Efficacy of Tuberculosis Treatment in Patients with Drug-Resistant Tuberculosis with the Use of Bedaquiline: The Experience of the Russian Federation
Abstract
:1. Introduction
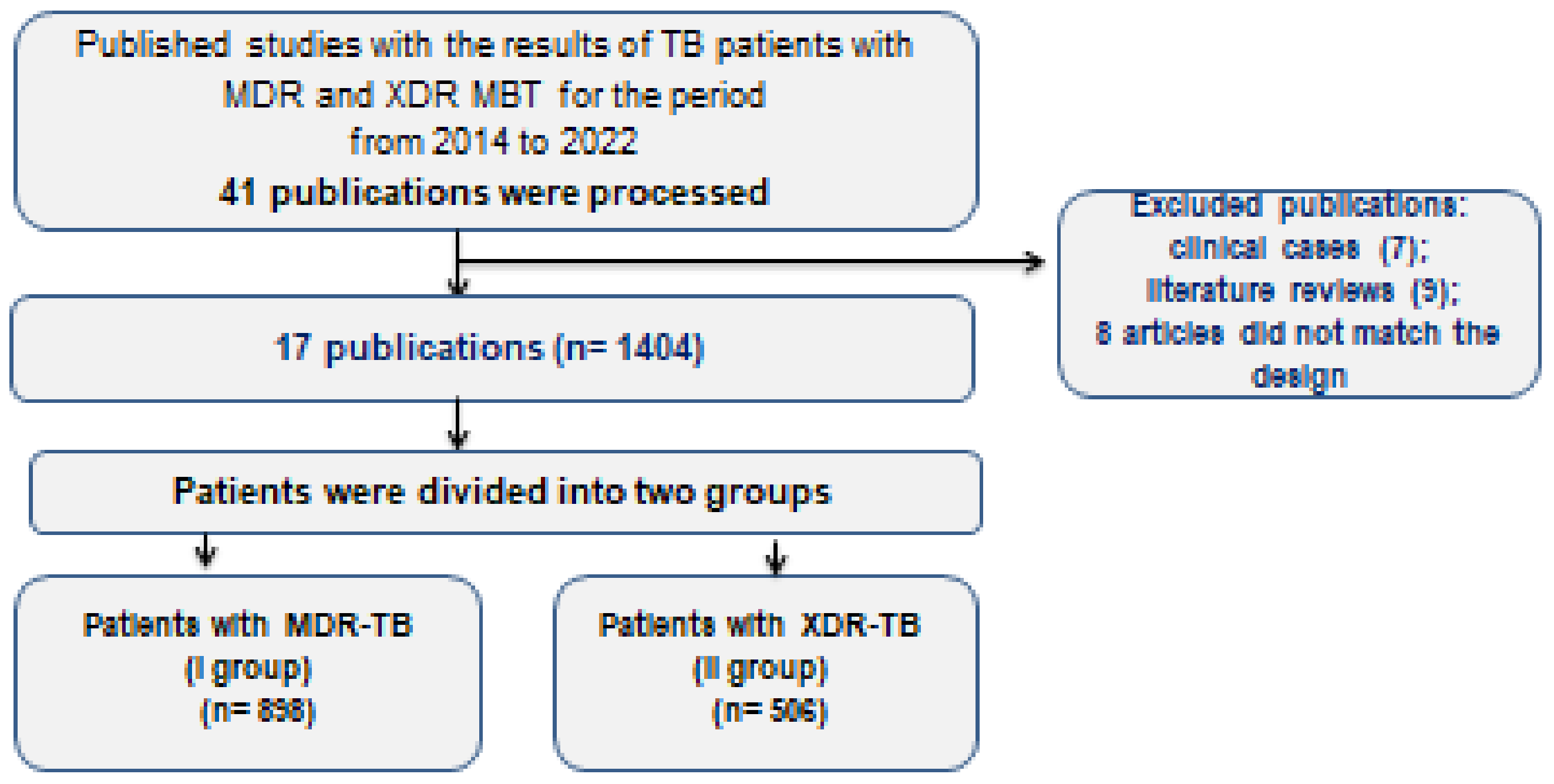
2. Materials and Methods
3. Criteria for the Efficacy of TB Treatment
4. Results of the Study
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2019; 283p, ISBN 978-92-4-156571-4. [Google Scholar]
- Trajman, A.; Felker, I.; Alves, L.C.; Coutinho, I.; Osman, M.; Meehan, S.-A.; Singh, U.B.; Schwartz, Y. The COVID-19 and TB syndemic: The way forward. Int. J. Tuberc. Lung Dis. 2022, 26, 710–719. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Lists of High Burden Countries for TB, Multidrug/Rifampicin-Resistant TB (MDR/RR-TB) and TB/HIV, 2021–2025; World Health Organization: Geneva, Switzerland, 2021; 16p, ISBN 978-92-4-002943-9. [Google Scholar]
- McQuaid, C.F.; McCreesh, N.; Read, J.; Sumner, T.; Houben, R.M.G.J.; White, R.G.; Harris, R.C. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur. Respir. J. 2020, 56, 2001718. [Google Scholar] [CrossRef]
- Nechaeva, O.B. The state and prospects of the anti-tuberculosis service in Russia during the COVID-19 period. Tuberc. Lung Dis. 2020, 98, 7–19. [Google Scholar] [CrossRef]
- Vasilyeva, I.A.; Testov, V.V.; Sterlikov, S.A. Tuberculosis epidemic situation during the COVID-19 pandemic—2020–2021. Tuberc. Lung Dis. 2022, 100, 6–12. [Google Scholar] [CrossRef]
- Nechaeva, O.B. The epidemiological situation of tuberculosis in Russia. Tuberc. Lung Dis. 2018, 96, 15–24. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Mbuagbaw, L.; Guglielmetti, L.; Hewison, C.; Bakare, N.; Bastard, M.; Caumes, E.; Fréchet-Jachym, M.; Robert, J.; Veziris, N.; Khachatryan, N.; et al. Outcomes of Bedaquiline Treatment in Patients with Multidrug-Resistant Tuberculosis. Emerg. Infect. Dis. 2019, 25, 936–943. [Google Scholar] [CrossRef] [Green Version]
- Zimina, V.N.; Viktorova, I.B. Delamanid—A new anti-tuberculosis drug: Application, limitations, prospects. Tuberc. Lung Dis. 2021, 99, 58–66. [Google Scholar] [CrossRef]
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382, 2376–2377. [Google Scholar] [CrossRef]
- WHO. The Use of Bedaquiline in the Treatment of Multidrug-Resistant Tuberculosis: Interim Policy Guidance; WHO: Geneva, Switzerland, 2013; 57p. [Google Scholar]
- Kudlay, D.A. Development and introduction into clinical practice of a new pharmacological substance from the class of diarylquinolines. Exp. Clin. Pharmacol. 2021, 84, 41–47. [Google Scholar] [CrossRef]
- WHO. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; WHO: Geneva, Switzerland, 2019; 96p, ISBN 978-92-4-155052-9. [Google Scholar]
- Chan, B.; Khadem, T.M.; Brown, J. A review of tuberculosis: Focus on bedaquiline. Am. J. Health Pharm. 2013, 70, 84–94. [Google Scholar] [CrossRef]
- Diacon, A.H.; Donald, P.R.; Pym, A.; Grobusch, M.; Patientia, R.F.; Mahanyele, R.; Bantubani, N.; Narasimooloo, R.; De Marez, T.; van Heeswijk, R.; et al. Randomized Pilot Trial of Eight Weeks of Bedaquiline (TMC207) Treatment for Multidrug-Resistant Tuberculosis: Long-Term Outcome, Tolerability, and Effect on Emergence of Drug Resistance. Antimicrob. Agents Chemother. 2012, 56, 3271–3276. [Google Scholar] [CrossRef] [Green Version]
- Diacon, A.H.; Pym, A.; Grobusch, M.; Patientia, R.; Rustomjee, R.; Page-Shipp, L.; Pistorius, C.; Krause, R.; Bogoshi, M.; Churchyard, G.; et al. The Diarylquinoline TMC207 for Multidrug-Resistant Tuberculosis. N. Engl. J. Med. 2009, 360, 2397–2405. [Google Scholar] [CrossRef] [Green Version]
- Diacon, A.H.; Pym, A.; Grobusch, M.P.; de los Rios, J.M.; Gotuzzo, E.; Vasilyeva, I.; Leimane, V.; Andries, K.; Bakare, N.; De Marez, T.; et al. Multidrug-Resistant Tuberculosis and Culture Conversion with Bedaquiline. N. Engl. J. Med. 2014, 371, 723–732. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment—Drug-Susceptible Tuberculosis Treatment; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. Multidrug-Resistant Tuberculosis in Children and Adolescents in the WHO European Region, Expert Opinion; WHO Regional Office for Europe: Copenhagen, Denmark, 2019. [Google Scholar]
- Borisov, S.E.; Dheda, K.; Enwerem, M.; Leyet, R.R.; D’Ambrosio, L.; Centis, R.; Sotgiu, G.; Tiberi, S.; Alffenaar, J.-W.; Maryandyshev, A.; et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: A multicentre study. Eur. Respir. J. 2017, 49, 1700387. [Google Scholar] [CrossRef] [Green Version]
- Borisov, S.E.; Ivanushkina, T.N.; Ivanova, D.A.; Filippov, A.V.; Litvinova, N.V.; Rodina, O.V.; Garmash, Y.Y.; Safonova, S.G.; Bogorodskaya, E.M. Efficacy and safety of six-month chemotherapy regimens including bedaquiline in patients with respiratory tuberculosis. Tuberc. Soc. Signif. Dis. 2015, 3, 30–49. [Google Scholar]
- Morozova, T.I.; Otpuschennikova, O.N.; Doktorova, N.P.; Danilov, A.N. Experience in the use of bedaquiline in the treatment of patients with drug-resistant pulmonary tuberculosis. Tuberc. Lung Dis. 2016, 94, 29–35. [Google Scholar]
- Balasanyants, G.S.; Federation, S.M.K. Experience of using bedaquiline in the complex treatment of patients with tuberculosis associated with HIV infection. Tuberc. Lung Dis. 2017, 95, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Vasilyeva, I.A.; Samoylova, A.G.; Lovacheva, O.; Chernousova, L.N.; Bagdasaryan, T.R. Effect of various anti-tuberculosis and antibacterial drugs on the effectiveness of treatment of patients with multidrug-resistant tuberculosi. Tuberc. Lung Dis. 2017, 95, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Lepshina, S.M.; Serdyuk, O.V.; Yurovskaya, E.I. Experience in the use of bedaquiline in patients with multidrug-resistant pulmonary tuberculosis. Univ. Clin. 2017, 4, 89–99. [Google Scholar]
- Konovalova, N.M.; Odinets, V.S.; Vasilenko, T.I.; Zadremaylova, T.A. Experience with the use of bedaquiline in the treatment of patients with pulmonary tuberculosis with multiple and extensive drug resistance of the pathogen. Tuberc. Lung Dis. 2017, 95, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Kondakova, M.N.; Khabirov, V.V.; Zhemkov, V.F.; Shpakovskaya, L.R.; Daynovets, A.V.; Elkin, A.V. The effect of bedaquiline on the effectiveness of complex therapy for respiratory tuberculosis. Tuberc. Lung Dis. 2018, 96, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Tikhonov, A.M.; Burakova, M.V.; Vaniev, E.V.; Romanov, V.V.; Vasilyeva, I.A. Effectiveness of chemotherapy with bedaquiline in patients with drug-resistant pulmonary tuberculosis. Tuberc. Lung Dis. 2018, 96, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Borisov, S.E.; Filippov, A.V.; Ivanova, D.A.; Ivanushkina, T.N.; Litvinova, N.V.; Garmash, Y.Y. Efficacy and safety of bedaquiline-based regimens chemotherapy in patients with respiratory tuberculosis: Immediate and final results. Tuberc. Lung Dis. 2019, 97, 28–40. [Google Scholar] [CrossRef]
- Golubchikov, P.N.; Kruk, E.A.; Mishustin, S.P.; Petrenko, T.I.; Kudlay, D.A. Experience in the treatment of patients with extensively drug-resistant tuberculosis, including long-term use of bedaquiline, in the Tomsk region: Immediate and long-term result. Tuberc. Lung Dis. 2019, 97, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Danilova, T.I.; Korneev, Y.V.; Kudlay, D.A.; Nikolenko, N.Y. The results of the use of bedaquiline-based therapy regimens in patients with MDR/XDR tuberculosis, including in combination with HIV-infection (experiment of the Leningrad region). Tuberc. Lung Dis. 2020, 98, 56–62. [Google Scholar] [CrossRef]
- Stavitskaya, N.V.; Felker, I.G.; Zhukova, E.M.; Tlif, I.; Doktorova, N.P.; Kudlay, D. Multivariate analysis of the results of the use of bedaquiline in the treatment of MDR/XDR pulmonary tuberculosis. Tuberc. Lung Dis. 2020, 98, 56–62. [Google Scholar] [CrossRef]
- Ivanova, D.A.; Borisov, S.E.; Rodina, O.V.; Filippov, A.V.; Ivanushkina, T.N.; Litvinova, N.V. Safety of treatment regimens for patients with multidrug-resistant tuberculosis according to new WHO recommendations 201. Tuberc. Lung Dis. 2020, 98, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Yablonsky, P.K.; Starshinova, A.A.; Nazarenko, M.M.; Chuzhov, A.L.; Alexeev, D.U.; Pavlova, M.V. Efficiency of new chemotherapy regimens in patients with extensive drug resistance of the pathogen. Bull. Mod. Clin. Med. 2022, 15, 67–75. [Google Scholar] [CrossRef]
- Starshinova, A.A.; Nazarenko, M.M.; Belyaeva, E.N.; Kudlay, D.A.; Pavlova, M.V.; Yablonskiy, P.K. Effectiveness of bedaquiline use in patients with multidrug and extensive drug TB. sustainability. Tuberc. Lung Dis. 2022, 100, 56–63. [Google Scholar] [CrossRef]
- Morozova, T.I.; Doktorova, N.P.; Otpushchennikova, O.N.; Nikolenko, N.Y. Bedaquiline in the treatment of extensively drugresistant tuberculosis. Med. Counc. 2022, 16, 90–96. [Google Scholar] [CrossRef]
- Federal Clinical Guidelines for the Diagnosis and Treatment of Extensively Drug-Resistant Respiratory Tuberculosis. Guideline, The Russian Federation Moscow. 2015. 56p. Available online: http://roftb.ru/structure (accessed on 9 October 2022).
- Yablonsky, P.K. Phthisiology. National Clinical Guidelines; GEOTAR-Media: Moscow, Russia, 2015; 240p. [Google Scholar]
- WHO. Definitions and Reporting Framework for Tuberculosis—2013 Revision; World Health Organization: Geneva, Switzerland, 2013; Revision: Updated December 2014 and January 2020; 40p. [Google Scholar]
- World Health Organization. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; WHO/CDS/TB/2019.3; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/ (accessed on 29 March 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org. (accessed on 30 November 2021).
- Max Gordon and Thomas Lumley. Forestplot: Advanced Forest Plot Using ‘grid’ Graphics. 2021. R Package Version 2.0.1. Available online: https://CRAN.R-project.org/package=forestplot (accessed on 9 October 2022).
- World Health Organization. Global Tuberculosis Report 2018; WHO/CDS/TB/2018.20; World Health Organization: Geneva, Switzerland, 2018; pp. 95–96. [Google Scholar]
- Fernandes, G.F.S.; Thompson, A.M.; Castagnolo, D.; Denny, W.A.; Dos Santos, J.L. Tuberculosis Drug Discovery: Challenges and New Horizons. J. Med. Chem. 2022, 65, 7489–7531. [Google Scholar] [CrossRef]
- Yablonsky, P.K.; Vinogradova, T.I.; Levashev, Y.N.; Pavlova, M.V.; Zilber, E.K.; Starshinova, A.A.; Sapozhnikova, N.V.; Chernokhaeva, I.V.; Archakova, L.I.; Zabolotnykh, N.V.; et al. Preclinical and clinical studies of a new anti-tuberculosis drug Perchloson. Ther. Arch. 2016, 88, 111–115. [Google Scholar]
- Pontali, E.; Sotgiu, G.; Tiberi, S.; Tadolini, M.; Visca, D.; D’Ambrosio, L.; Centis, R.; Spanevello, A.; Migliori, G.B. Combined treatment of drug-resistant tuberculosis with bedaquiline and delamanid: A systematic review. Eur. Respir. J. 2018, 52, 1800934. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Ahuja, S.D.; Akkerman, O.W.; Alffenaar, J.C.; Anderson, L.F.; Baghaei, P.; Bang, D.; Barry, P.M.; Bastos, M.L.; Behera, D.; et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: An individual patient data meta-analysis. Lancet 2018, 392, 821–834. [Google Scholar] [CrossRef]
- Dan, T.; Hu, X.; Wu, J.; Chi, C.; Xu, L.; Hu, R.; Qian, J.; Yun, L.; Du, J. Sixty-seven MDR-, and XDR-PTB cases with sputum culture conversion then treated with bedaquiline-containing regimens: A singal arm, single center observational study. Chin. J. Antituberc. 2021, 43, 1146–1152. [Google Scholar] [CrossRef]
- Guglielmetti, L.; Veziris, N.; Aubry, A.; Brossier, F.; Bernard, C.; Sougakoff, W.; Jarlier, V.; Robert, J.; Surveillan, F.T.L. Risk factors for extensive drug resistance in multidrug-resistant tuberculosis cases: A case-case study. Int. J. Tuberc. Lung Dis. 2018, 22, 54–59. [Google Scholar] [CrossRef]
- Klevno, N.I.; Aksenova, V.A.; Kazakov, A.V.; Kovalevskaya, E.B. Short course chemotherapy in children suffering from drug resistant tuberculosis. Tuberc. Lung Dis. 2021, 99, 34–39. [Google Scholar] [CrossRef]
- Koirala, S.; Borisov, S.; Danila, E.; Mariandyshev, A.; Shrestha, B.; Lukhele, N.; Dalcolmo, M.; Shakya, S.; Miliauskas, S.; Kuksa, L.; et al. Outcome of treatment of MDR-TB or drug-resistant patients treated with bedaquiline and delamanid: Results from a large global cohort. Pulmonology 2021, 27, 403–412. [Google Scholar] [CrossRef]
- Hatami, H.; Sotgiu, G.; Bostanghadiri, N.; Abadi, S.S.D.; Mesgarpour, B.; Goudarzi, H.; Migliori, G.B.; Nasiri, M.J. Bedaquiline-containing regimens and multidrug-resistant tuberculosis: A systematic review and meta-analysis. J. Bras. Pneumol. 2022, 48, e20210384. [Google Scholar] [CrossRef]
- Mokrousov, I.; Akhmedova, G.; Molchanov, V.; Fundovnaya, E.; Kozlova, E.; Ostankova, Y.; Semenov, A.; Maslennikova, N.; Leontev, D.; Zhuravlev, V.; et al. Frequent acquisition of bedaquiline resistance by epidemic extensively drug-resistant Mycobacterium tuberculosis strains in Russia during long-term treatment. Clin. Microbiol. Infect. 2020, 27, 478–480. [Google Scholar] [CrossRef]
- Mokrousov, I.; Akhmedova, G.; Polev, D.; Molchanov, V.; Vyazovaya, A. Acquisition of bedaquiline resistance by extensively drug-resistant Mycobacterium tuberculosis strain of Central Asian Outbreak clade. Clin. Microbiol. Infect. 2019, 25, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, M.V.; Ershova, E.S.; Chernokhaeva, I.V.; Sapozhnikova, N.V.; Archakova, L.I. Adverse reactions in the treatment of respiratory tuberculosis with new generation drugs. Med. Alliance 2018, 2, 23–37. [Google Scholar]
- Pavlova, M.V.; Vinogradova, T.I.; Zabolotnykh, N.V.; Ershova, E.S.; Sapozhnikova, N.V.; Chernokhaeva, I.V.; Archakova, L.I.; Vitovskaya, M.L.; Dogonadze, M.Y.; Starshinova, A.A.; et al. Prospects for the use of new generation anti-tuberculosis drugs in the treatment of drug-resistant tuberculosis (experimental clinical study). Rev. Clin. Pharmacol. Drug Ther. 2018, 16, 33–40. [Google Scholar] [CrossRef]
- Motta, I.; Cozzi, S.N.; Pontali, E. QT prolongation for old and new drugs: How much should we really worry? Int. J. Tuberc. Lung Dis. 2022, 26, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, E.M.; Vokhminova, L.G.; Kudlay, D.A. Influence of modern chemotherapy of tuberculosis with MDR/XDR on changes in the QT interval on the ECG in patient. Tuberc. Lung Dis. 2019, 97, 19–22. [Google Scholar] [CrossRef]
- Guglielmetti, L.; Tiberi, S.; Burman, M.; Kunst, H.; Wejse, C.; Togonidze, T.; Bothamley, G.; Lange, C. QT prolongation and cardiac toxicity of new tuberculosis drugs in Europe: A Tuberculosis Network European Trialsgroup (TBnet) study. Eur. Respir. J. 2018, 52, 1800537. [Google Scholar] [CrossRef] [Green Version]
- Borisov, S.; Danila, E.; Maryandyshev, A.; Dalcolmo, M.; Miliauskas, S.; Kuksa, L.; Manga, S.; Skrahina, A.; Diktanas, S.; Codecasa, L.R.; et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: First global report. Eur. Respir. J. 2019, 54, 1901522. [Google Scholar] [CrossRef]
- Martinez, E.; Hennessy, D.; Jelfs, P.; Crighton, T.; Chen, S.C.-A.; Sintchenko, V. Mutations associated with in vitro resistance to bedaquiline in Mycobacterium tuberculosis isolates in Australia. Tuberculosis 2018, 111, 31–34. [Google Scholar] [CrossRef]
- Pontali, E.; D’Ambrosio, L.; Centis, R.; Sotgiu, G.; Migliori, G.B. Multidrug-resistant tuberculosis and beyond: An updated analysis of the current evidence on bedaquiline. Eur. Respir. J. 2017, 49, 1700146. [Google Scholar] [CrossRef]





| N | Author Surname, First Name, Patronymic | Year | Number of Patients | Follow-Up Period (Weeks) | MDR MTB (n) | % | XDR MTB (n) | % |
|---|---|---|---|---|---|---|---|---|
| 1 | Borisov S.E. et al. [22] | 2015 | 54 | 24 | 23 | 42.6 | 31 | 57.4 |
| 2 | Morozova T.I. et al. [23] | 2016 | 49 | 22 | 16 | 32.7 | 33 | 67.3 |
| 3 | Balasanyantzh G.S. [24] | 2017 | 14 | 26 | 2 | 14.3 | 12 | 85.7 |
| 4 | Vasiljeva I.A. et al. [25] | 2017 | 412 | 84 | 237 | 57.6 | 175 | 42.4 |
| 5 | Lepshina S.M., Serduk O.V., Urovskaya E.I. [26] | 2017 | 34 | 24 | 34 | 100 | 0 | 0 |
| 6 | Konovalova N. M. et al. [27] | 2017 | 21 | 80 | 10 | 41.6 | 11 | 52.3 |
| 7 | Kondakova M.N. et al. [28] | 2018 | 38 | 24 | 0 | 0 | 38 | 100 |
| 8 | Tihonova L.U. et al. [29] | 2018 | 23 | 24 | 13 | 56.5 | 10 | 43.5 |
| 9 | Borisov S.E. et al. [30] | 2019 | 315 | 24 | 315 | 100 | n | n |
| 10 | Golubchikov P.N. et al. [31] | 2019 | 39 | 48 | 8 | 20.5 | 31 | 79.5 |
| 11 | Danilova T.I. et al. [32] | 2020 | 46 | 24 | 16 | 34.8 | 30 | 65.2 |
| 12 | Danilova T.I. et al. [32] | 2020 | 34 | 24 | 10 | 29.4 | 24 | 70.6 |
| 13 | Stavickaya N.V. et al. [33] | 2020 | 70 | 70 | 70 | 100 | 0 | 0 |
| 14 | Ivanova D.A. et al. [34] | 2020 | 122 | 96 | 122 | 100 | 0 | 0 |
| 15 | Yablonskiy P.K. et al. [35] | 2022 | 23 | 96 | 0 | 0 | 23 | 100 |
| 16 | Starshinova A.A. et al. [36] | 2022 | 22 | 96 | 22 | 100 | 0 | 0 |
| 17 | Morozova T.I. et al. [37] | 2022 | 88 | 24 | 0 | 0 | 88 | 100 |
| Total | 1404 | 898 | 61.6 | 506 | 38.4 | |||
| Patient Characteristics | Number of Patients with Symptoms/Total Number of Patients | % |
|---|---|---|
| Pulmonary TB | ||
| Infiltrative TB | 384/954 | 40.3 |
| Fibrous-cavernous TB | 295/954 | 30.9 |
| Disseminated TB | 80/954 | 8.3 |
| Multiple tuberculomas | 34/954 | 3.5 |
| Caseous pneumonia | 21/954 | 2.2 |
| Extrapulmonary TB | ||
| Intrathoracic lymphadenopathy | 24/954 | 2.5 |
| Generalized tuberculosis | 14/954 | 1.4 |
| Incident cases | 266/909 | 29.3 |
| Disease recurrence | 213/909 | 23.4 |
| Bacterial excretion | 808/884 | 91.4 |
| Concomitant pathology | 703/851 | 82.6 |
| HIV infection | 42/703 | 5.9 |
| Addictions | ||
| Tobacco smoking | 294/505 | 58.2 |
| Alcohol addiction | 139/694 | 20.1 |
| Drug addiction | 12/379 | 3.1 |
| Cure Rates | With MDR/XDR TB Patients | |
|---|---|---|
| % | 95% Cl | |
| Clinical Efficacy | 78.2 | 75.1–81.1 |
| X-ray dynamic | 72.6 | 67.3–77.5 |
| Closure of cavities | 34.9 | 28.1–42.2 |
| Cessation of bacterial excretion | 79.5 | 76.5–82.3 |
| Treatment success | 75.5 | 72.3–78.5 |
| Recovery after a course of therapy | 82.0 | 78.6–85.1 |
| Departure from treatment | 9.8 | 7.9–12.2 |
| Death | 6.5 | 4.9–8.3 |
| TB Patients | Group I—Patients with MDR Tuberculosis | Group II—Patients with XDR Tuberculosis | ||
|---|---|---|---|---|
| % | 95% Cl | % | 95% Cl | |
| The efficiency of treatment by the end of the course of therapy | 89.9 | 85.9–93.2 | 71.9 | 66.2–77.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starshinova, A.; Dovgalyk, I.; Belyaeva, E.; Glushkova, A.; Osipov, N.; Kudlay, D. Efficacy of Tuberculosis Treatment in Patients with Drug-Resistant Tuberculosis with the Use of Bedaquiline: The Experience of the Russian Federation. Antibiotics 2022, 11, 1622. https://doi.org/10.3390/antibiotics11111622
Starshinova A, Dovgalyk I, Belyaeva E, Glushkova A, Osipov N, Kudlay D. Efficacy of Tuberculosis Treatment in Patients with Drug-Resistant Tuberculosis with the Use of Bedaquiline: The Experience of the Russian Federation. Antibiotics. 2022; 11(11):1622. https://doi.org/10.3390/antibiotics11111622
Chicago/Turabian StyleStarshinova, Anna, Irina Dovgalyk, Ekaterina Belyaeva, Anzhela Glushkova, Nikolay Osipov, and Dmitry Kudlay. 2022. "Efficacy of Tuberculosis Treatment in Patients with Drug-Resistant Tuberculosis with the Use of Bedaquiline: The Experience of the Russian Federation" Antibiotics 11, no. 11: 1622. https://doi.org/10.3390/antibiotics11111622
APA StyleStarshinova, A., Dovgalyk, I., Belyaeva, E., Glushkova, A., Osipov, N., & Kudlay, D. (2022). Efficacy of Tuberculosis Treatment in Patients with Drug-Resistant Tuberculosis with the Use of Bedaquiline: The Experience of the Russian Federation. Antibiotics, 11(11), 1622. https://doi.org/10.3390/antibiotics11111622







