Effectiveness of the Fungal Metabolite 3-O-Methylfunicone towards Canine Coronavirus in a Canine Fibrosarcoma Cell Line (A72)
Abstract
:1. Introduction
2. Results
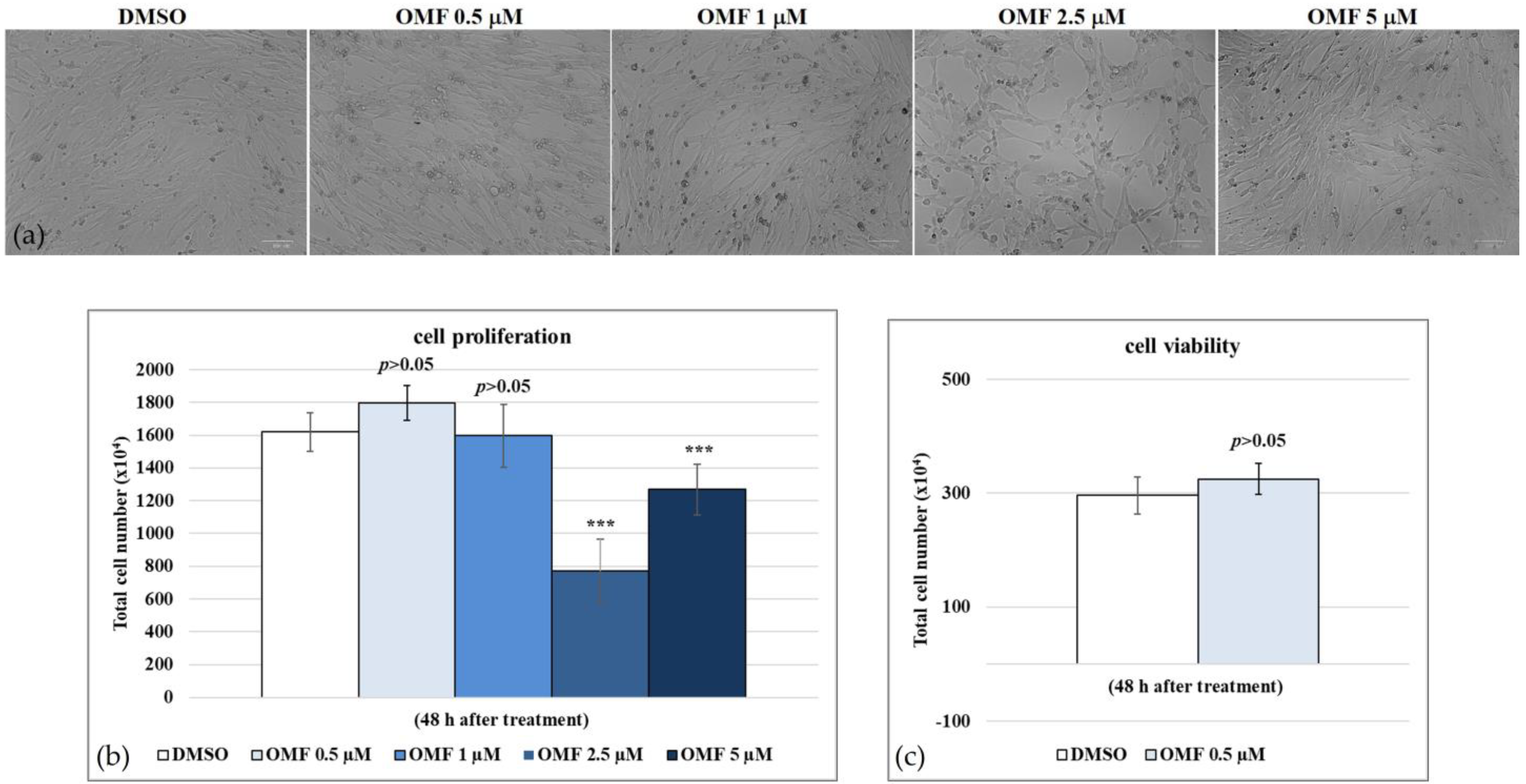
2.1. 3-O-Methylfunicone (OMF)
2.2. OMF Increased Cell Viability during CCoV Infection
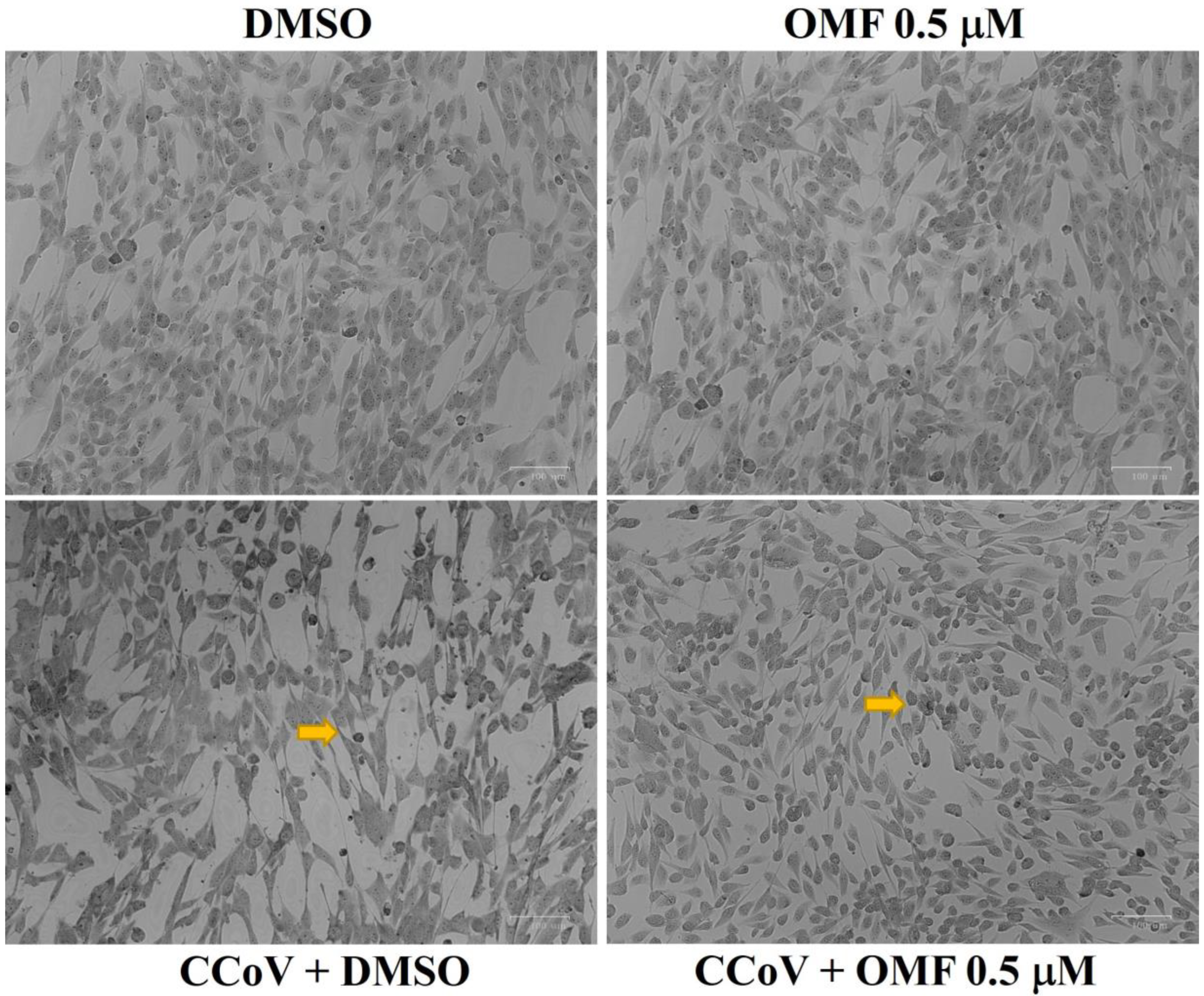
2.3. OMF Reduced Signs of Morphological Cell Death during CCoV Infection in A72 Cells
2.4. OMF Reduced Virus Yield during CCoV Infection in A72 Cells
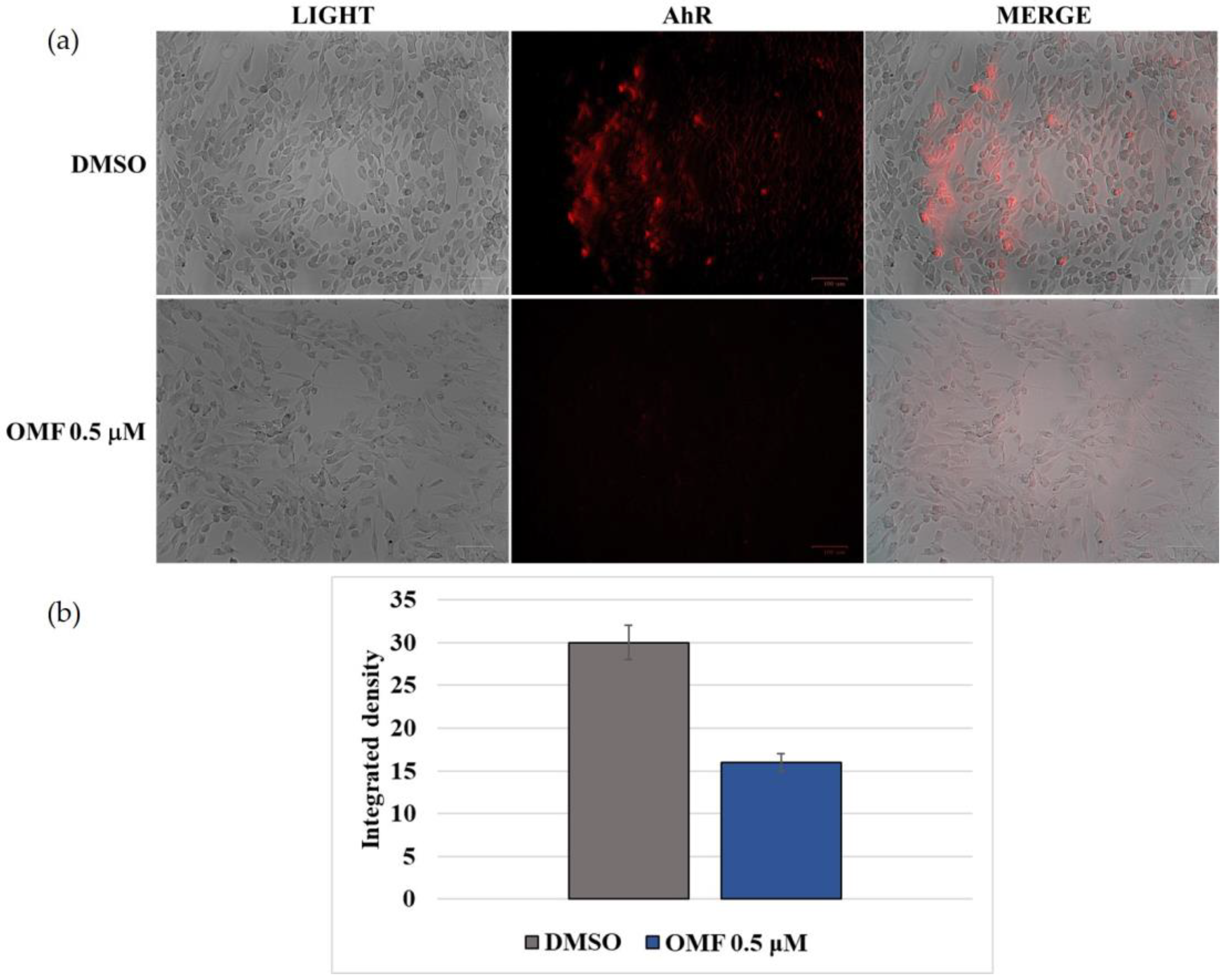
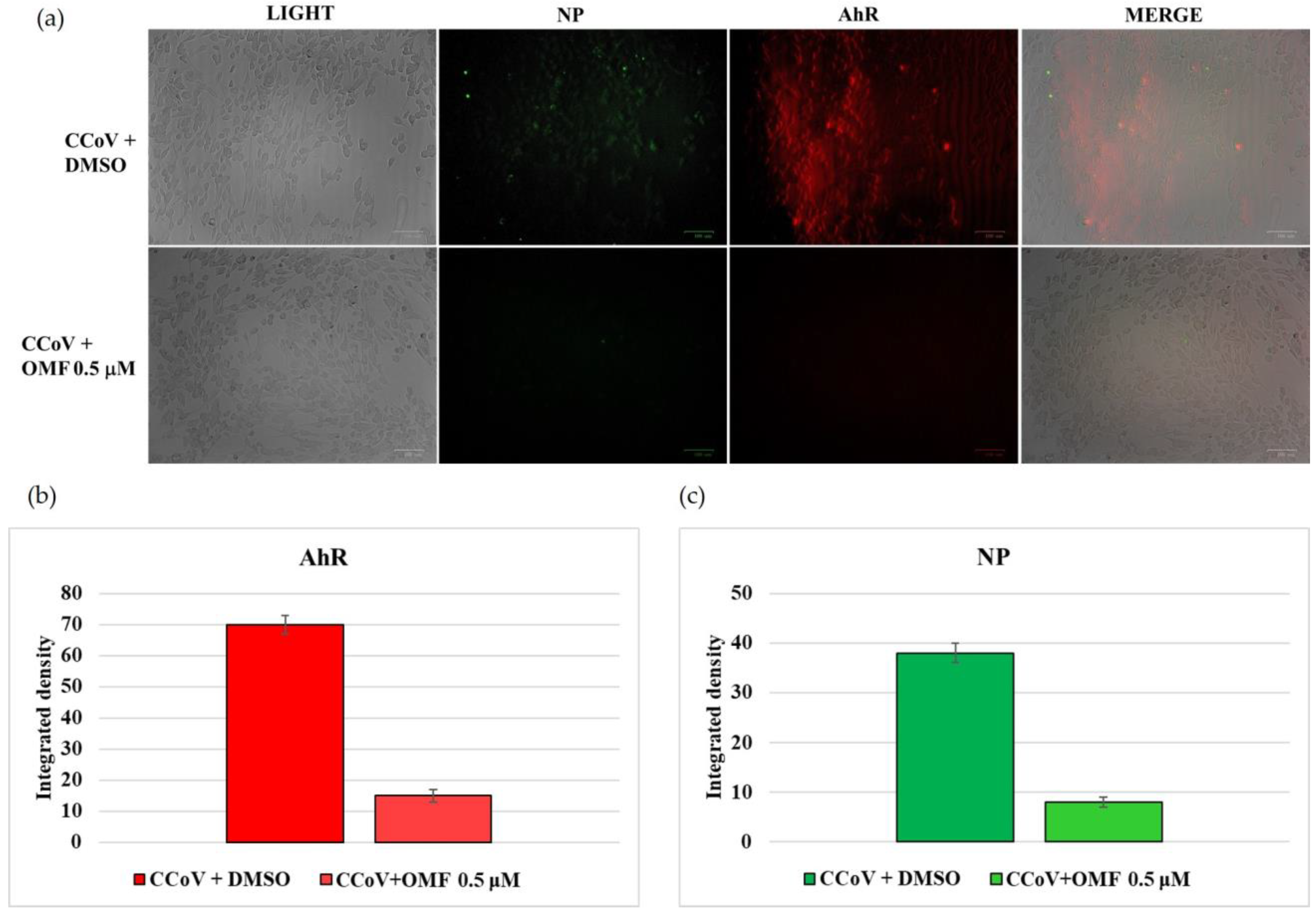
2.5. OMF Downregulated the Expression of AhR and NP during CCoV Infection
3. Discussion
4. Materials and Methods
4.1. Production and Isolation of OMF
4.2. Cell Cultures and Virus Infection
4.3. Cell Proliferation
4.4. Cell Viability
4.5. Examination of Cell Morphology
4.6. IF Staining
4.7. Virus Production
4.8. Viral Nucleic Acids Extraction Procedures
4.9. Real-Time RT-PCR for CCoV Quantification
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenney, S.P.; Wang, Q.; Vlasova, A.; Jung, K.; Saif, L. Naturally occurring animal coronaviruses as models for studying highly pathogenic human coronaviral disease. Vet. Pathol. 2021, 58, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Timurkan, M.O.; Aydin, H.; Dincer, E.; Coskun, N. Molecular characterization of canine coronaviruses: An enteric and pantropic approach. Arch. Virol. 2021, 166, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, A.; Tempesta, M.; Elia, G.; Martella, V.; Decaro, N.; Buonavoglia, C. The knotty biology of canine coronavirus: A worrying model of coronaviruses’ danger. Res. Vet. Sci. 2022, 144, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Torun, S.; Yilmaz, Z.; Pratelli, A. A serological evidence of minute virus of canines (MVC; canine parvovirus type-1) in dogs in Turkey. Turk. J. Vet. Anim. Sci. 2005, 29, 923–925. [Google Scholar]
- Buonavoglia, C.; Decaro, N.; Martella, V.; Elia, G.; Campolo, M.; Desario, C.; Castagnaro, M.; Tempesta, M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006, 12, 492–494. [Google Scholar] [CrossRef] [Green Version]
- Decaro, N.; Mari, V.; von Reitzenstein, M.; Lucente, M.S.; Cirone, F.; Elia, G.; Martella, V.; King, V.L.; Di Bello, A.; Varello, K.; et al. A pantropic canine coronavirus genetically related to the prototype isolate CB/05. Vet. Microbiol. 2012, 159, 239–244. [Google Scholar] [CrossRef]
- Alfano, F.; Fusco, G.; Mari, V.; Occhiogrosso, L.; Miletti, G.; Brunetti, R.; Galiero, G.; Desario, C.; Cirilli, M.; Decaro, N. Circulation of pantropic canine coronavirus in autochthonous and imported dogs, Italy. Transbound. Emerg. Dis. 2020, 67, 1991–1999. [Google Scholar] [CrossRef] [Green Version]
- Vlasova, A.N.; Diaz, A.; Damtie, D.; Xiu, L.; Toh, T.H.; Lee, J.S.Y.; Saif, L.J.; Gray, G.C. Novel canine coronavirus isolated from a hospitalized patient with pneumonia in East Malaysia. Clin. Infect. Dis. 2022, 74, 446–454. [Google Scholar] [CrossRef]
- Lednicky, J.A.; Tagliamonte, M.S.; White, S.K.; Blohm, G.M.; Alam, M.M.; Iovine, N.M.; Salemi, M.; Mavian, C.; Morris, J.G. Isolation of a novel recombinant canine coronavirus from a visitor to Haiti: Further evidence of transmission of coronaviruses of zoonotic origin to humans. Clin. Infect. Dis. 2022, 75, e1184–e1187. [Google Scholar] [CrossRef]
- Pratelli, A.; Tempesta, M.; Elia, G.; Martella, V.; Decaro, N.; Buonavoglia, C.; Buonavoglia, A.; Lanave, G.; Tempesta, M.; Camero, M.; et al. One world, one health, one virology of the mysterious labyrinth of coronaviruses: The canine coronavirus affair. Res. Vet. Sci. 2021, 2, e646–e647. [Google Scholar] [CrossRef]
- Linnakoski, R.; Reshamwala, D.; Veteli, P.; Cortina-Escribano, M.; Vanhanen, H.; Marjomäki, V. Antiviral agents from fungi: Diversity, mechanisms and potential applications. Front. Microbiol. 2018, 9, 2325. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.G. Potential of small-molecule fungal metabolites in antiviral chemotherapy. Antivir. Chem. Chemother. 2017, 25, 20–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicoletti, R.; Manzo, E.; Ciavatta, M.L. Occurrence and bioactivities of funicone-related compounds. Int. J. Mol. Sci. 2009, 10, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Dellagreca, M.; Andolfi, A. New insights into chemical and biological properties of funicone-like compounds. Toxins. 2022, 14, 466. [Google Scholar] [CrossRef]
- Nicoletti, R.; Scognamiglio, M.; Fiorentino, A. Structural and bioactive properties of 3-O-methylfunicone. Mini Rev. Med. Chem. 2014, 14, 1043–1047. [Google Scholar] [CrossRef]
- Nakajima, S.; Watashi, K.; Kamisuki, S.; Tsukuda, S.; Takemoto, K.; Matsuda, M.; Suzuki, R.; Aizaki, H.; Sugawara, F.; Wakita, T. Specific inhibition of hepatitis C virus entry into host hepatocytes by fungi-derived sulochrin and its derivatives. Biochem. Biophys. Res. Commun. 2013, 440, 515–520. [Google Scholar] [CrossRef]
- Fiorito, F.; Cerracchio, C.; Salvatore, M.M.; Serra, F.; Pucciarelli, A.; Amoroso, M.G.; Nicoletti, R.; Andolfi, A. Antiviral property of the fungal metabolite 3-O-methylfunicone in bovine Herpesvirus 1 infection. Microorganisms 2022, 10, 188. [Google Scholar] [CrossRef]
- Salvatore, M.M.; DellaGreca, M.; Nicoletti, R.; Salvatore, F.; Vinale, F.; Naviglio, D.; Andolfi, A. Talarodiolide, a new 12-membered macrodiolide, and GC/MS investigation of culture filtrate and mycelial extracts of Talaromyces pinophilus. Molecules 2018, 23, 950. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Cerracchio, C.; Serra, F.; Amoroso, M.G.; Fiorito, F. Canine coronavirus activates aryl hydrocarbon receptor during in vitro infection. Viruses 2022, 14, 2437. [Google Scholar] [CrossRef]
- Thiemann, S.; Smit, N.; Strowig, T. Antibiotic resistance: Problems and new opportunities. In How to Overcome the Antibiotic Crisis Facts, Challenges, Technologies and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 2016; Volume 398, pp. 1–236. ISBN 978-3-642-54826-0 978-3-642-54827-7. [Google Scholar]
- Nicoletti, R. Antitumor and Immunomodulatory Compounds from Fungi. Encyclopedia of Mycology 2021, 2, 683–709. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Barbosa, B.V.R.; Lima, M.T.N.S.; Cardoso, P.G.; Contigli, C.; Pimenta, L.P.S. Antiviral fungal metabolites and some insights into their contribution to the current COVID-19 pandemic. Bioorganic Med. Chem. 2021, 46, 116366. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.M.; El-Maradny, Y.A.; Osman, A.A.; Darwish, A.M.G.; Nahas, H.H.A.; Niedbała, G.; Piekutowska, M.; Abdel-Rahman, M.A.; Balbool, B.A.; Abdel-Azeem, A.M. A comprehensive review about the molecular structure of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Insights into natural products against COVID-19. Pharmaceutics 2021, 13, 1759. [Google Scholar] [CrossRef]
- Belachew, A.M.; Feyisa, A.; Mohamed, S.B.; W/Mariam, J.F. Investigating fungi-derived bioactive molecules as inhibitor of the SARS coronavirus papain like protease: Computational based study. Front. Med. 2021, 8, 752095. [Google Scholar] [CrossRef]
- Rao, P.; Shukla, A.; Parmar, P.; Rawal, R.M.; Patel, B.; Saraf, M.; Goswami, D. Reckoning a fungal metabolite, pyranonigrin A as a potential Main protease (Mpro) inhibitor of novel SARS-CoV-2 virus identified using docking and molecular dynamics simulation. Biophys. Chem. 2020, 264, 106425. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Patel, R.; Shukla, A.; Parmar, P.; Rawal, R.M.; Saraf, M.; Goswami, D. Identifying structural–functional analogue of GRL0617, the only well-established inhibitor for papain-like protease (PLpro) of SARS-CoV2 from the pool of fungal metabolites using docking and molecular dynamics simulation. Mol. Divers. 2022, 26, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, K.S.; Ansari, M.; Hosseyni Moghaddam, M.S.; Ebrahimi, Z.; Salehi, Z.; Shahlaei, M.; Moradi, S. In silico investigation on the inhibitory effect of fungal secondary metabolites on RNA dependent RNA polymerase of SARS-CoV-II: A docking and molecular dynamic simulation study. Comput. Biol. Med. 2021, 135, 104613. [Google Scholar] [CrossRef]
- Watashi, K.; Hijikata, M.; Hosaka, M.; Yamaji, M.; Shimotohno, K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology 2003, 38, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S.; Kuok, D.I.T.; Cheung, M.C.; Ng, M.M.T.; Ng, K.C.; Hui, K.P.Y.; Peiris, J.S.M.; Chan, M.C.W.; Nicholls, J.M. Effect of interferon alpha and cyclosporine treatment separately and in combination on Middle East Respiratory Syndrome Coronavirus (MERS-CoV) replication in a human in-vitro and ex-vivo culture model. Antivir. Res. 2018, 155, 89–96. [Google Scholar] [CrossRef]
- de Wilde, A.H.; Zevenhoven-Dobbe, J.C.; van der Meer, Y.; Thiel, V.; Narayanan, K.; Makino, S.; Snijder, E.J.; van Hemert, M.J. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011, 92, 2542–2548. [Google Scholar] [CrossRef]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA—J. Am. Med. Assoc. 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Jayasuriya, H.; Dewey, R.; Polishook, J.D.; Dombrowski, A.W.; Zink, D.L.; Guan, Z.; Collado, J.; Platas, G.; Pelaez, F.; et al. Isolation, structure, and HIV-1-integrase inhibitory activity of structurally diverse fungal metabolites. J. Ind. Microbiol. Biotechnol. 2003, 30, 721–731. [Google Scholar] [PubMed]
- Singh, S.; Malik, B.K.; Sharma, D.K. Targeting HIV-1 through molecular modeling and docking studies of CXCR4: Leads for therapeutic development. Chem. Biol. Drug Des. 2007, 69, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.; Di Trani, L.; Gatto, I.; Franco, M.; Vignolo, E.; Bedini, B.; Elia, G.; Buonavoglia, C. Canine coronavirus induces apoptosis in cultured cells. Vet. Microbiol. 2007, 121, 64–72. [Google Scholar] [CrossRef]
- De Martino, L.; Marfé, G.; Longo, M.; Fiorito, F.; Montagnaro, S.; Iovane, V.; Decaro, N.; Pagnini, U. Bid cleavage, cytochrome c release and caspase activation in canine coronavirus-induced apoptosis. Vet. Microbiol. 2010, 141, 36–45. [Google Scholar] [CrossRef]
- Marfè, G.; Tafani, M.; Fiorito, F.; Pagnini, U.; Iovane, G.; de Martino, L. Involvement of FOXO transcription factors, TRAIL-FasL/Fas, and Sirtuin proteins family in canine coronavirus type II-induced apoptosis. PLoS ONE 2011, 6, e27313. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.S.F.; Chan, K.H.; Cheng, V.C.C.; Yuen, K.Y. Comparative host gene transcription by microarray analysis early after infection of the Huh7 cell line by SARS coronavirus and human coronavirus 229E. Hong Kong Med. J. 2005, 15, 23–26. [Google Scholar]
- Grunewald, M.E.; Shaban, M.G.; Mackin, S.R.; Fehr, A.R.; Perlman, S. Murine coronavirus infection activates the aryl hydrocarbon TCDD-inducible-PARP expression. J. Virol. 2020, 94, e01743-19. [Google Scholar] [CrossRef] [Green Version]
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Dávola, M.E.; Elizalde, M.; Paletta, A.; Ashkar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148. [Google Scholar] [CrossRef]
- Guarnieri, T. Hypothesis: Emerging roles for aryl hydrocarbon receptor in orchestrating CoV-2-related inflammation. Cells 2022, 11, 648. [Google Scholar] [CrossRef]
- Torti, M.F.; Giovannoni, F.; Quintana, F.J.; García, C.C. The aryl hydrocarbon receptor as a modulator of anti-viral immunity. Front. Immunol. 2021, 12, 624293. [Google Scholar] [CrossRef]
- Fiorito, F.; Santamaria, R.; Irace, C.; De Martino, L.; Iovane, G. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and the viral infection. Environ. Res. 2017, 153, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Parga, T.; Suryawanshi, A.; Rouse, B.T. Controlling viral immuno-inflammatory lesions by modulating aryl hydrocarbon receptor signaling. PLoS Pathog. 2011, 7, e1002427. [Google Scholar] [CrossRef] [Green Version]
- Fiorito, F.; Pagnini, U.; De Martino, L.; Montagnaro, S.; Ciarcia, R.; Florio, S.; Pacilio, M.; Fucito, A.; Rossi, A.; Iovane, G.; et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases bovine herpesvirus type-1 (BHV-1) replication in Madin-Darby Bovine Kidney (MDBK) cells in vitro. J. Cell. Biochem. 2008, 103, 221–233. [Google Scholar] [CrossRef]
- Fiorito, F.; Ciarcia, R.; Granato, G.E.; Marfe, G.; Iovane, V.; Florio, S.; De Martino, L.; Pagnini, U. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induced autophagy in a bovine kidney cell line. Toxicology 2011, 290, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, R.; Fiorito, F.; Irace, C.; De Martino, L.; Maffettone, C.; Granato, G.E.; Di Pascale, A.; Iovane, V.; Pagnini, U.; Colonna, A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin impairs iron homeostasis by modulating iron-related proteins expression and increasing the labile iron pool in mammalian cells. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 704–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorito, F.; Nocera, F.P.; Cantiello, A.; Iovane, V.; Lambiase, S.; Piccolo, M.; Ferraro, M.G.; Santamaria, R.; De Martino, L. Bovine herpesvirus-1 infection in mouse neuroblastoma (Neuro-2A) cells. Vet. Microbiol. 2020, 247, 108762. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Quinta-Costa, M.; Leite, P.S.; Guimarães, J.E. Critical evaluation of techniques to detect and measure cell death—Study in a model of UV radiation of the leukaemic cell line HL60. Anal. Cell. Pathol. 1999, 19, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [Green Version]
- Zakeri, Z.; Lockshin, R.A. Cell death: History and future. Adv. Exp. Med. Biol. 2008, 615, 1–11. [Google Scholar]
- Altamura, G.; Power, K.; Martano, M.; degli Uberti, B.; Galiero, G.; De Luca, G.; Maiolino, P.; Borzacchiello, G. Felis catus papillomavirus type-2 E6 binds to E6AP, promotes E6AP/p53 binding and enhances p53 proteasomal degradation. Sci. Rep. 2018, 8, 17529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decaro, N.; Elia, G.; Martella, V.; Campolo, M.; Mari, V.; Desario, C.; Lucente, M.S.; Lorusso, E.; Kanellos, T.; Gibbons, R.H.; et al. Immunity after natural exposure to enteric canine coronavirus does not provide complete protection against infection with the new pantropic CB/05 strain. Vaccine 2010, 28, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.G.; Di Concilio, D.; Langellotti, A.L.; Martello, A.; Cioffi, B.; Suffredini, E.; Cozzi, L.; Russo, V.; Mauriello, G.; Di Pasquale, S.; et al. Quantitative Real-Time PCR and digital PCR to evaluate residual quantity of HAV in experimentally depurated mussels. Food Environ. Virol. 2021, 13, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Tofani, S.; Ianiro, G.; De Sabato, L.; Monini, M.; Angeloni, G.; Ponterio, E.; D’Agostino, C.; Di Bari, M.A.; Valeri, M.; Di Bartolo, I. Detection and whole genome sequencing of murine norovirus in animal facility in Italy. Anim. Biotechnol. 2021, 1–8. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerracchio, C.; Iovane, V.; Salvatore, M.M.; Amoroso, M.G.; Dakroub, H.; DellaGreca, M.; Nicoletti, R.; Andolfi, A.; Fiorito, F. Effectiveness of the Fungal Metabolite 3-O-Methylfunicone towards Canine Coronavirus in a Canine Fibrosarcoma Cell Line (A72). Antibiotics 2022, 11, 1594. https://doi.org/10.3390/antibiotics11111594
Cerracchio C, Iovane V, Salvatore MM, Amoroso MG, Dakroub H, DellaGreca M, Nicoletti R, Andolfi A, Fiorito F. Effectiveness of the Fungal Metabolite 3-O-Methylfunicone towards Canine Coronavirus in a Canine Fibrosarcoma Cell Line (A72). Antibiotics. 2022; 11(11):1594. https://doi.org/10.3390/antibiotics11111594
Chicago/Turabian StyleCerracchio, Claudia, Valentina Iovane, Maria Michela Salvatore, Maria Grazia Amoroso, Hiba Dakroub, Marina DellaGreca, Rosario Nicoletti, Anna Andolfi, and Filomena Fiorito. 2022. "Effectiveness of the Fungal Metabolite 3-O-Methylfunicone towards Canine Coronavirus in a Canine Fibrosarcoma Cell Line (A72)" Antibiotics 11, no. 11: 1594. https://doi.org/10.3390/antibiotics11111594
APA StyleCerracchio, C., Iovane, V., Salvatore, M. M., Amoroso, M. G., Dakroub, H., DellaGreca, M., Nicoletti, R., Andolfi, A., & Fiorito, F. (2022). Effectiveness of the Fungal Metabolite 3-O-Methylfunicone towards Canine Coronavirus in a Canine Fibrosarcoma Cell Line (A72). Antibiotics, 11(11), 1594. https://doi.org/10.3390/antibiotics11111594








