Cytotoxicity and Antibacterial Efficacy of AgCu and AgFe NanoAlloys: A Comparative Study
Abstract
1. Introduction
- The atomic radii of Fe and Cu are similar;
- They are both first transition series elements;
- Both Cu and Fe are mutually immiscible with Ag;
- Contact killing, in which there is direct, ostensibly dry contact between the NP and bacterium. In fact, as recently noted [23], there is always a nanoscopic layer of water deposited on the NP surface from the ambient humidity. This serves to hydrolyze and ionize the NP surface oxide, which attacks the bacterium;
- Killing in liquids that support the ionization of the NP surface. This appears to be the case in many toxicological evaluations. The metal surface ionizes, leading to ionic diffusion. Ultimately, NPs from the metal are found in the outer membrane surfaces of the bacteria, as well as within the cytoplasm [6];
- Killing in liquids that do not support the ionization of the NP surface. This is the case for human body fluids, such as blood and lymph. They are highly saturated and also contain substances that can reduce metal ions to zero valency [24]. The attack of the bacterium occurs through the protein corona that forms about the NP [23].
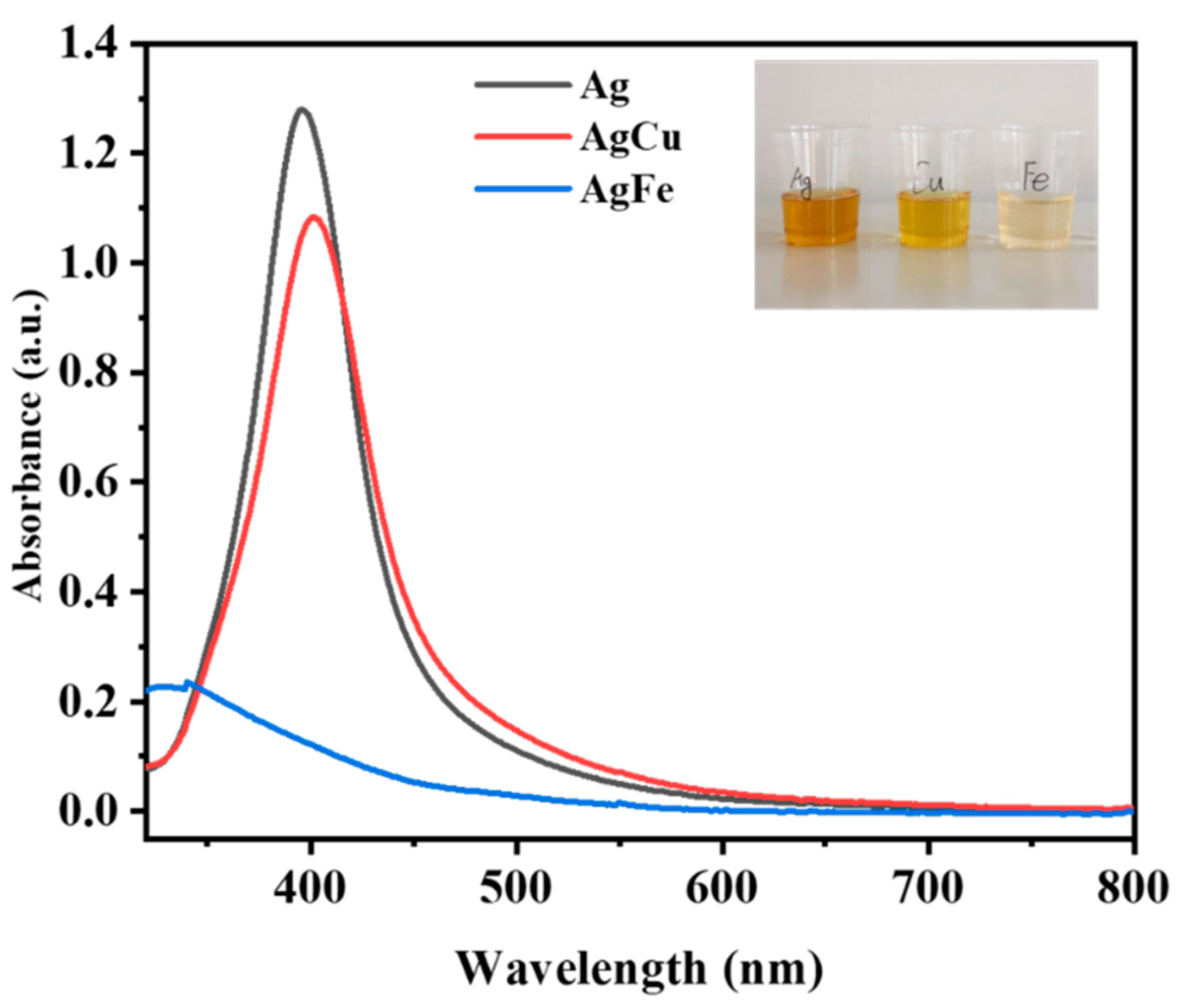
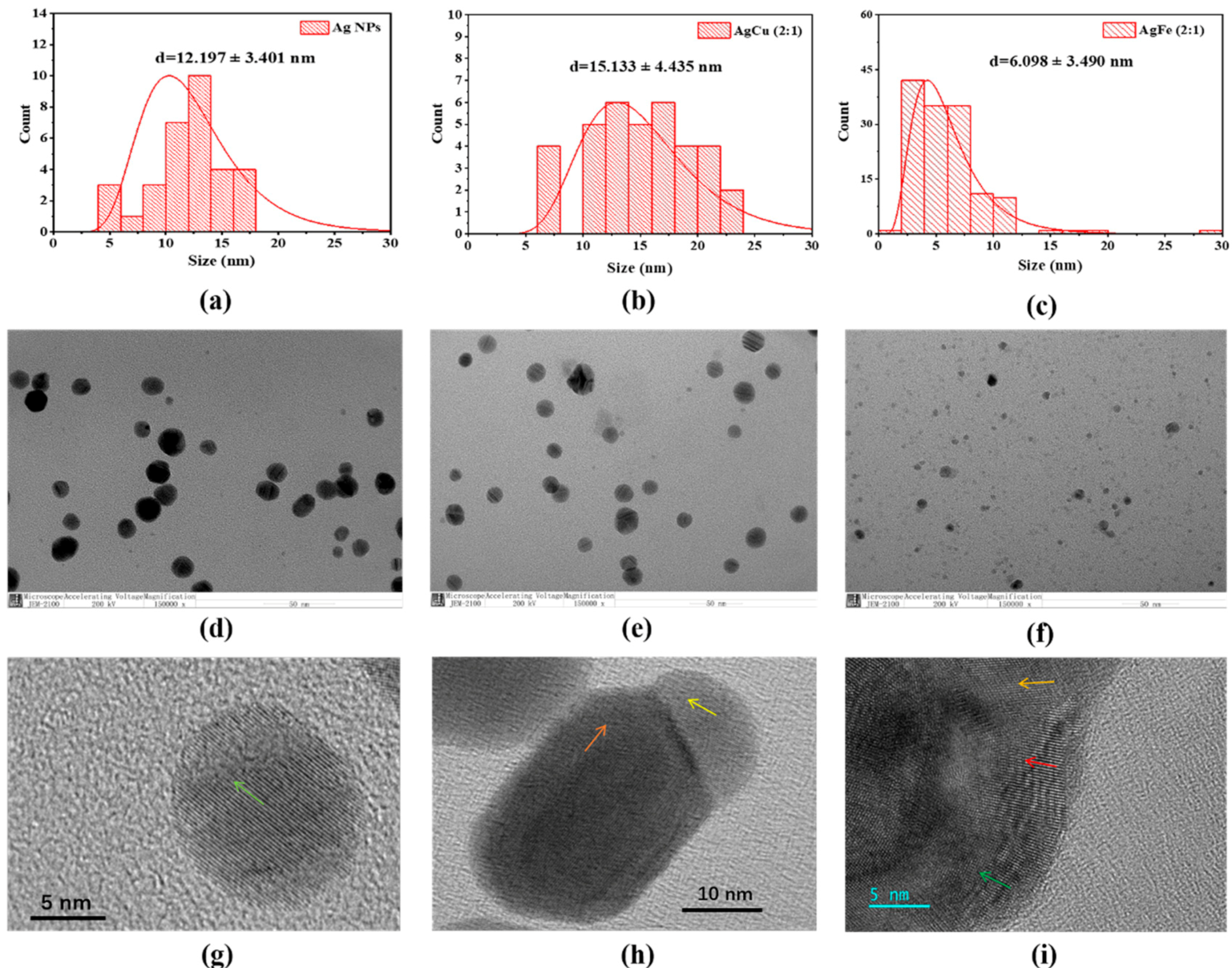
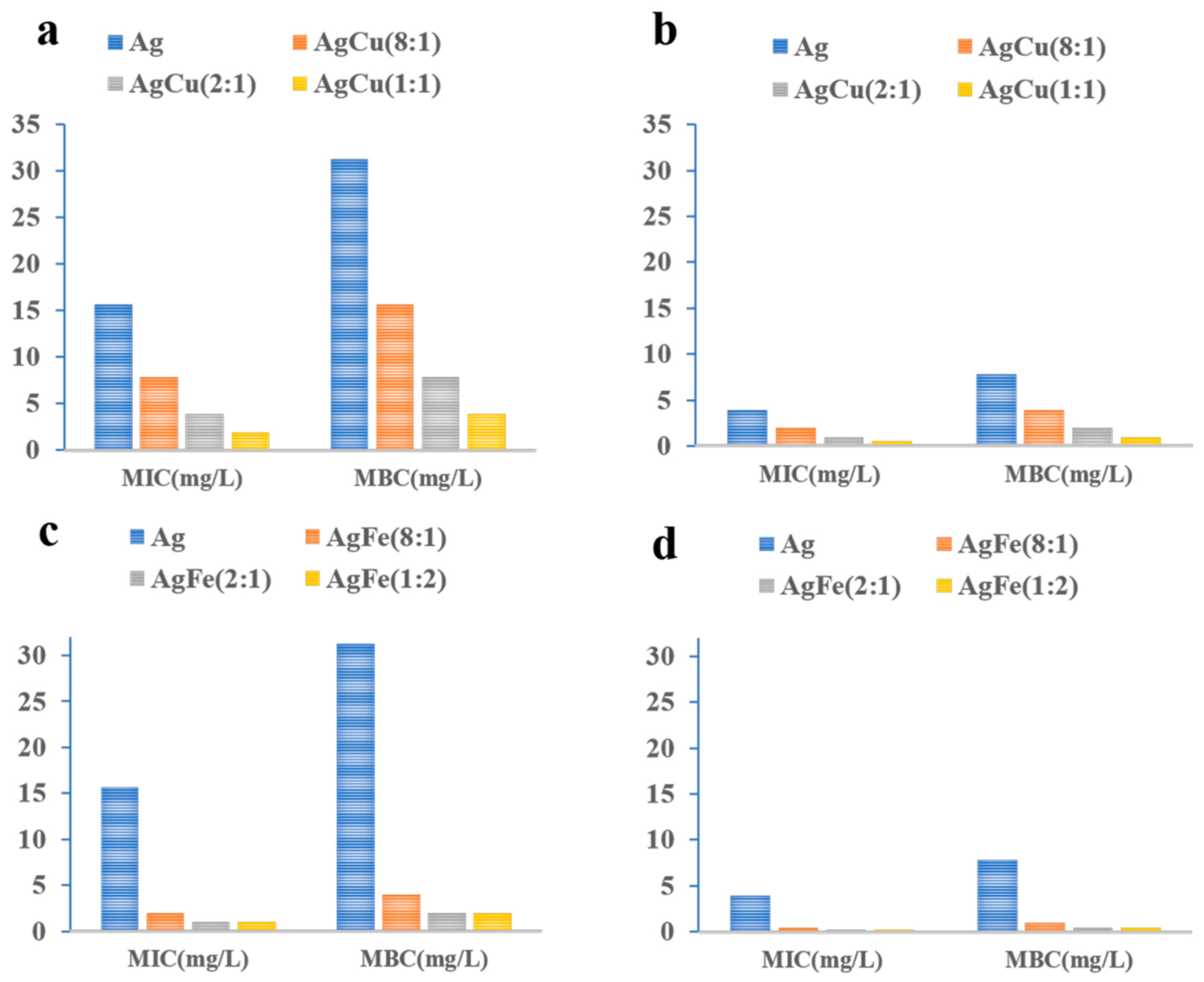
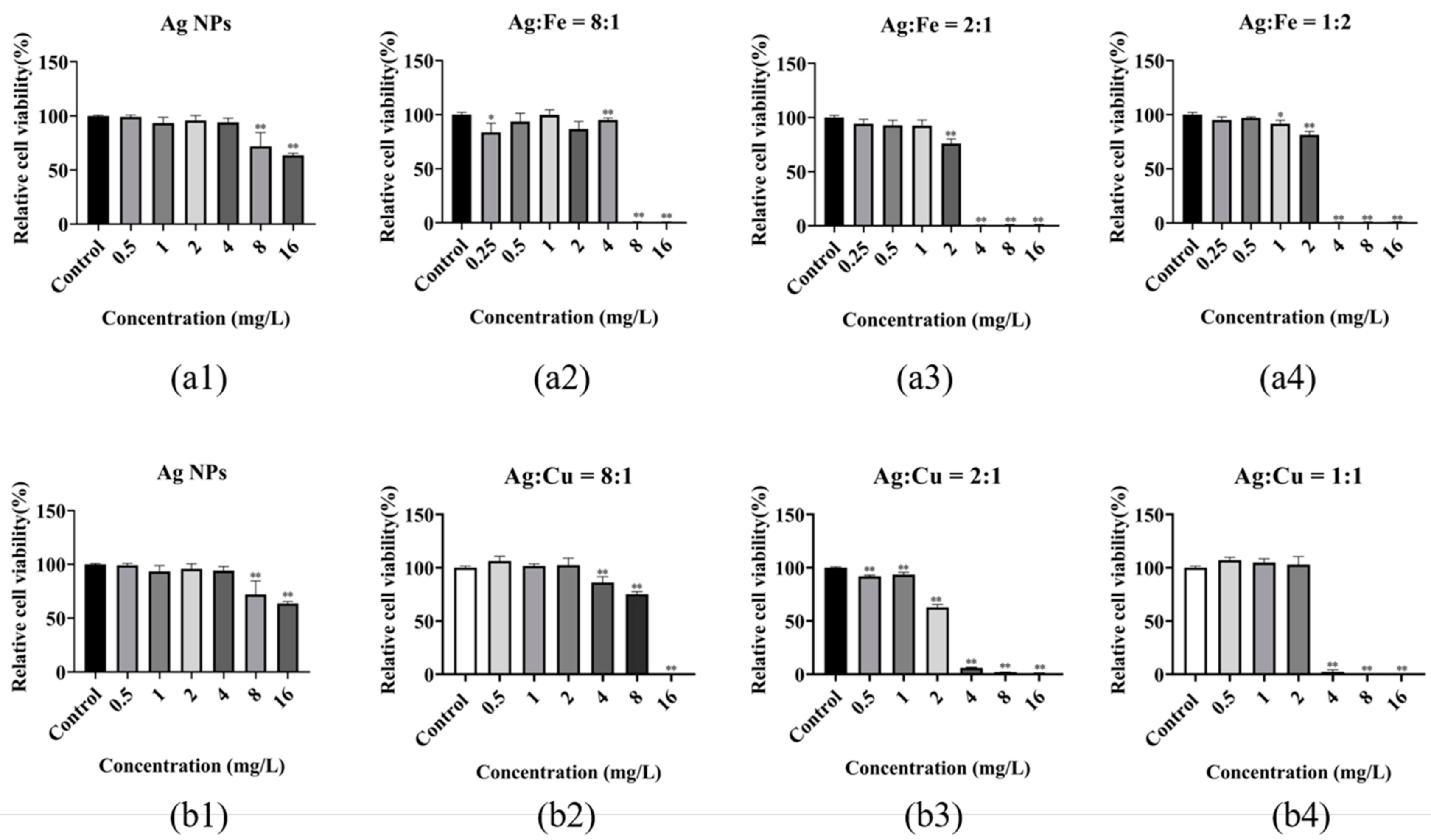
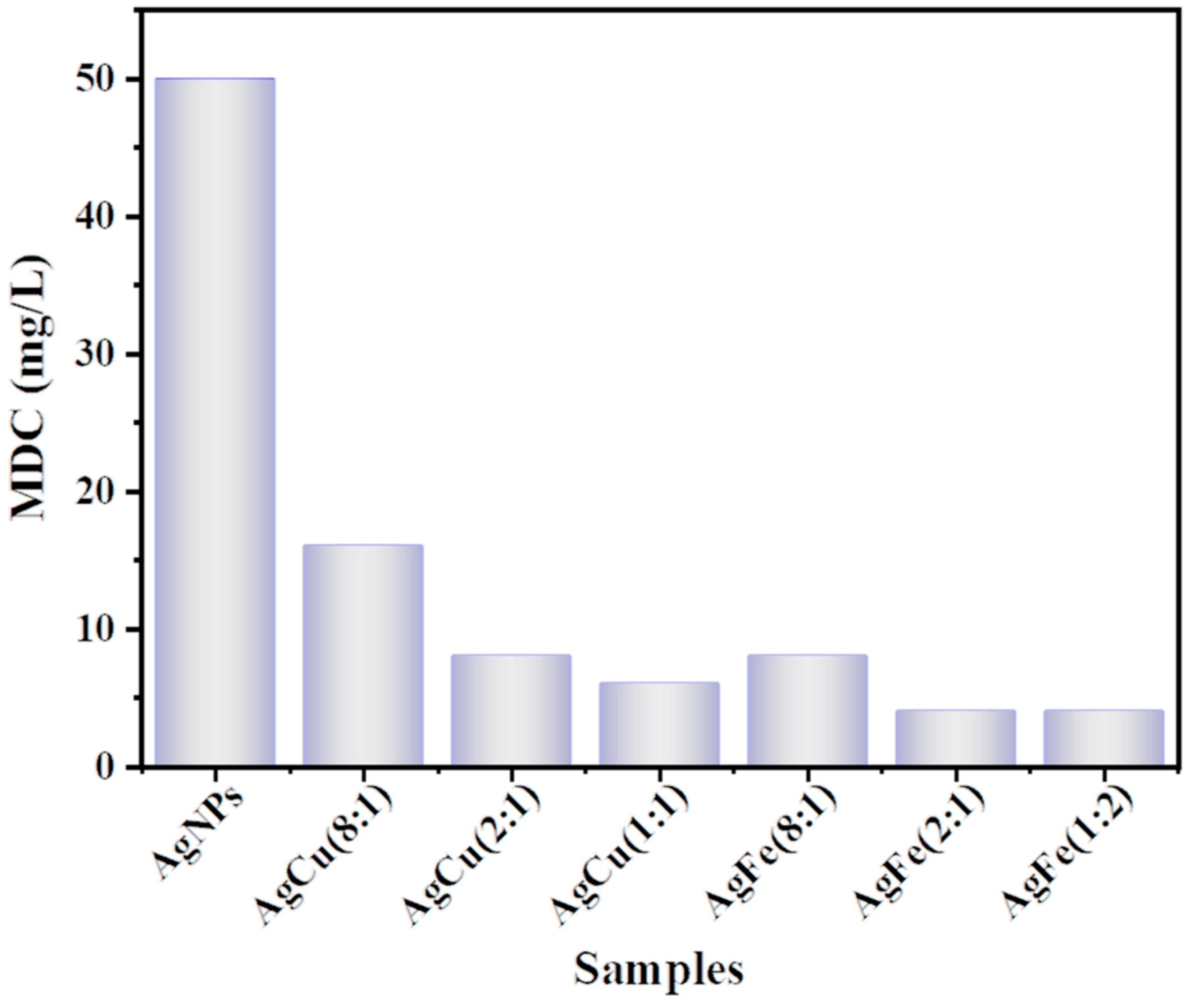
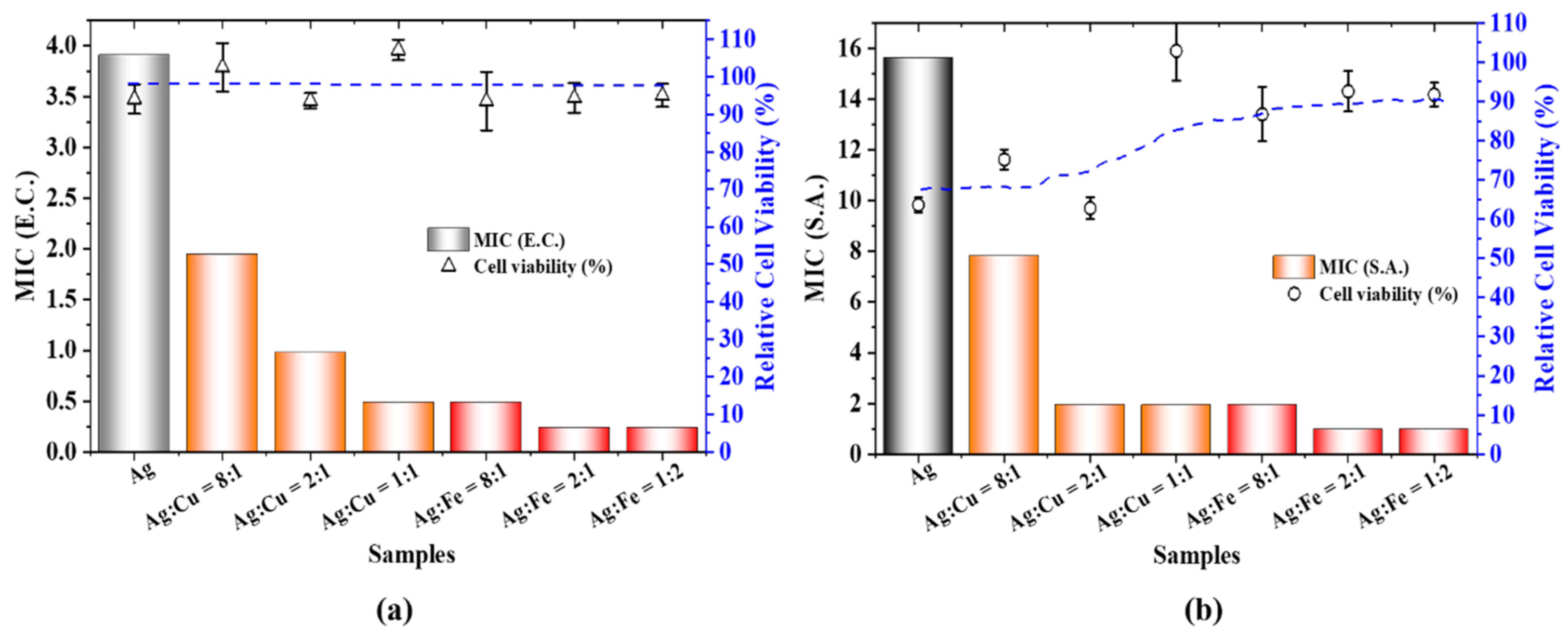
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
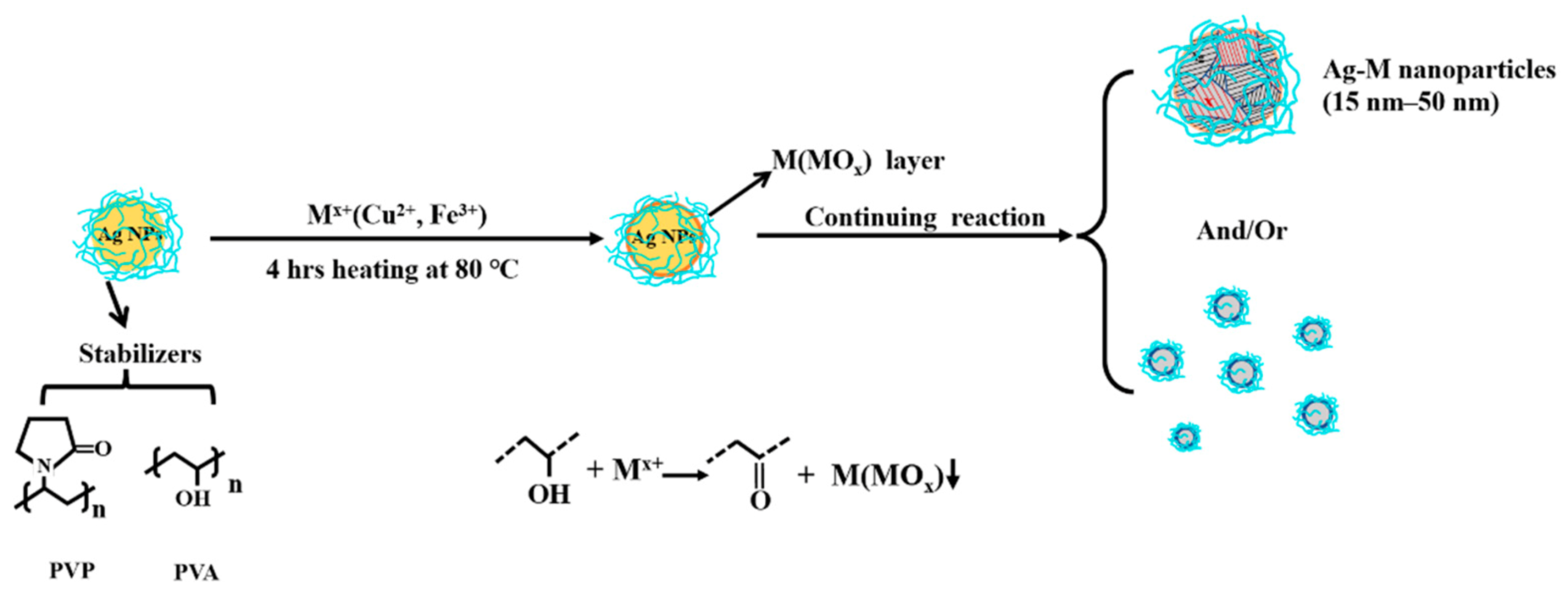
4.2. Synthesis of AgM NAs
4.3. Characterizations
4.4. Antibacterial Efficacy (Minimum Inhibitory Concentration Tests)
4.5. Cytotoxicity
4.6. ICP-MS Analysis
4.7. Antioxidation Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Antibiotic Resistance Threats in the United States; U.S. Departmnet of Health and Human Services: Washington DC, USA, 2019.
- Marambio-Jones, C.; Hoek, E. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Marassi, V.; Di Cristo, L.; Smith, S.G.; Ortelli, S.; Blosi, M.; Costa, A.L.; Reschiglian, P.; Volkov, Y.; Prina-Mello, A. Silver nanoparticles as a medical device in healthcare settings: A five-step approach for candidate screening of coating agents. R. Soc. Open Sci. 2018, 5, 171113. [Google Scholar] [CrossRef]
- McShan, D.; Ray, P.C.; Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yahia, L.H.; Sacher, E. Antimicrobial properties of the Ag, Cu nanoparticle system. Biology 2021, 10, 137. [Google Scholar] [CrossRef]
- Taner, M.; Sayar, N.; Yulug, I.G.; Suzer, S. Synthesis, characterization and antibacterial investigation of silver–copper nanoalloys. J. Mater. Chem. 2011, 21, 13150–13154. [Google Scholar] [CrossRef]
- Tan, K.S.; Cheong, K.Y. Advances of Ag, Cu, and Ag–Cu alloy nanoparticles synthesized via chemical reduction route. J. Nanopart. Res. 2013, 15, 1537. [Google Scholar] [CrossRef]
- Moradi, F.; Sedaghat, S.; Moradi, O.; Arab Salmanabadi, S.J.I.; Chemistry, N.-M. Review on green nano-biosynthesis of silver nanoparticles and their biological activities: With an emphasis on medicinal plants. Inorg. Nano-Met. Chem. 2021, 51, 133–142. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R.d. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Długosz, O.; Sochocka, M.; Ochnik, M.; Banach, M. Metal and bimetallic nanoparticles: Flow synthesis, bioactivity and toxicity. J. Colloid Interface Sci. 2021, 586, 807–818. [Google Scholar] [CrossRef]
- Amendola, V.; Guadagnini, A.; Agnoli, S.; Badocco, D.; Pastore, P.; Fracasso, G.; Gerosa, M.; Vurro, F.; Busato, A.; Marzola, P. Polymer-coated silver-iron nanoparticles as efficient and biodegradable MRI contrast agents. J. Colloid Interface Sci. 2021, 596, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Serpooshan, V. Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. ACS Nano 2012, 6, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zhao, N.; Yang, R.; Yang, J.; Cheng, X.; Zhou, Y. Ultrafine Ag–Fe alloys with graphene-based cellular monolith as a novel antimicrobial material. J. Alloys Compd. 2020, 816, 152592. [Google Scholar] [CrossRef]
- Panchal, D.; Pal, S. Photo-Induced Synthesis of Coral-Like Hierarchical Ag–Fe Bimetallic Multifunctional Nanostructures. Chem. Mater. 2021, 33, 6501–6513. [Google Scholar] [CrossRef]
- de Oliveira Gonçalves, K.; Vieira, D.P.; Levy, D.; Bydlowski, S.P.; Courrol, L.C. Uptake of silver, gold, and hybrids silver-iron, gold-iron and silver-gold aminolevulinic acid nanoparticles by MCF-7 breast cancer cells. Photodiagn. Photodyn. Ther. 2020, 32, 102080. [Google Scholar] [CrossRef]
- Prucek, R.; Tuček, J.; Kilianová, M.; Panáček, A.; Kvítek, L.; Filip, J.; Kolář, M.; Tománková, K.; Zbořil, R. The targeted antibacterial and antifungal properties of magnetic nanocomposite of iron oxide and silver nanoparticles. Biomaterials 2011, 32, 4704–4713. [Google Scholar] [CrossRef]
- Al-Asfar, A.; Zaheer, Z.; Aazam, E.S. Eco-friendly green synthesis of Ag@ Fe bimetallic nanoparticles: Antioxidant, antimicrobial and photocatalytic degradation of bromothymol blue. J. Photochem. Photobiol. B Biol. 2018, 185, 143–152. [Google Scholar] [CrossRef]
- Sandupatla, R.; Dongamanti, A.; Koyyati, R. Antimicrobial and antioxidant activities of phytosynthesized Ag, Fe and bimetallic Fe-Ag nanoparticles using Passiflora edulis: A comparative study. Mater. Today Proc. 2021, 44, 2665–2673. [Google Scholar] [CrossRef]
- Loran, S.; Yelon, A.; Sacher, E. Unexpected findings on the physicochemical characterization of the silver nanoparticle surface. Appl. Surf. Sci. 2018, 428, 1079–1081. [Google Scholar] [CrossRef]
- Loran, S.; Cheng, S.; Botton, G.; L’H, Y.; Yelon, A.; Sacher, E. The physicochemical characterization of the Cu nanoparticle surface, and of its evolution on atmospheric exposure: Application to antimicrobial bandages for wound dressings. Appl. Surf. Sci. 2019, 473, 25–30. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. Do iron oxide nanoparticles have significant antibacterial properties? Antibiotics 2021, 10, 884. [Google Scholar] [CrossRef]
- Sacher, E. A Pragmatic Perspective of the Initial Stages of the Contact Killing of Bacteria on Copper-Containing Surfaces. Appl. Microbiol. 2022, 2, 449–452. [Google Scholar] [CrossRef]
- Kang, F.; Alvarez, P.J.; Zhu, D. Microbial extracellular polymeric substances reduce Ag+ to silver nanoparticles and antagonize bactericidal activity. Environ. Sci. Technol. 2014, 48, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Huang, X.; Ye, N.; Lu, Q.; Zhang, G.; Peng, S.; Wang, H.; Liu, Y. Cytotoxicity of Metal-Based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022, 9, 2106049. [Google Scholar] [CrossRef]
- Golbamaki, N.; Rasulev, B.; Cassano, A.; Robinson, R.L.M.; Benfenati, E.; Leszczynski, J.; Cronin, M.T. Genotoxicity of metal oxide nanomaterials: Review of recent data and discussion of possible mechanisms. Nanoscale 2015, 7, 2154–2198. [Google Scholar] [CrossRef]
- Durán, N.; Seabra, A.B.; Lima, R.d. Cytotoxicity and Genotoxicity of Biogenically Synthesized Silver Nanoparticles. In Nanotoxicology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 245–263. [Google Scholar]
- Technologies, S. Technological Report, N20180646. 2018; (available on written request). [Google Scholar]
- Mireles, L.K.; Wu, M.-R.; Saadeh, N.; Yahia, L.H.; Sacher, E. Physicochemical characterization of polyvinyl pyrrolidone: A tale of two polyvinyl pyrrolidones. ACS Omega 2020, 5, 30461–30467. [Google Scholar] [CrossRef]
- Liu, Y.-J. Synthetic self-assembled homogeneous network hydrogels with high mechanical and recoverable properties for tissue replacement. J. Mater. Chem. B 2016, 4, 4847–4854. [Google Scholar] [CrossRef]
- Cassu, S.N.; Felisberti, M.I. Poly(vinyl alcohol) and poly(vinyl pyrrolidone) blrnds: Miscibility, mictoheterogeneity and free volume change. Polymer 1997, 38, 3907–3911. [Google Scholar] [CrossRef]
- Zhou, F.; Zhu, Y.; Yang, L.; Yang, D.-Q.; Sacher, E. Ag NP catalysis of Cu ions in the preparation of AgCu NPs and the mechanism of their enhanced antibacterial efficacy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127831. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, F.; Hu, J.; Yang, L.; Yang, D.-Q.; Sacher, E. A facile route to prepare colorless Ag-Cu nanoparticle dispersions with elevated antibacterial effects. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127116. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, F.; Wang, K.; Yang, D.; Sacher, E. AgCu NP Formation by the Ag NP Catalysis of Cu Ions at Room Temperature and Their Antibacterial Efficacy: A Kinetic Study. Molecules 2022, 27, 6951. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhu, Y.; Yang, D.-Q.; Sacher, E. Ultrahigh antibacterial effect of easily obtained AgFe NPs with prominent catalytic properties and biological activities. Manuscr. Prep. 2022. [Google Scholar]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Carroll, K.C.; Butel, J.; Morse, S. Jawetz Melnick and Adelbergs Medical Microbiology 27 E; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Chen, H. Responsive and synergistic antibacterial coatings: Fighting against bacteria in a smart and effective way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef]
- Niño-Martínez, N.; Salas Orozco, M.F.; Martínez-Castañón, G.-A.; Torres Méndez, F.; Ruiz, F. Molecular mechanisms of bacterial resistance to metal and metal oxide nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Garg, A.; Pandit, S.; Mokkapati, V.; Mijakovic, I. Antimicrobial effects of biogenic nanoparticles. Nanomaterials 2018, 8, 1009. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Lv, H.; Cui, S.; Yang, Q.; Song, X.; Wang, D.; Hu, J.; Zhou, Y.; Liu, Y. AgNPs-incorporated nanofiber mats: Relationship between AgNPs size/content, silver release, cytotoxicity, and antibacterial activity. Mater. Sci. Eng. C 2021, 118, 111331. [Google Scholar] [CrossRef]
- Avalos, A.; Haza, A.I.; Mateo, D.; Morales, P. Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J. Appl. Toxicol. 2014, 34, 413–423. [Google Scholar] [CrossRef]
- Barbasz, A.; Oćwieja, M.; Piergies, N.; Duraczyńska, D.; Nowak, A. Antioxidant-modulated cytotoxicity of silver nanoparticles. J. Appl. Toxicol. 2021, 41, 1863–1878. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Abu Habib, M.M.; Badria, F.A. A new hexagalloyl compound from Emblica officinalis Gaertn.: Antioxidant, cytotoxicity, and silver ion reducing activities. Chem. Pap. 2021, 75, 6509–6518. [Google Scholar] [CrossRef]
- Shriniwas, P.P.; Subhash, T.K. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar]
- Monprasit, P.; Lawanprasert, S.; Maniratanachote, R. Effects of silver, silver-copper and silver-tin nanoparticles in THP-1 cells. TJPS 2018, 2018, 42. [Google Scholar]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol. Vitr. 2013, 27, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Samberger, C.; Kueznik, T.; Absenger, M.; Roblegg, E.; Zimmer, A.; Pieber, T.R. Cytotoxicity of nanoparticles independent from oxidative stress. J. Toxicol. Sci. 2009, 34, 363–375. [Google Scholar] [CrossRef]
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, 1119S–1124S. [Google Scholar] [CrossRef] [PubMed]
- Mb, N.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.N.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Ahmadi, S. Mathematical modeling of cytotoxicity of metal oxide nanoparticles using the index of ideality correlation criteria. Chemosphere 2019, 242, 125192. [Google Scholar] [CrossRef] [PubMed]
- Gotzmann, G.; Jorsch, C.; Wetzel, C.; Funk, H. Antimicrobial effects and dissolution properties of silver copper mixed layers. Surf. Coat. Technol. 2018, 336, 22–28. [Google Scholar] [CrossRef]
- Chang, T.L.; Zhou, X.; Liang, J. Synthesis and characterization of Ag-Cu alloy nanoparticles for antimicrobial applications: A polydopamine chemistry application. Mater. Sci. Eng. C 2019, 98, 675–684. [Google Scholar] [CrossRef]
- Yang, M.; Lu, F.; Zhou, T.; Zhao, J.; Ding, C.; Fakhri, A.; Gupta, V.K. Biosynthesis of nano bimetallic Ag/Pt alloy from Crocus sativus L. extract: Biological efficacy and catalytic activity. J. Photochem. Photobiol. B Biol. 2020, 212, 112025. [Google Scholar] [CrossRef]
- Hertadi, R.; Amari, M.M.S.; Ratnaningsih, E. Enhancement of antioxidant activity of levan through the formation of nanoparticle systems with metal ions. Heliyon 2020, 6, e04111. [Google Scholar] [CrossRef]



| Samples | Ag NPs | M Ions | Ratio of Ag/M Ions | Heating (80 °C) | Heating Time |
|---|---|---|---|---|---|
| Ag | 500 ppm | 0 | - | No | - |
| AgCu1 | 500 ppm | 62.5 ppm | 8:1 | Yes | 4 h |
| AgCu2 | 500 ppm | 250 ppm | 2:1 | Yes | 4 h |
| AgCu3 | 500 ppm | 500 ppm | 1:1 | Yes | 4 h |
| AgFe1 | 500 ppm | 62.5 ppm | 8:1 | Yes | 4 h |
| AgFe2 | 500 ppm | 250 ppm | 2:1 | Yes | 4 h |
| AgFe3 | 500 ppm | 1000 ppm | 1:2 | Yes | 4 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Kostantin, E.; Yang, D.-Q.; Sacher, E. Cytotoxicity and Antibacterial Efficacy of AgCu and AgFe NanoAlloys: A Comparative Study. Antibiotics 2022, 11, 1737. https://doi.org/10.3390/antibiotics11121737
Zhou F, Kostantin E, Yang D-Q, Sacher E. Cytotoxicity and Antibacterial Efficacy of AgCu and AgFe NanoAlloys: A Comparative Study. Antibiotics. 2022; 11(12):1737. https://doi.org/10.3390/antibiotics11121737
Chicago/Turabian StyleZhou, Fang, Elie Kostantin, De-Quan Yang, and Edward Sacher. 2022. "Cytotoxicity and Antibacterial Efficacy of AgCu and AgFe NanoAlloys: A Comparative Study" Antibiotics 11, no. 12: 1737. https://doi.org/10.3390/antibiotics11121737
APA StyleZhou, F., Kostantin, E., Yang, D.-Q., & Sacher, E. (2022). Cytotoxicity and Antibacterial Efficacy of AgCu and AgFe NanoAlloys: A Comparative Study. Antibiotics, 11(12), 1737. https://doi.org/10.3390/antibiotics11121737







