: Kinetics of Antimicrobial Activity on Clinical Isolates. Antibiotics, 11(3), 361. https://doi.org/10.3390/antibiotics11030361










