Abstract
Ventilator-associated pneumonia is a frequent cause of ICU-acquired infections. These infections are associated with high morbidity and mortality. The increase in antibiotic resistance, particularly among Gram-negative bacilli, makes the choice of empiric antibiotic therapy complex for physicians. Multidrug-resistant organisms (MDROs) related infections are associated with a high risk of initial therapeutic inadequacy. It is, therefore, necessary to quickly identify the bacterial species involved and their susceptibility to antibiotics. New diagnostic tools have recently been commercialized to assist in the management of these infections. Moreover, the recent enrichment of the therapeutic arsenal effective on Gram-negative bacilli raises the question of their place in the therapeutic management of these infections. Most national and international guidelines recommend limiting their use to microbiologically documented infections. However, many clinical situations and, in particular, the knowledge of digestive or respiratory carriage by MDROs should lead to the discussion of the use of these new molecules, especially the new combinations with beta-lactamase inhibitors in empirical therapy. In this review, we present the current epidemiological data, particularly in terms of MDRO, as well as the clinical and microbiological elements that may be taken into account in the discussion of empirical antibiotic therapy for patients managed for ventilator-associated pneumonia.
1. Introduction
Ventilator-associated pneumonia (VAP) is one of the most frequent causes of intensive care unit (ICU)-acquired infections [1]. In French ICUs, 8% of patients developed hospital-acquired pneumonia in 2016, and 88.7% among them were ventilator-associated pneumonia [2].
For more than a decade, we have been confronted with the spread of multi-resistant bacteria in hospitals [3,4,5] and the community [6,7,8].
The increasing prevalence of resistance among bacteria, particularly gram-negative bacilli (GNB) and especially Enterobacterales, makes harder the choice of antibiotics, in case of infection. Several factors seem to be associated with a higher risk of infection related to multidrug-resistant organisms (MDRO), such as the local prevalence, previous antibiotic therapy, time of occurrence of the infection, and previous MDRO colonization.
In our clinical practice, the spread of MDRO resistance leads to a higher risk of antibiotic inadequation. Indeed, there are two opposing risks when choosing an empirical antibiotic therapy. On one side is the individual level, reflected by the risk to choose a narrow spectrum antibiotic with potentially important consequences in terms of mortality and morbidity. On the other side is the collective level, reflected by the choice of a broad-spectrum antibiotic which could contribute to the amplification of resistance.
In the last four years, medical research has been driven by the discovery of new beta-lactamase inhibitors and the marketing of new antibiotics with broad-spectrum activity.
Numerous authors suggest and encourage rational use of these new antibiotics out of fear of the emergence of new resistance mechanisms. However, certain clinical situations require empirical choices. It, therefore, seemed important to assess relevant factors and variables at the time of prescribing in order to choose the most appropriate molecule, both in terms of spectrum and possible ecological effects.
2. HCAP, HAP, VAP: Clinical Concepts and Historical Perspective
From the first consensus conference in 1996 until now, the history of concepts and definitions around nosocomial pneumonia has never been a long calm river. Its evolution is intrinsically linked to epidemiological, diagnostic, and therapeutic advances in the context of precision medicine [9]. A remarkable example would be the path of the Healthcare-Associated Pneumonia (HCAP) category in guidelines. If the term was initially defined in the 2005 ATS recommendations [10] in order to avoid inappropriate empiric antimicrobial therapy after the emergence of the “golden hours” concept in the intensive care unit (ICU) [11], it has finally proved to be irrelevant. By HCAP, it was meant “any patient who was hospitalized in an acute care hospital for 2 or more days within 90 days of the infection”. This categorization resulted in the increasing usage of broad-spectrum antibiotics in a population which eventually appeared to be no more infected with MDRO pathogens than patients with community-acquired pneumonia (CAP) [12]. Furthermore, this category failed to predict mortality [13]. The starting point was a retrospective cohort study based on more than 4000 patients with proven bacterial pneumonia within the 48 h following admission and transfer from a healthcare facility which showed an incidence of a quarter of admitted patients with Methicillin-Resistant Staphylococcus aureus (MRSA) infection, with the same incidence for Pseudomonas aeruginosa (PA) [14]. These findings, which led to treating CAP with risk factors of MDRO organisms carriage the same way as ventilator-associated pneumonia (VAP) regardless of the severity status, were later largely overruled [15]. Indeed, recent studies found no major difference in terms of bacterial epidemiology between CAP and HCAP [16], and antibiotic-resistant organisms were found to be rare whatever population at risk of carriage was analyzed [17].
Then, a deeper knowledge of bacterial epidemiology stood out as the key challenge of nosocomial pneumonia management for future years.
3. Definitions and Issues of Nosocomial Pneumonia
Hospital-Acquired Pneumonia (HAP) is defined as new pneumonia (a lower respiratory tract infection verified by the presence of a new pulmonary infiltrate on imaging) in non-intubated patients, that develops more than 48 h after admission. When it develops after 48 h of endotracheal intubation, it is categorized as a VAP. In the ICU, VAPs are the most present entity, with an overwhelming majority (more than 95%) of reported cases of pneumonia [18].
VAP is a major problem in the ICU due to its frequency and short-term consequences. It is the main source of healthcare-associated infections (HAI) in these departments, with an incidence reaching 40% of patients with up to 16 episodes per 1000 days of mechanical ventilation [19]. It is associated with significant morbidity since it is complicated in 30 to 50% of cases by septic shock, in 10 to 15% of cases by acute respiratory distress syndrome (ARDS), and in 10 to 15% of cases by multi-organ failure (MOF) [20,21,22,23,24]. It also has an impact on the duration of mechanical ventilation (increased from 7 to 11 days), the length of hospital stay (increased from 11 to 13 days), and health economics (EUR 30,000 to 40,000/per episode) [25,26,27]. Finally, the overall mortality of patients who experienced a VAP in the ICU is considerable (20 to 40% depending on the series), with an average attributable mortality (to the VAP episode alone) of 13% [28].
4. Epidemiology
HAP and VAP may be caused by a variety of pathogens and can be polymicrobial. Common pathogens include aerobic GNB (e.g., Escherichia coli, Klebsiella pneumoniae, Enterobacter spp., PA, Acinetobacter spp.) and Gram-positive cocci (e.g., Staphylococcus aureus, including MRSA, Streptococcus spp.) [29,30]. Furthermore, there is increasing recognition that a substantial fraction of nosocomial pneumonia may be due to viruses [29].
Among 8474 cases of VAP reported in the United States Centers for Disease Control and Prevention, the distribution of pathogens associated were Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella species, Enterobacter species, Acinetobacter baumannii, and E. coli in, respectively, 24.1%, 16.6%, 10.1%, 8.6%, 6.6%, and 5.9% of cases [31]. In a prospective observational study evaluating 158,519 patients admitted to the University of North Carolina Hospital over a 4-year period, a total of 282 episodes of documented VAP and 190 episodes of documented HAP in non-ventilated patients were identified (Table 1) [32].

Table 1.
Frequency of isolation of pathogens from patients with Ventilator-Associated Pneumonia (VAP) and non-ventilated patients with Hospital-Acquired Pneumonia (HAP) [32].
These findings are similar to those observed in a meta-analysis of 24 studies performed during the development of the 2016 Infectious Disease Society of America guidelines [33].
The etiology of HAP and VAP depends largely upon whether the patient has risk factors for MDRO pathogens [33]. The frequency of specific MDRO pathogens varies among hospitals, within hospitals, and between different patient populations. One of the major problems lies in the spread of MDROs and, specifically, extended-spectrum beta-lactamase-producing Enterobacterales (ESBLEs) in the community. This diffusion has consequences in hospitals and in intensive care units where, among hospitalized patients, between 5 and 25% are ESBL producing Enterobacterales carriers [34,35,36].
4.1. Bacterial Epidemiology: A Practical Approach in ICU
Choosing the right antibiotic lies in anticipating both the species and the resistance mechanisms that will be involved in the infection (Figure 1). If the local epidemiology weighs heavily on the species involved, it remains that certain clinical situations and data specific to the medical history of the patient must lead to considering certain species.
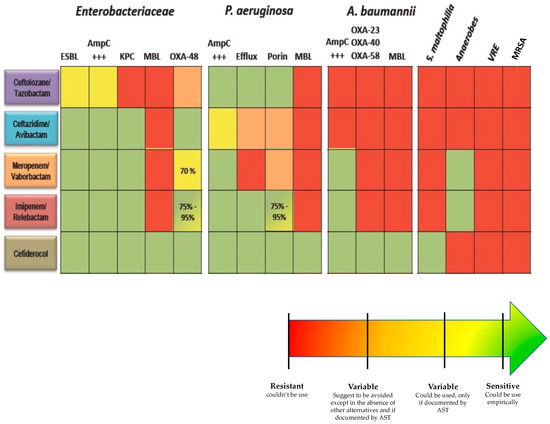
Figure 1.
Spectrum of activity of new antibiotics. AST: Antibiotic susceptibility test, ESBL: Extended-spectrum beta-lactamase, AmpC: Cephalosporinase, KPC: Klebsiella pneumoniae carbapenemase, MBL: metallo-betalactamase, VRE: vancomycin resistant Enterococci, MRSA: Methicillin-resistant Staphylococcus aureus.
4.2. Profiling Bacterial Species in Pneumonia: Born to Be Wild
4.2.1. Methicillin Susceptible Staphylococcus aureus (MSSA)
Among the population, 30 to 50% are permanently or intermittently colonized with Staphylococcus aureus species. Moreover, the risk of secondary infection seems to be higher among previously colonized patients [37,38,39]. Indeed, in 2017 post-hoc analysis of two cohort studies of more than 9000 critically ill patients found that patients colonized with Staphylococcus aureus at ICU admission had an up to 15 times increased risk for developing this outcome compared with non-colonized patients [40]. Staphylococcus aureus should be considered in the case of HAP that complicates influenza infection or in the case of early HAP in patients known to be previously colonized with MSSA. Several authors suggested a higher risk in a specific population, such as traumatic and non-traumatic brain injury patients [41]. Indeed, the authors suggested that MSSA is most frequent in this specific population, accounting for up to 40–50% of VAP. In a recent study focusing specifically on bacteriological aspects of Staphylococcus aureus VAP, the authors highlighted that nearly 74% of the patients had severe head trauma or a priori history of coma [41,42].
4.2.2. Enterobacterales
Enterobacterales remain the most frequent species found in HAP and VAP patients. These species must be systematically considered in the choice of antibiotics, whatever the circumstances. The only question that should be asked is if the antibiotic spectrum should include resistant bacteria. In this perspective, the time of occurrence of the event should be addressed as major information. Indeed, early HAP and VAP seem to be related to sensitive species, whereas the duration of hospitalization and previous antibiotic therapy seem to be associated with more resistant species [43].
4.2.3. Pseudomonas aeruginosa
Assessing the specific risk of PA infection is highly relevant since the mortality attributable to this GNB seems more important than others (both because of the multi-resistant nature of the germ inducing a delay in appropriate antibiotic therapy and because it more often affects more severe patients) [44]. Pseudomonas aeruginosa colonization remains rare even in critically ill patients in the ICU. Indeed, in developed countries, the PA colonization rate at ICU admission is close to 10% in several studies [45,46,47]. However, PA is a highly prevalent causative pathogen in HAP and VAP. An experience of the French national surveillance, REA-RAISIN, found that a higher probability of PA-VAP is regularly associated with higher age, length of mechanical ventilation before pneumonia, antibiotics at admission, and admission in a ward with a higher incidence of patients with PA infection. Interestingly, transfer from a medical unit or ICU was also found to be associated with a higher probability of PA-VAP (45). Hospital admission should thus be considered a turning point in the colonization pressure experienced by the patient (and not only the admission in ICU). Lower probability of PA pneumonia was associated with traumatism and, as expected, with admission in a ward with higher patient turnover. Some populations seem to be more at risk, such as patients with COPD, cystic fibrosis, or bronchiectasis. Old studies suggested a higher prevalence of late VAP [48]. Indeed, as PA is a saprophytic species specifically linked to water, acquisition requires a contaminated environment and selection pressure.
4.2.4. Acinetobacter baumannii
Although found with a low worldwide prevalence, Acinetobacter baumannii is one of the most antibiotic-resistant pathogens, with 50% of carbapenem-resistant isolates in US intensive care units, including a vast majority of extreme drug-resistant (XDR) strains [49]. Moreover, the survival of Acinetobacter baumanii in the biofilm makes their treatment difficult [50,51].
Acinetobacter are ubiquitous organisms recovered from soil or surface water. Acinetobacter are rarely found in the microbiota of patients in the northern hemisphere. Indeed, several studies suggested a low rate of Acinetobacter baumannii carriage in the communities in Germany and France [52], even in the population of patients admitted to the intensive care unit [53]. However, authors highlighted higher carriage rates in other parts of the globe, such as Hong Kong, the Asia-Pacific region, and other countries with hot and humid climates. In these locations, Acinetobacter baumannii has emerged as a cause of severe community-acquired infections [54]. Classically-found risk factors for Acinetobacter baumannii infection are tropical or sub-tropical climate, excessive alcohol consumption, smoking, or having an underlying health condition (diabetes mellitus or chronic lung disease) with a different weight of each risk depending on the location [55]. In the ICU, a difference must be made according to the epidemiological data. Whereas Acinetobacter baumannii is associated with early-onset HAP/VAP in southern countries, it seems rarely isolated in northern countries and is usually associated with several risk factors. Indeed, factors independently associated with Acinetobacter baumannii infection are commonly found to be immunosuppression, previous antimicrobial therapy, previous sepsis in the ICU, and a history of recent invasive procedures [56].
4.2.5. Stenotrophomonas maltophilia
HAP or VAP related to Stenotrophomonas maltophilia (SM) are rare [30]. SM is an environmental bacterium found in aqueous habitats, including plant rhizospheres, animals, foods, and water sources. It is not a highly virulent pathogen, but it has emerged as an important nosocomial pathogen. The incidence of SM hospital-acquired infections (HAI) is increasing, particularly in the immunocompromised patient population [57,58]. Risk factors for this infection include chronic respiratory diseases (especially cystic fibrosis), hematologic malignancy, chemotherapy-induced neutropenia, organ transplant patients, human immunodeficiency virus (HIV) infection, hemodialysis patients, and neonates [59]. Furthermore, hospital settings, prolonged intensive care unit stays, mechanical ventilation, tracheostomies, central venous catheters, severe traumatic injuries, significant burns, mucositis or mucosal barrier damaging factors, and the use of broad-spectrum antibiotic courses were shown to increase the risk of infection [60,61].
4.3. Risk Factors Associated with MDRO
As highlighted before, three main factors seem to be associated with MDRO-related pulmonary infection. Firstly, previous antibiotic therapy is one of the major risk factors as it is the source of selecting/inducing MDRO, and it paves the field of acquisition of resistant bacteria from the environment. Thus, before considering a species or a particular resistance mechanism, it is essential to trace the history of specific antibiotic exposure that can help the practitioner assess the risk of dealing with a specific species or resistance mechanism (Figure 2). For instance, carbapenem exposure and exposure to β-lactams inactive against Pseudomonas aeruginosa have been strongly correlated to the emergence of Carbapenem-Resistant Pseudomonas aeruginosa isolation, due to the repression or inactivation of the OprD gene encoding porin OprD2 [62,63]. As for species, Stenotrophomas maltophilia-related pneumonia has been found to be associated with previous exposure to Meropenem [64,65] and Enterococcus with previous exposure to third-generation cephalosporins [66].
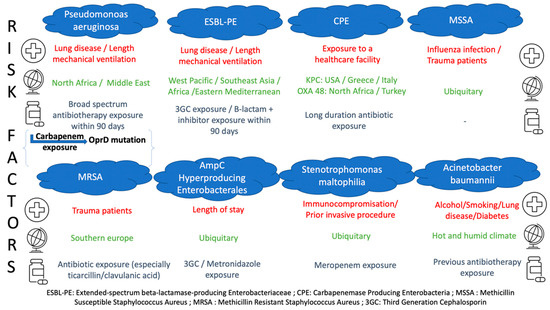
Figure 2.
Risk factors for MDRO-related infections.
Secondly, the length of hospitalization seems to be an important risk factor as it corresponds to a duration of exposure to a particular (bacterial) environment, responsible for the modification of the patients’ microbiota [67]. Indeed, several authors suggested [68,69] that duration of hospitalization and antibiotic therapy were the two main factors associated with MDRO-related pneumonia.
Thirdly, prior colonization with MDROs seems to be an indispensable prerequisite for the occurrence of MDRO infection [37,70,71]. In light of the spread of MDRO and the increase in the number of colonized patients [35], it seems more and more difficult to interpret the weight of MDRO colonization as a risk factor for MDRO infection. Several authors suggested that rare are the patients infected with MDRO among carriers [72,73]. In a prospective study among carriers, only 6% developed ICU-acquired pneumonia related to ESBL producing Enterobacterales.
A large study conducted in a French ICU suggested that the first infection episode rates in EBSL-PE carriers vary from 10% to 42% [70,74,75,76]. Whereas, the rate of the second episode rises from 10% to 30% [73]. Klebsiella pneumoniae carriage has also been found to carry a specific risk of colonization/infection transition in a surgical population of liver transplanted patients [77]. As for antibiotic use, a carbapenem exposure within the preceding three days has been reported to have a protective effect on ESBL PE VAP in one study [78]. Finally, a retrospective cohort study of more than 500 ICU patients with suspected VAP analyzed sensitivity and specificity of prior ESBL-PE colonization as a predictor of ESBL-PE-VAP and found, respectively, 85.0% and 95.7%. The positive and negative predictive values were 41.5% and 99.4%, respectively, with a positive likelihood ratio of 19.8. Moreover, no data support an impact of ESBL carriage on mortality which is a supplementary argument in favor of a “wait and see” strategy [79].
It, therefore, seems necessary to be able to identify among patients carrying multidrug-resistant bacteria those at risk of infection. Studies that occurred outside the ICU [80] and in the ICU [81,82] have suggested that relative abundance was the main risk factor associated with secondary bacteremia and VAP. Besides the risk due to the significant biomass of multidrug-resistant bacteria, it seems that colonization with non-E. coli species was associated with a higher risk of secondary infection [82]. Indeed, it has been shown that a high relative fecal abundance of ESBL-producing Enterobacterales is associated with a higher risk of ESBL-producing Enterobacterales associated VAP [81]. One study showed that in ICU patients colonized with ESBL-producing Enterobacterales, the onset of ESBL-producing Enterobacterales throat carriage preceded the occurrence of ESBL-producing Enterobacterales associated VAP [82].
4.4. Extended-Spectrum Beta-Lactamase-Producing Enterobacterales (ESBL-PE)
A recent meta-analysis found an overall prevalence of ESBL-producing Enterobacterales in a community of 14% among healthy individuals with an increasing annual rate of approximately 5%. The most impacted locations were in the West Pacific, Southeast Asia, Africa, and the eastern Mediterranean [83]. In Europe, Italy has a particularly high rate of ESBL, with 26% of Escherichia coli displaying resistance to the third-generation cephalosporins in 2013 [84]. Within a country, the prevalence can be very different from one region to another; in some locations, we observed the endemic situation in the community [35], whereas others were scarcely affected [35]. A 2012 French prospective study in a medical ICU showed a 15% ESBL-producing Enterobacterales carriage rate, mostly of Escherichia coli (62%). Transfer from another ICU, previous hospital admission in another country, surgery within the past year, prior neurologic disease, and prior administration of third-generation cephalosporin (within 3–12 months before ICU admission) have been found to be risk factors of ESBL-producing Enterobacterales carriage at ICU admission. Furthermore, advanced age, male gender, colonization pressure (defined as the sum of the daily proportion of patients in the unit colonized with ESBL-PE during the days preceding acquisition or ICU discharge), 3GC within the past three months, and B-lactam + inhibitor within three months were associated with the ESBL-PE-acquired carriage in the ICU in the same study [73].
4.5. AmpC Hyperproducing Enterobacterales (AHE)
In a retrospective study of more than a thousand ICU patients, the prevalence of intestinal colonization with AHE evolved from 2% at admission to 30% in patients with lengths of stay (LOS) exceeding four weeks. Metronidazole, cephalosporin use, and the LOS were found to be independently associated with acquired carriage in ICU patients [85]. It has been known for over 50 years that commensal anaerobes confer protection against exogenous pathogens, which may explain why metronidazole, by its impact on colonization resistance, favors the emergence of such mutants from subdominant, wild-type Enterobacterales populations. Therefore, AHE community prevalence could be considered insignificant, whereas its emergence in the ICU should not. Currently, information on the digestive carriage of AHE is not systematically provided to the clinician and differs from one center to another, even though studies have shown the value of this information for initial therapeutic adequacy in the case of sepsis [86].
4.6. Carbapenemase-Producing Enterobacterales (CPE)
The distribution of the different types of CPE is very heterogeneous on a global scale [87]. In communities, Klebsiella-producing Carbapenemase (KPC) is widespread in the United States and endemic in some European countries, such as Greece and Italy [88]. Among the metallo-β-lactamases (MBL), New Delhi metallo-β-lactamase (NDM), Verona integron-encoded metallo-β-lactamase (VIM), and imipenemase metallo-β-lactamase (IMP) enzymes are the most frequently identified worldwide [89]. IMP producing Gram-negative bacteria are mainly located in eastern Asia and Australia, mostly in Acinetobacter baumannii. VIM producers are most often found in Italy and Greece (Enterobacterales) and in Russia (Pseudomonas aeruginosa) [90,91]. OXA-48–producing Enterobacterales are endemic in Turkey and are frequently encountered in several European countries and across North Africa [92]. Reported risk factors for community carriage of CPE are, as expected, geographical location and recent antibiotic use. In the ICU, the prevalence of CPE varies from 6% to 37%, depending on the unit location [93,94]. A recent five-year case control study found the length of hospital admission >20 days, hospital admission within the previous year, exposure to a healthcare facility in a country with high carbapenem-resistant Enterobacterales prevalence 3 months before admission, and the use of antibiotics longer than 10 days to be independent predictors of CPE carriage.
4.7. Methicillin-Resistant Staphylococcus aureus (MRSA)
Several risk factors of MRSA acquisition during a hospital stay have been described as LOS, presence of patients colonized with MRSA in the same ICU at the same time, previous antibiotic use (especially ticarcillin/clavulanic acid), central venous catheter insertion, and period of nurse understaffing [95,96]. Interestingly, the specific population of trauma patients have been found to be particularly at risk of MRSA acquisition. In this very population, road traffic accident victims were at greater risk of acquiring MRSA than patients who had suffered other mechanisms of injury, probably because of more skin defects, such as open versus closed fractures, or more surgical procedures [97].
5. When to Use Broad-Spectrum Antibiotics, What Tools to Guide Us?
Colonization of the upper respiratory tract is a precondition to VAP in almost all patients [98]. However, prior colonization is not systematically responsible for the infection. Carriage should be interpreted solely as a risk factor as it could not be responsible for an infection on its own [99,100]. It is important to emphasize this point, considering the fact that knowledge of colonization misleads physicians in an overprescribing path [101].
5.1. Moving from an Empirical to Oriented Antimicrobial Choices
The conventional microbiological approach for HAP-VAP diagnosis consisted of cultures coupled with antimicrobial susceptibility testing, requiring approximately 48 h to 72 h from sampling to results delivery to physicians. It is important to notice that the implementation of MALDI-TOF MS in microbiology laboratories has already shown an impact on HAP-VAP management [102].
New strategies need to be implemented to reduce the pathogen identification time and MDRO-genes because of frequently unappropriated empirical therapy. Molecular techniques, such as syndromic m-PCR panels, have introduced a considerable change in antibiomicrobial stewardship intervention, accelerating targeted therapy in different conditions such as HAP-VAP.
Among these, the BioFire® FilmArray® Pneumonia Panel (FA-PN) (bioMérieux SA, Marcy-l’Étoile, France) is the widely used one. It is a Food and Drug Administration (FDA) syndromic m-PCR that simultaneously identifies 33 targets: 15 typical and 3 atypical bacterial pathogens, 8 respiratory viruses and 7 resistance genes in BAL/mini-BAL, tracheal aspirates (ETA), and sputum specimens.
Two recent multicentric studies on performance evaluation demonstrated excellent positive percentage agreement and negative percentage agreement values when compared with conventional culture methods [103,104]. In all these studies, it is important that the prevalence of bacteria off-panel is non-negligible and should be kept in mind by physicians and laboratory staff.
Many prospective and retrospective studies, so-called “real-life studies”, have been published to evaluate the clinical impact of this approach. Caméléna et al. showed a considerably reduced sample-to-result time compared to conventional approach (5.5 h vs. 25.9 h for cultures (p < 0.001) and 57 h for AST (p < 0.001), respectively) [105]. During the COVID-19 pandemic, Maataaoui et al. revealed in a prospective cohort of 112 episodes (104 HAP-VAP) an early empirical therapeutic change in 34% of HAP-VAP episodes (of which 46.3% were withdrawn) when this panel was performed [106]. Another recent prospective study showed among COVID-19 ICU patients that antibiotics were initiated in 87 (72.5%) of 120 pneumonia episodes and were not administered in 80 (87.0%) of 92 non-pneumonia episodes based on FA results [107].
Because of the high cost of this approach, some studies suggested scores to rationalize performing such a test. A comprehensive study suggested that both clinical (temperature and Clinical Pulmonary Infection Score) and biological parameters (WBC BAL count and % of PMNs) were correlated with FA-PN with or without conventional culture results [108]. In some cases, interpretation of results remains a challenge for physicians and laboratory staff. Based on a retrospective non-interventional study, Novy et al. designed an algorithm helping antimicrobial stewardship prescription with FA-PN results in cases of HAP-VAP suspicion and confirmed the poor reliability of ETA samples because of over-detection of the microbial and viral genome [109].
Other panels exist, such as the syndromic m-PCR panel for HAP-VAP developed by Curetis (Curetis GmbH, Holzgerlingen, Germany) with The Unyvero P55 Pneumonia panel, capable of identifying 20 pathogens of lower respiratory tract infections (LRTI) and 19 resistance genes. With a longer turn-around time of 5 h, performances seemed to be lower than previously described panels. However, a non-interventional study recently showed that this test could have led to modifications of empirical therapy in 60% (57/95) of HAP-VAP episodes [110]. “In-house” multiplex PCRs have been customized by several laboratories, such as the custom-designed multi-pathogen TaqMan Array Cards (TAC; Thermo Fisher Scientific, Waltham, MA, USA) in a UK center with good analytical performance [111].
This kind of approach has the advantage of identifying multiple pathogens with a shorter turn-around time, including those which are fastidious and pathogens that cannot be retrieved by conventional cultures. This remains particularly true when antimicrobials have already been started before sampling.
However, the reliance on the presence of resistance genes should be interpreted with caution. Importantly, these methods can only detect antibiotic resistance genes which have been chosen by industrials, and the limits of these assays need to be well known by physicians and microbiology labs. Conventional culture methods shall be continued because of the significant prevalence of uncovered pathogens.
5.2. How to Choose the Empirical Antibiotic: “Because It Was Him, Because It Was Me”
The ICU carries multiple specificities making the choice of empiric antimicrobial therapy a singular decision for each patient. Indeed, multiple parameters must be considered for critically ill patients, including the severity of illness, the seriousness of the situation, the certainty of the diagnosis, the local microbial ecology, and MDR prevalence in the unit. However, once these parameters are settled, the first legitimate question would be: does the treatment have to be empirical?
5.3. Rusher or Dragger?
Delayed initiation of antibiotic therapy has often been cited as a major risk factor for excess mortality, supporting the idea that “a large antimicrobial spectrum” should be provided to ICU patients. As the global rise of MDR incidence has become more widely known among practitioners, a “structural” tension has arisen between the need not to delay antibiotic therapy and the need to choose the right one. The idea that all ICU patients should be started on antibiotics as soon as possible implies that all patients admitted in those units have the same level of severity which is obviously inaccurate [112]. Studies have shown that this increased risk of mortality due to delayed initiation of antibiotic therapy was effective, especially for the most severe patients [113]. It would, therefore, seem appropriate, when the patient’s condition allows it, to wait for the germ identification and antibiogram.
5.4. Under Pressure
Local epidemiological knowledge is crucial. As seen previously, there is a heterogeneity in the distribution of MDRs, which suggests different considerations when choosing antibiotics, depending on the unit location [114]. If MDR carriage does not mandate any antibiotic therapy, it is well documented that it is a necessary step prior to infection [115]. Hence, each practitioner should be aware of the bacterial epidemiology of the hospital and unit in which they work [33].
Colonization pressure described in 1994 by Bonten et al. [116] is a fundamental concept that needs to be addressed in order to choose an adequate antibiotic. A study led by Trouillet et al. in 1998 was the first trial to link the changing bacterial epidemiology according to mechanical ventilation (MV) duration and previous antibiotic therapy. Firstly, it showed that patients who were not exposed to antibiotics and who underwent MV for less than seven days (in other terms, patients who had very low colonization pressure) were infected with the “usual” germs present in oropharyngeal and respiratory microbiota (Streptococcus, Haemophilus influenzae, Enterobacterales). On the other hand, when they underwent MV for more than seven days and had greater antibiotic exposure, non-fermenting gram-negative bacilli (PA, Stenotrophomonas, Acinetobacter) were more frequent. Multiple lessons could be drawn from this study with a remarkable reproducibility in the following years. It showed the importance of colonization pressure in VAP bacterial epidemiology and raised awareness about the fundamtental importance of selection pressure, which has been later confirmed in other studies [117]. MDR prevalence and antibiotic usage have risen in the 20 years since this historical study. If the “five days after ICU admission cut off” is since then usually used to materialize the risk of resistance [111,118], shifting the clinical and epidemiological reasoning in time could be an appropriate current adaptation. Indeed, according to geographical locations and hospital epidemiological situations, the patient admitted to the ICU or already in the ICU and undergoing MV could have experienced colonization and selection pressure for several days before. Thus, considering this pressure from the first contact with the healthcare facility (Emergency Room, medical ward, ICU), the question “what antibiotic should I use for this VAP?” as a continuum might be more relevant.
In conclusion, the reasoned choice of antibiotics to treat HAP/VAP requires the consideration of many variables ranging from local epidemiological data to the patient’s personal history, including prior antibiotic therapy and length of stay (Figure 3). The new microbiological diagnostic methods make it possible to move from an empirical prescription to an oriented prescription, reducing the delay for adequate antibiotic therapy.
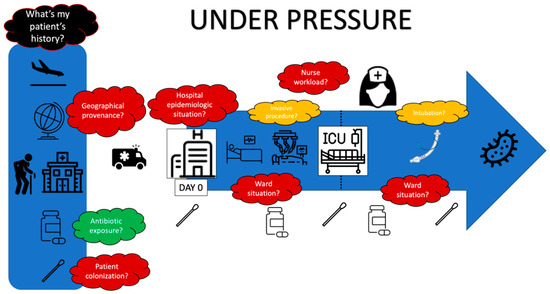
Figure 3.
Under pressure.
Author Contributions
Conceptualization: K.C., G.P.d.P., J.-R.Z., B.P.; Writing original draft: K.C., G.P.d.P., B.P.; Writing review and editing: K.C., G.P.d.P., L.D., J.-R.Z., B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef]
- Rello, J.; Vidaur, L.; Sandiumenge, A.; Rodríguez, A.; Gualis, B.; Boque, C.; Diaz, E. De-escalation therapy in ventilator-associated pneumonia. Crit. Care Med. 2004, 32, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Hayakawa, K.; Silverman, E.; Haider, S.; Alluri, K.C.; Datla, S.; Diviti, S.; Kuchipudi, V.; Muppavarapu, K.S.; Lephart, P.R.; et al. Risk factors for colonization due to carbapenem-resistant Enterobacteriaceae among patients exposed to long-term acute care and acute care facilities. Infect. Control Hosp. Epidemiol. 2014, 35, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.-S.; Chang, Y.-C.; Lin, W.-C.; Lee, W.-S.; Hsueh, P.-R.; Hsu, C.-W. Epidemiology, Treatment, and Prevention of Nosocomial Bacterial Pneumonia. J. Clin. Med. 2020, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Schwaber, M.J.; Carmeli, Y. Carbapenem-resistant Enterobacteriaceae: A potential threat. JAMA 2008, 300, 2911–2913. [Google Scholar] [CrossRef]
- Kelly, A.M.; Mathema, B.; Larson, E.L. Carbapenem-resistant Enterobacteriaceae in the community: A scoping review. Int. J. Antimicrob. Agents 2017, 50, 127–134. [Google Scholar] [CrossRef]
- Pitout, J.D.D. Enterobacteriaceae that produce extended-spectrum β-lactamases and AmpC β-lactamases in the community: The tip of the iceberg? Curr. Pharm. Des. 2013, 19, 257–263. [Google Scholar] [CrossRef]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community. Infect. Dis. Clin. North Am. 2020, 34, 709–722. [Google Scholar] [CrossRef]
- Ladner, J.T.; Grubaugh, N.D.; Pybus, O.G.; Andersen, K.G. Precision epidemiology for infectious disease control. Nat. Med. 2019, 25, 206–211. [Google Scholar] [CrossRef]
- American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [CrossRef]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.E.; Jones, M.M.; Huttner, B.; Stoddard, G.; Brown, K.A.; Stevens, V.W.; Greene, T.; Sauer, B.; Madaras-Kelly, K.; Rubin, M.; et al. Trends in Antibiotic Use and Nosocomial Pathogens in Hospitalized Veterans With Pneumonia at 128 Medical Centers, 2006-2010. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Rother, C.; Salih, W.; Ewig, S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: A systematic review and meta-analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Shorr, A.; Tabak, Y.P.; Gupta, V.; Liu, L.Z.; Johannes, R.S. Epidemiology and outcomes of health-care-associated pneumonia: Results from a large US database of culture-positive pneumonia. Chest 2005, 128, 3854–3862. [Google Scholar] [CrossRef]
- Shindo, Y.; Ito, R.; Kobayashi, D.; Ando, M.; Ichikawa, M.; Shiraki, A.; Goto, Y.; Fukui, Y.; Iwaki, M.; Okumura, J.; et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2013, 188, 985–995. [Google Scholar] [CrossRef]
- Schweitzer, V.A.; van Werkhoven, C.H.; van Heijl, I.; Smits, R.F.; Boel, C.H.E.; Bonten, M.J.M.; Postma, D.F.; Oosterheert, J.J. Relevance of healthcare-associated pneumonia for empirical antibiotic therapy in the Netherlands. Neth. J. Med. 2018, 76, 389–396. [Google Scholar] [PubMed]
- Garcia-Vidal, C.; Viasus, D.; Roset, A.; Adamuz, J.; Verdaguer, R.; Dorca, J.; Gudiol, F.; Carratalà, J. Low incidence of multidrug-resistant organisms in patients with healthcare-associated pneumonia requiring hospitalization. Clin. Microbiol. Infect. 2011, 17, 1659–1665. [Google Scholar] [CrossRef]
- Healthcare-associated infections in intensive care units—Annual Epidemiological Report for 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-intensive-care-units-annual-epidemiological-1 (accessed on 10 September 2021).
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-associated pneumonia in adults: A narrative review. Intensiv. Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Di Pasquale, M.; Ferrer, M.; Esperatti, M.; Crisafulli, E.; Giunta, V.; Li Bassi, G.; Rinaudo, M.; Blasi, F.; Niederman, M.; Torres, A. Assessment of severity of ICU-acquired pneumonia and association with etiology. Crit. Care Med. 2014, 42, 303–312. [Google Scholar] [CrossRef]
- Arvanitis, M.; Anagnostou, T.; Kourkoumpetis, T.K.; Ziakas, P.D.; Desalermos, A.; Mylonakis, E. The Impact of Antimicrobial Resistance and Aging in VAP Outcomes: Experience from a Large Tertiary Care Center. PLoS ONE 2014, 9, e89984. [Google Scholar] [CrossRef]
- Blot, S.; Koulenti, D.; Dimopoulos, G.; Martin, C.; Komnos, A.; Krueger, W.A.; Spina, G.; Armaganidis, A.; Rello, J. EU-VAP Study Investigators Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients*. Crit. Care Med. 2014, 42, 601–609. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Torres, A.; Rinaudo, M.; Terraneo, S.; de Rosa, F.; Ramirez, P.; Diaz, E.; Fernández-Barat, L.; Li Bassi, G.L.; Ferrer, M. Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J. Infect. 2015, 70, 213–222. [Google Scholar] [CrossRef]
- Franchineau, G.; Luyt, C.E.; Combes, A.; Schmidt, M. Ventilator-associated pneumonia in extracorporeal membrane oxygenation-assisted patients. Ann. Transl. Med. 2018, 6, 427. [Google Scholar] [CrossRef]
- Muscedere, J.G.; Day, A.; Heyland, D.K. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin. Infect. Dis. 2010, 51 (Suppl. S1), S120–S125. [Google Scholar] [CrossRef]
- Kollef, M.H.; Hamilton, C.W.; Ernst, F.R. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect. Control Hosp. Epidemiol. 2012, 33, 250–256. [Google Scholar] [CrossRef]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.H.; Bergmans, D.C.J.J.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Jones, R.N. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin. Infect. Dis. 2010, 51 (Suppl. S1), S81–S87. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S. National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E.; Samsa, G.P.; Brown, V.; Niederman, M.S. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect. Control Hosp. Epidemiol. 2007, 28, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Derde, L.P.G.; Cooper, B.S.; Goossens, H.; Malhotra-Kumar, S.; Willems, R.J.L.; Gniadkowski, M.; Hryniewicz, W.; Empel, J.; Dautzenberg, M.J.D.; Annane, D.; et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: An interrupted time series study and cluster randomised trial. Lancet Infect. Dis. 2014, 14, 31–39. [Google Scholar] [CrossRef]
- Pilmis, B.; Cattoir, V.; Lecointe, D.; Limelette, A.; Grall, I.; Mizrahi, A.; Marcade, G.; Poilane, I.; Guillard, T.; Bourgeois Nicolaos, N.; et al. Carriage of ESBL-producing Enterobacteriaceae in French hospitals: The PORTABLSE study. J. Hosp. Infect. 2018, 98, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Zahar, J.-R.; Blot, S.; Nordmann, P.; Martischang, R.; Timsit, J.-F.; Harbarth, S.; Barbier, F. Screening for Intestinal Carriage of Extended-spectrum Beta-lactamase-producing Enterobacteriaceae in Critically Ill Patients: Expected Benefits and Evidence-based Controversies. Clin. Infect. Dis. 2019, 68, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.L.; Vos, M.C.; Ott, A.; van Belkum, A.; Voss, A.; Kluytmans, J.A.J.W.; van Keulen, P.H.J.; Vandenbroucke-Grauls, C.M.J.E.; Meester, M.H.M.; Verbrugh, H.A. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet Lond. Engl. 2004, 364, 703–705. [Google Scholar] [CrossRef]
- Wertheim, H.F.L.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Wertheim, H.F.L.; Verbrugh, H.A. Global prevalence of meticillin-resistant Staphylococcus aureus. Lancet Lond. Engl. 2006, 368, 1866. [Google Scholar] [CrossRef]
- Paling, F.P.; Wolkewitz, M.; Bode, L.G.M.; Klein Klouwenberg, P.M.C.; Ong, D.S.Y.; Depuydt, P.; de Bus, L.; Sifakis, F.; Bonten, M.J.M.; Kluytmans, J.A.J.W. Staphylococcus aureus colonization at ICU admission as a risk factor for developing S. aureus ICU pneumonia. Clin. Microbiol. Infect. 2017, 23, e9–e49. [Google Scholar] [CrossRef]
- Launey, Y.; Asehnoune, K.; Lasocki, S.; Dahyot-Fizelier, C.; Huet, O.; Le Pabic, E.; Malejac, B.; Seguin, P. AtlanRéa Group Risk factors for ventilator-associated pneumonia due to Staphylococcus aureus in patients with severe brain injury: A multicentre retrospective cohort study. Anaesth. Crit. Care Pain Med. 2021, 40, 100785. [Google Scholar] [CrossRef]
- Tilouche, L.; Ben Dhia, R.; Boughattas, S.; Ketata, S.; Bouallegue, O.; Chaouch, C.; Boujaafar, N. Staphylococcus aureus Ventilator-Associated Pneumonia: A Study of Bacterio-Epidemiological Profile and Virulence Factors. Curr. Microbiol. 2021, 78, 2556–2562. [Google Scholar] [CrossRef]
- Zahar, J.-R.; Lesprit, P.; Ruckly, S.; Eden, A.; Hikombo, H.; Bernard, L.; Harbarth, S.; Timsit, J.-F.; Brun-Buisson, C. BacterCom Study Group Predominance of healthcare-associated cases among episodes of community-onset bacteraemia due to extended-spectrum β-lactamase-producing Enterobacteriaceae. Int. J. Antimicrob. Agents 2017, 49, 67–73. [Google Scholar] [CrossRef]
- Cillóniz, C.; Gabarrús, A.; Ferrer, M.; Puig de la Bellacasa, J.; Rinaudo, M.; Mensa, J.; Niederman, M.S.; Torres, A. Community-Acquired Pneumonia Due to Multidrug- and Non-Multidrug-Resistant Pseudomonas aeruginosa. Chest 2016, 150, 415–425. [Google Scholar] [CrossRef]
- Venier, A.G.; Gruson, D.; Lavigne, T.; Jarno, P.; L’hériteau, F.; Coignard, B.; Savey, A.; Rogues, A.M. REA-RAISIN group Identifying new risk factors for Pseudomonas aeruginosa pneumonia in intensive care units: Experience of the French national surveillance, REA-RAISIN. J. Hosp. Infect. 2011, 79, 44–48. [Google Scholar] [CrossRef]
- Paling, F.P.; Wolkewitz, M.; Depuydt, P.; de Bus, L.; Sifakis, F.; Bonten, M.J.M.; Kluytmans, J.A.J.W. P. aeruginosa colonization at ICU admission as a risk factor for developing P. aeruginosa ICU pneumonia. Antimicrob. Resist. Infect. Control 2017, 6, 38. [Google Scholar] [CrossRef]
- Harris, A.D.; Jackson, S.S.; Robinson, G.; Pineles, L.; Leekha, S.; Thom, K.A.; Wang, Y.; Doll, M.; Pettigrew, M.M.; Johnson, J.K. Pseudomonas aeruginosa Colonization in the Intensive Care Unit: Prevalence, Risk Factors, and Clinical Outcomes. Infect. Control Hosp. Epidemiol. 2016, 37, 544–548. [Google Scholar] [CrossRef]
- Craven, D.E.; Steger, K.A. Nosocomial pneumonia in mechanically ventilated adult patients: Epidemiology and prevention in 1996. Semin. Respir. Infect. 1996, 11, 32–53. [Google Scholar]
- Spellberg, B.; Bonomo, R.A. Combination Therapy for Extreme Drug Resistant (XDR) Acinetobacter baumannii: Ready for Prime-Time? Crit. Care Med. 2015, 43, 1332–1334. [Google Scholar] [CrossRef]
- Sarshar, M.; Behzadi, P.; Scribano, D.; Palamara, A.T.; Ambrosi, C. Acinetobacter baumannii: An Ancient Commensal with Weapons of a Pathogen. Pathogens 2021, 10, 387. [Google Scholar] [CrossRef]
- Zeighami, H.; Valadkhani, F.; Shapouri, R.; Samadi, E.; Haghi, F. Virulence characteristics of multidrug resistant biofilm forming Acinetobacter baumannii isolated from intensive care unit patients. BMC Infect. Dis. 2019, 19, 629. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; van Aken, E.; Shunburne, L.; van der Reijden, T.J.K.; Bernards, A.T.; Nemec, A.; Towner, K.J. Prevalence of Acinetobacter baumannii and other Acinetobacter spp. in faecal samples from non-hospitalised individuals. Clin. Microbiol. Infect. 2005, 11, 329–332. [Google Scholar] [CrossRef]
- Chatellier, D.; Burucoa, C.; Pinsard, M.; Frat, J.-P.; Robert, R. Prevalence of Acinetobacter baumannii carriage in patients of 53 French intensive care units on a given day. Med. Mal. Infect. 2007, 37, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Z.; Hsueh, P.R.; Lee, L.N.; Yu, C.J.; Yang, P.C.; Luh, K.T. Severe community-acquired pneumonia due to Acinetobacter baumannii. Chest 2001, 120, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Dexter, C.; Murray, G.L.; Paulsen, I.T.; Peleg, A.Y. Community-acquired Acinetobacter baumannii: Clinical characteristics, epidemiology and pathogenesis. Expert Rev. Anti Infect. Ther. 2015, 13, 567–573. [Google Scholar] [CrossRef]
- García-Garmendia, J.-L.; Ortiz-Leyba, C.; Garnacho-Montero, J.; Jiménez-Jiménez, F.-J.; Pérez-Paredes, C.; Barrero-Almodóvar, A.E.; Miner, M.G. Risk Factors for Acinetobacter baumannii Nosocomial Bacteremia in Critically Ill Patients: A Cohort Study. Clin. Infect. Dis. 2001, 33, 939–946. [Google Scholar] [CrossRef]
- Lee, M.-R.; Wang, H.-C.; Yang, C.-Y.; Lin, C.-K.; Kuo, H.-Y.; Ko, J.-C.; Sheng, W.-H.; Lee, L.-N.; Yu, C.-J.; Hsueh, P.-R. Clinical characteristics and outcomes of patients with pleural infections due to Stenotrophomonas maltophilia at a medical center in Taiwan, 2004–2012. Eur. J. Clin. Microbiol. 2014, 33, 1143–1148. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.C.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Choi, J.Y.; Yeom, J.-S.; Song, Y.G. Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia and clinical impact of quinolone-resistant strains. BMC Infect. Dis. 2019, 19, 754. [Google Scholar] [CrossRef]
- Ibn Saied, W.; Merceron, S.; Schwebel, C.; Le Monnier, A.; Oziel, J.; Garrouste-Orgeas, M.; Marcotte, G.; Ruckly, S.; Souweine, B.; Darmon, M.; et al. Ventilator-associated pneumonia due to Stenotrophomonas maltophilia: Risk factors and outcome. J. Infect. 2020, 80, 279–285. [Google Scholar] [CrossRef]
- Guerci, P.; Bellut, H.; Mokhtari, M.; Gaudefroy, J.; Mongardon, N.; Charpentier, C.; Louis, G.; Tashk, P.; Dubost, C.; Ledochowski, S.; et al. Outcomes of Stenotrophomonas maltophilia hospital-acquired pneumonia in intensive care unit: A nationwide retrospective study. Crit. Care Lond. Engl. 2019, 23, 371. [Google Scholar] [CrossRef]
- Jeon, Y.D.; Jeong, W.Y.; Kim, M.H.; Jung, I.Y.; Ahn, M.Y.; Ann, H.W.; Ahn, J.Y.; Han, S.H.; Choi, J.Y.; Song, Y.G.; et al. Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia. Medicine 2016, 95, e4375. [Google Scholar] [CrossRef]
- Coppry, M.; Jeanne-Leroyer, C.; Noize, P.; Dumartin, C.; Boyer, A.; Bertrand, X.; Dubois, V.; Rogues, A.-M. Antibiotics associated with acquisition of carbapenem-resistant Pseudomonas aeruginosa in ICUs: A multicentre nested case-case-control study. J. Antimicrob. Chemother. 2019, 74, 503–510. [Google Scholar] [CrossRef]
- Paramythiotou, E.; Lucet, J.-C.; Timsit, J.-F.; Vanjak, D.; Paugam-Burtz, C.; Trouillet, J.-L.; Belloc, S.; Kassis, N.; Karabinis, A.; Andremont, A. Acquisition of multidrug-resistant Pseudomonas aeruginosa in patients in intensive care units: Role of antibiotics with antipseudomonal activity. Clin. Infect. Dis. 2004, 38, 670–677. [Google Scholar] [CrossRef]
- Dewart, C.M.; Hebert, C.; Pancholi, P.; Stevenson, K. 482. Time Series Analysis of Antimicrobial Consumption and Pseudomonas aeruginosa Resistance in an Academic Medical Center in the United States (2013–2018). Open Forum Infect. Dis. 2019, 6, S236. [Google Scholar] [CrossRef]
- Hotta, G.; Matsumura, Y.; Kato, K.; Nakano, S.; Yunoki, T.; Yamamoto, M.; Nagao, M.; Ito, Y.; Takakura, S.; Ichiyama, S. Risk factors and outcomes of Stenotrophomonas maltophilia bacteraemia: A comparison with bacteraemia caused by Pseudomonas aeruginosa and Acinetobacter species. PLoS ONE 2014, 9, e112208. [Google Scholar] [CrossRef]
- Pallares, R.; Pujol, M.; Peña, C.; Ariza, J.; Martin, R.; Gudiol, F. Cephalosporins as risk factor for nosocomial Enterococcus faecalis bacteremia. A matched case-control study. Arch. Intern. Med. 1993, 153, 1581–1586. [Google Scholar] [CrossRef]
- Planquette, B.; Timsit, J.-F.; Misset, B.Y.; Schwebel, C.; Azoulay, E.; Adrie, C.; Vesin, A.; Jamali, S.; Zahar, J.-R.; Allaouchiche, B.; et al. Pseudomonas aeruginosa ventilator-associated pneumonia. predictive factors of treatment failure. Am. J. Respir. Crit. Care Med. 2013, 188, 69–76. [Google Scholar] [CrossRef]
- Pettigrew, M.M.; Gent, J.F.; Kong, Y.; Halpin, A.L.; Pineles, L.; Harris, A.D.; Johnson, J.K. Gastrointestinal Microbiota Disruption and Risk of Colonization With Carbapenem-resistant Pseudomonas aeruginosa in Intensive Care Unit Patients. Clin. Infect. Dis. 2019, 69, 604–613. [Google Scholar] [CrossRef]
- Ravi, A.; Halstead, F.D.; Bamford, A.; Casey, A.; Thomson, N.M.; van Schaik, W.; Snelson, C.; Goulden, R.; Foster-Nyarko, E.; Savva, G.M.; et al. Loss of microbial diversity and pathogen domination of the gut microbiota in critically ill patients. Microb. Genomics 2019, 5. [Google Scholar] [CrossRef]
- Bruyère, R.; Vigneron, C.; Bador, J.; Aho, S.; Toitot, A.; Quenot, J.-P.; Prin, S.; Emmanuel Charles, P. Significance of Prior Digestive Colonization With Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae in Patients With Ventilator-Associated Pneumonia. Crit. Care Med. 2016, 44, 699–706. [Google Scholar] [CrossRef]
- Dubinsky-Pertzov, B.; Temkin, E.; Harbarth, S.; Fankhauser-Rodriguez, C.; Carevic, B.; Radovanovic, I.; Ris, F.; Kariv, Y.; Buchs, N.C.; Schiffer, E.; et al. Carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae and the risk of surgical site infection after colorectal surgery: A prospective cohort study. Clin. Infect. Dis. 2018, 68, 1699–1704. [Google Scholar] [CrossRef]
- Razazi, K.; Mekontso Dessap, A.; Carteaux, G.; Jansen, C.; Decousser, J.-W.; de Prost, N.; Brun-Buisson, C. Frequency, associated factors and outcome of multi-drug-resistant intensive care unit-acquired pneumonia among patients colonized with extended-spectrum β-lactamase-producing Enterobacteriaceae. Ann. Intensiv. Care 2017, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Razazi, K.; Derde, L.P.G.; Verachten, M.; Legrand, P.; Lesprit, P.; Brun-Buisson, C. Clinical impact and risk factors for colonization with extended-spectrum β-lactamase-producing bacteria in the intensive care unit. Intensiv. Care Med. 2012, 38, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Pommier, C.; Essaied, W.; Garrouste-Orgeas, M.; Schwebel, C.; Ruckly, S.; Dumenil, A.-S.; Lemiale, V.; Mourvillier, B.; Clec’h, C.; et al. Colonization and infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in ICU patients: What impact on outcomes and carbapenem exposure? J. Antimicrob. Chemother. 2016, 71, 1088–1097. [Google Scholar] [CrossRef]
- Jalalzaï, W.; Boutrot, M.; Guinard, J.; Guigon, A.; Bret, L.; Poisson, D.-M.; Boulain, T.; Barbier, F. Cessation of screening for intestinal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in a low-endemicity intensive care unit with universal contact precautions. Clin. Microbiol. Infect. 2018, 24, 429.e7–429.e12. [Google Scholar] [CrossRef]
- Vodovar, D.; Marcadé, G.; Rousseau, H.; Raskine, L.; Vicaut, E.; Deye, N.; Baud, F.J.; Mégarbane, B. Predictive factors for extended-spectrum beta-lactamase producing Enterobacteriaceae causing infection among intensive care unit patients with prior colonization. Infection 2014, 42, 743–748. [Google Scholar] [CrossRef]
- Logre, E.; Bert, F.; Khoy-Ear, L.; Janny, S.; Giabicani, M.; Grigoresco, B.; Toussaint, A.; Dondero, F.; Dokmak, S.; Roux, O.; et al. Risk Factors and Impact of Perioperative Prophylaxis on the Risk of Extended-spectrum β-Lactamase-producing Enterobacteriaceae-related Infection Among Carriers Following Liver Transplantation. Transplantation 2021, 105, 338–345. [Google Scholar] [CrossRef]
- Barbier, F.; Bailly, S.; Schwebel, C.; Papazian, L.; Azoulay, É.; Kallel, H.; Siami, S.; Argaud, L.; Marcotte, G.; Misset, B.; et al. Infection-related ventilator-associated complications in ICU patients colonised with extended-spectrum β-lactamase-producing Enterobacteriaceae. Intensiv. Care Med. 2018, 44, 616–626. [Google Scholar] [CrossRef]
- Repessé, X.; Artiguenave, M.; Paktoris-Papine, S.; Espinasse, F.; Dinh, A.; Charron, C.; El Sayed, F.; Geri, G.; Vieillard-Baron, A. Epidemiology of extended-spectrum beta-lactamase-producing Enterobacteriaceae in an intensive care unit with no single rooms. Ann. Intensiv. Care 2017, 7, 73. [Google Scholar] [CrossRef]
- Ruppé, E.; Lixandru, B.; Cojocaru, R.; Büke, C.; Paramythiotou, E.; Angebault, C.; Visseaux, C.; Djuikoue, I.; Erdem, E.; Burduniuc, O.; et al. Relative fecal abundance of extended-spectrum-β-lactamase-producing Escherichia coli strains and their occurrence in urinary tract infections in women. Antimicrob. Agents Chemother. 2013, 57, 4512–4517. [Google Scholar] [CrossRef]
- Pilmis, B.; Mizrahi, A.; Péan de Ponfilly, G.; Philippart, F.; Bruel, C.; Zahar, J.-R.; Le Monnier, A. Relative faecal abundance of extended-spectrum β-lactamase-producing Enterobacterales and its impact on infections among intensive care unit patients: A pilot study. J. Hosp. Infect. 2021, 112, 92–95. [Google Scholar] [CrossRef]
- Andremont, O.; Armand-Lefevre, L.; Dupuis, C.; de Montmollin, E.; Ruckly, S.; Lucet, J.-C.; Smonig, R.; Magalhaes, E.; Ruppé, E.; Mourvillier, B.; et al. Semi-quantitative cultures of throat and rectal swabs are efficient tests to predict ESBL-Enterobacterales ventilator-associated pneumonia in mechanically ventilated ESBL carriers. Intensiv. Care Med. 2020, 46, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Karanika, S.; Karantanos, T.; Arvanitis, M.; Grigoras, C.; Mylonakis, E. Fecal Colonization With Extended-spectrum Beta-lactamase–Producing Enterobacteriaceae and Risk Factors Among Healthy Individuals: A Systematic Review and Metaanalysis. Clin. Infect. Dis. 2016, 63, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Mondain, V.; Secondo, G.; Guttmann, R.; Ferrea, G.; Dusi, A.; Giacomini, M.; Courjon, J.; Pradier, C. A toolkit for the management of infection or colonization by extended-spectrum beta-lactamase producing Enterobacteriaceae in Italy: Implementation and outcome of a European project. Eur. J. Clin. Microbiol. 2018, 37, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Poignant, S.; Guinard, J.; Guigon, A.; Bret, L.; Poisson, D.-M.; Boulain, T.; Barbier, F. Risk Factors and Outcomes for Intestinal Carriage of AmpC-Hyperproducing Enterobacteriaceae in Intensive Care Unit Patients. Antimicrob. Agents Chemother. 2015, 60, 1883–1887. [Google Scholar] [CrossRef]
- Manquat, E.; Le Dorze, M.; Pean De Ponfilly, G.; Benmansour, H.; Amarsy, R.; Cambau, E.; Soyer, B.; Chousterman, B.G.; Jacquier, H. Impact of systematic screening for AmpC-hyperproducing Enterobacterales intestinal carriage in intensive care unit patients. Ann. Intensiv. Care 2020, 10, 149. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. 2018, 66, 1290–1297. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Palzkill, T. Metallo-β-lactamase structure and function. Ann. N. Y. Acad. Sci. 2013, 1277, 91–104. [Google Scholar] [CrossRef]
- Edelstein, M.V.; Skleenova, E.N.; Shevchenko, O.V.; D’souza, J.W.; Tapalski, D.V.; Azizov, I.S.; Sukhorukova, M.V.; Pavlukov, R.A.; Kozlov, R.S.; Toleman, M.A.; et al. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: A longitudinal epidemiological and clinical study. Lancet Infect. Dis. 2013, 13, 867–876. [Google Scholar] [CrossRef]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-beta-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef]
- Potron, A.; Poirel, L.; Dortet, L.; Nordmann, P. Characterisation of OXA-244, a chromosomally-encoded OXA-48-like β-lactamase from Escherichia coli. Int. J. Antimicrob. Agents 2016, 47, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Soria-Segarra, C.; Soria-Segarra, C.; Catagua-González, A.; Gutiérrez-Fernández, J. Carbapenemase producing Enterobacteriaceae in intensive care units in Ecuador: Results from a multicenter study. J. Infect. Public Health 2020, 13, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Song, J.E.; Kim, M.H.; Choi, H.; Kim, J.K.; Ann, H.W.; Kim, J.H.; Jeon, Y.; Jeong, S.J.; Kim, S.B.; et al. Risk factors for the acquisition of carbapenem-resistant Escherichia coli at a tertiary care center in South Korea: A matched case-control study. Am. J. Infect. Control 2014, 42, 621–625. [Google Scholar] [CrossRef]
- Oztoprak, N.; Cevik, M.A.; Akinci, E.; Korkmaz, M.; Erbay, A.; Eren, S.S.; Balaban, N.; Bodur, H. Risk factors for ICU-acquired methicillin-resistant Staphylococcus aureus infections. Am. J. Infect. Control 2006, 34, 1–5. [Google Scholar] [CrossRef]
- Dancer, S.J.; Coyne, M.; Speekenbrink, A.; Samavedam, S.; Kennedy, J.; Wallace, P.G.M. MRSA acquisition in an intensive care unit. Am. J. Infect. Control 2006, 34, 10–17. [Google Scholar] [CrossRef]
- Marshall, C.; Wolfe, R.; Kossmann, T.; Wesselingh, S.; Harrington, G.; Spelman, D. Risk factors for acquisition of methicillin-resistant Staphylococcus aureus (MRSA) by trauma patients in the intensive care unit. J. Hosp. Infect. 2004, 57, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.J.; Bergmans, D.C.; Ambergen, A.W.; de Leeuw, P.W.; van der Geest, S.; Stobberingh, E.E.; Gaillard, C.A. Risk factors for pneumonia, and colonization of respiratory tract and stomach in mechanically ventilated ICU patients. Am. J. Respir. Crit. Care Med. 1996, 154, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Rottier, W.C.; Bamberg, Y.R.P.; Dorigo-Zetsma, J.W.; van der Linden, P.D.; Ammerlaan, H.S.M.; Bonten, M.J.M. Predictive value of prior colonization and antibiotic use for third-generation cephalosporin-resistant enterobacteriaceae bacteremia in patients with sepsis. Clin. Infect. Dis. 2015, 60, 1622–1630. [Google Scholar] [CrossRef]
- Hagel, S.; Makarewicz, O.; Hartung, A.; Weiß, D.; Stein, C.; Brandt, C.; Schumacher, U.; Ehricht, R.; Patchev, V.; Pletz, M.W. ESBL colonization and acquisition in a hospital population: The molecular epidemiology and transmission of resistance genes. PLoS ONE 2019, 14, e0208505. [Google Scholar] [CrossRef]
- Hayon, J.; Figliolini, C.; Combes, A.; Trouillet, J.-L.; Kassis, N.; Dombret, M.C.; Gibert, C.; Chastre, J. Role of serial routine microbiologic culture results in the initial management of ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 41–46. [Google Scholar] [CrossRef]
- Mok, J.H.; Eom, J.S.; Jo, E.J.; Kim, M.H.; Lee, K.; Kim, K.U.; Park, H.-K.; Yi, J.; Lee, M.K. Clinical utility of rapid pathogen identification using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in ventilated patients with pneumonia: A pilot study. Respirol. Carlton Vic 2016, 21, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gastli, N.; Loubinoux, J.; Daragon, M.; Lavigne, J.-P.; Saint-Sardos, P.; Pailhoriès, H.; Lemarié, C.; Benmansour, H.; d’Humières, C.; Broutin, L.; et al. Multicentric evaluation of BioFire FilmArray Pneumonia Panel for rapid bacteriological documentation of pneumonia. Clin. Microbiol. Infect. 2021, 27, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Ginocchio, C.C.; Garcia-Mondragon, C.; Mauerhofer, B.; Rindlisbacher, C.; The EME Evaluation Program Collaborative. Multinational evaluation of the BioFire® FilmArray® Pneumonia plus Panel as compared to standard of care testing. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Caméléna, F.; Moy, A.-C.; Dudoignon, E.; Poncin, T.; Deniau, B.; Guillemet, L.; Le Goff, J.; Budoo, M.; Benyamina, M.; Chaussard, M.; et al. Performance of a multiplex polymerase chain reaction panel for identifying bacterial pathogens causing pneumonia in critically ill patients with COVID-19. Diagn. Microbiol. Infect. Dis. 2021, 99, 115183. [Google Scholar] [CrossRef]
- Maataoui, N.; Chemali, L.; Patrier, J.; Tran Dinh, A.; Le Fèvre, L.; Lortat-Jacob, B.; Marzouk, M.; d’Humières, C.; Rondinaud, E.; Ruppé, E.; et al. Impact of rapid multiplex PCR on management of antibiotic therapy in COVID-19-positive patients hospitalized in intensive care unit. Eur. J. Clin. Microbiol. 2021, 40, 2227–2234. [Google Scholar] [CrossRef]
- Posteraro, B.; Cortazzo, V.; Liotti, F.M.; Menchinelli, G.; Ippoliti, C.; De Angelis, G.; La Sorda, M.; Capalbo, G.; Vargas, J.; Antonelli, M.; et al. Diagnosis and Treatment of Bacterial Pneumonia in Critically Ill Patients with COVID-19 Using a Multiplex PCR Assay: A Large Italian Hospital’s Five-Month Experience. Microbiol. Spectr. 2021, 9, e0069521. [Google Scholar] [CrossRef]
- Rand, K.H.; Beal, S.G.; Cherabuddi, K.; Houck, H.; Lessard, K.; Tremblay, E.E.; Couturier, B.; Lingenfelter, B.; Rindlisbacher, C.; Jones, J. Relationship of Multiplex Molecular Pneumonia Panel Results With Hospital Outcomes and Clinical Variables. Open Forum Infect. Dis. 2021, 8, ofab368. [Google Scholar] [CrossRef]
- Novy, E.; Goury, A.; Thivilier, C.; Guillard, T.; Alauzet, C. Algorithm for rational use of Film Array Pneumonia Panel in bacterial coinfections of critically ill ventilated COVID-19 patients. Diagn. Microbiol. Infect. Dis. 2021, 101, 115507. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Bouadma, L.; Mathy, V.; Allouche, K.; Patrier, J.; Reboul, M.; Montravers, P.; Timsit, J.-F.; Armand-Lefevre, L. Performance and impact of a multiplex PCR in ICU patients with ventilator-associated pneumonia or ventilated hospital-acquired pneumonia. Crit. Care Lond. Engl. 2020, 24, 366. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Correction to: Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care Lond. Engl. 2021, 25, 130. [Google Scholar] [CrossRef]
- Chang, D.W.; Dacosta, D.; Shapiro, M.F. Priority Levels in Medical Intensive Care at an Academic Public Hospital. JAMA Intern. Med. 2017, 177, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Hranjec, T.; Rosenberger, L.H.; Swenson, B.; Metzger, R.; Flohr, T.R.; Politano, A.D.; Riccio, L.M.; Popovsky, K.A.; Sawyer, R.G. Aggressive versus conservative initiation of antimicrobial treatment in critically ill surgical patients with suspected intensive-care-unit-acquired infection: A quasi-experimental, before and after observational cohort study. Lancet Infect. Dis. 2012, 12, 774–780. [Google Scholar] [CrossRef]
- Falcone, M.; Russo, A.; Gentiloni Silverj, F.; Marzorati, D.; Bagarolo, R.; Monti, M.; Velleca, R.; D’Angelo, R.; Frustaglia, A.; Zuccarelli, G.C.; et al. Predictors of mortality in nursing-home residents with pneumonia: A multicentre study. Clin. Microbiol. Infect. 2018, 24, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Arzilli, G.; Scardina, G.; Casigliani, V.; Moi, M.; Lucenteforte, E.; Petri, D.; Rello, J.; Manissero, D.; Lopalco, P.L.; Tavoschi, L. Risk of infection in antimicrobial-resistant Gram-negative bacteria carriers: A systematic review. Eur. J. Public Health 2020, 30, ckaa165.319. [Google Scholar] [CrossRef]
- Bonten, M.J.; Gaillard, C.A.; Johanson, W.G.; van Tiel, F.H.; Smeets, H.G.; van der Geest, S.; Stobberingh, E.E. Colonization in patients receiving and not receiving topical antimicrobial prophylaxis. Am. J. Respir. Crit. Care Med. 1994, 150, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.J.; Dascomb, K.; Stenehjem, E.; Vikram, H.R.; Agrwal, N.; Sakata, K.; Williams, K.; Bockorny, B.; Bagavathy, K.; Mirza, S.; et al. Derivation and Multicenter Validation of the Drug Resistance in Pneumonia Clinical Prediction Score. Antimicrob. Agents Chemother. 2016, 60, 2652–2663. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Peterson, J.; Fernandez, J.F.; Qin, Z.; Fisher, A.C.; Nicholson, S.C. Comparison of the bacterial etiology of early-onset and late-onset ventilator-associated pneumonia in subjects enrolled in 2 large clinical studies. Respir. Care 2013, 58, 1220–1225. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).