pH Alteration in Plant-Mediated Green Synthesis and Its Resultant Impact on Antimicrobial Properties of Silver Nanoparticles (AgNPs)
Abstract
1. Introduction
2. Results
2.1. Sample Preparation
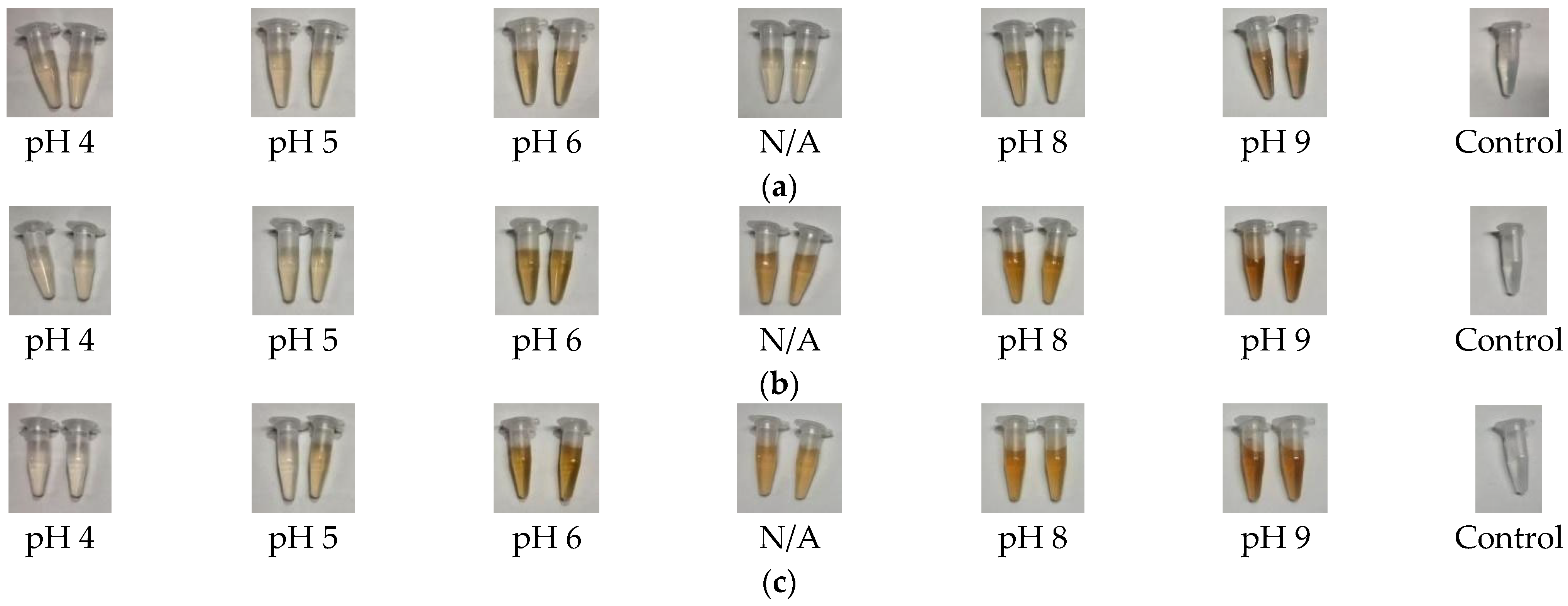
2.1.1. Visual Observation
2.1.2. UV-Vis Spectroscopy Analysis
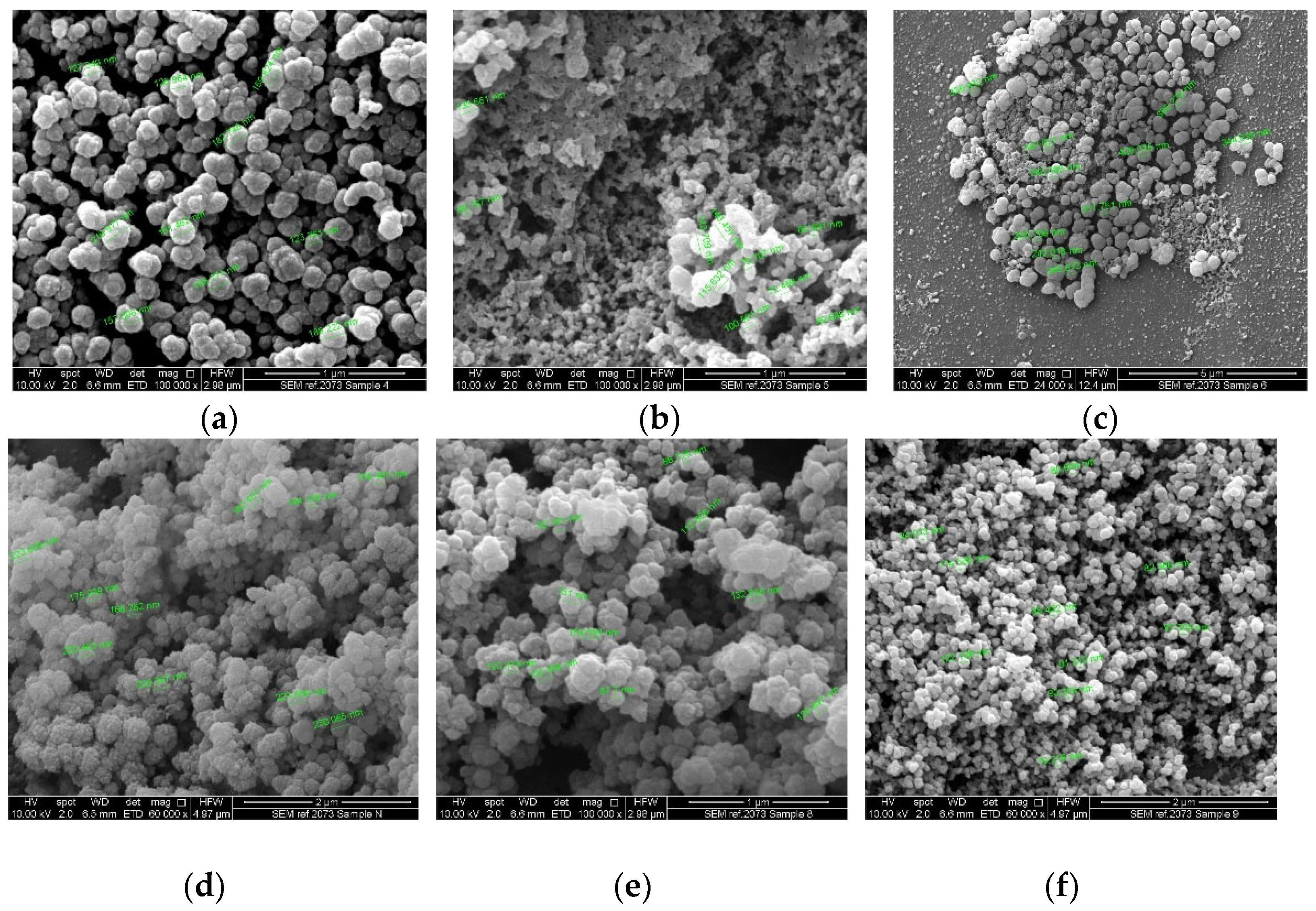
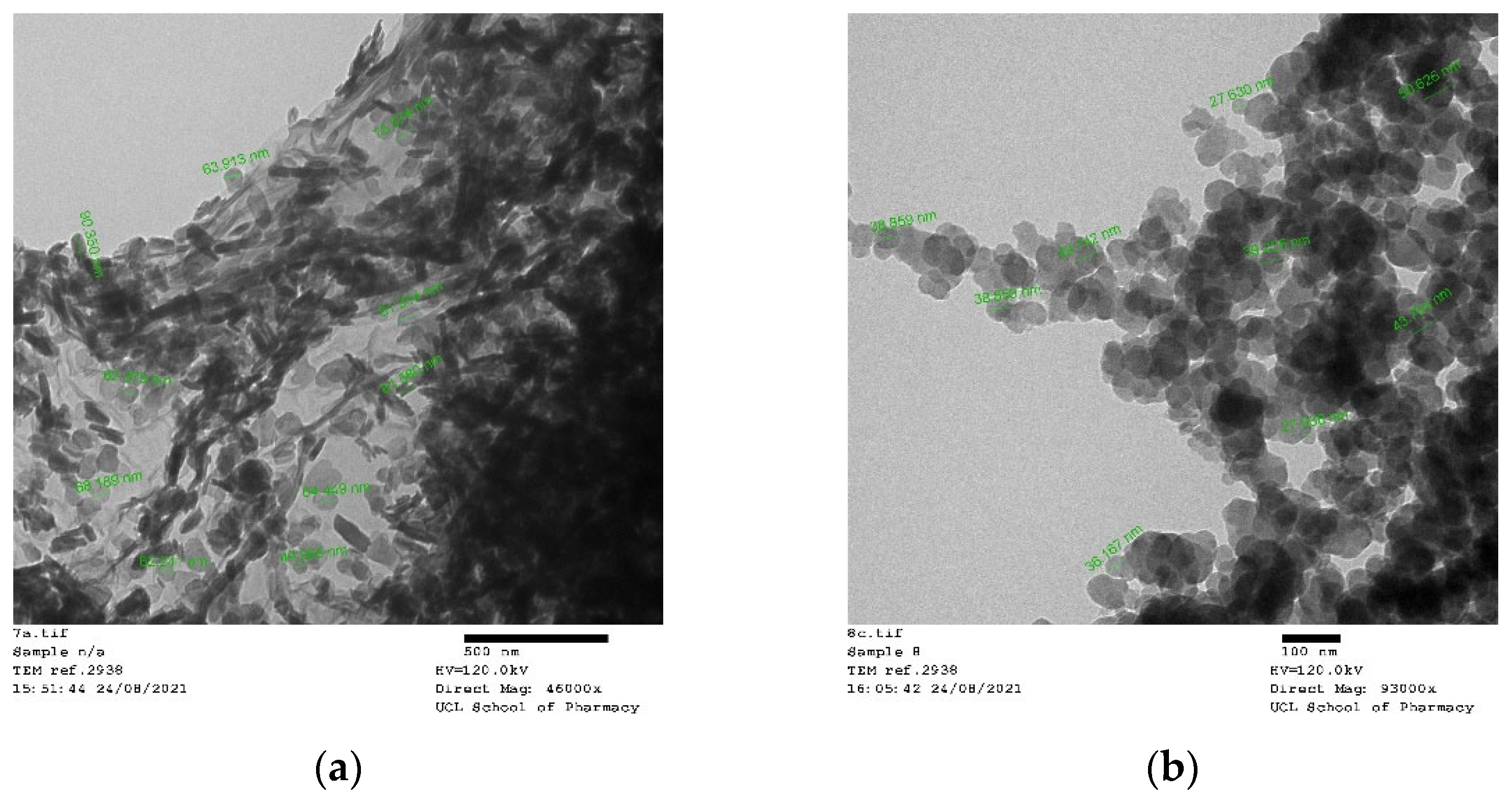
2.2. Nanoparticle Characterization
2.2.1. Dynamic Light Scattering (DLS) Analysis
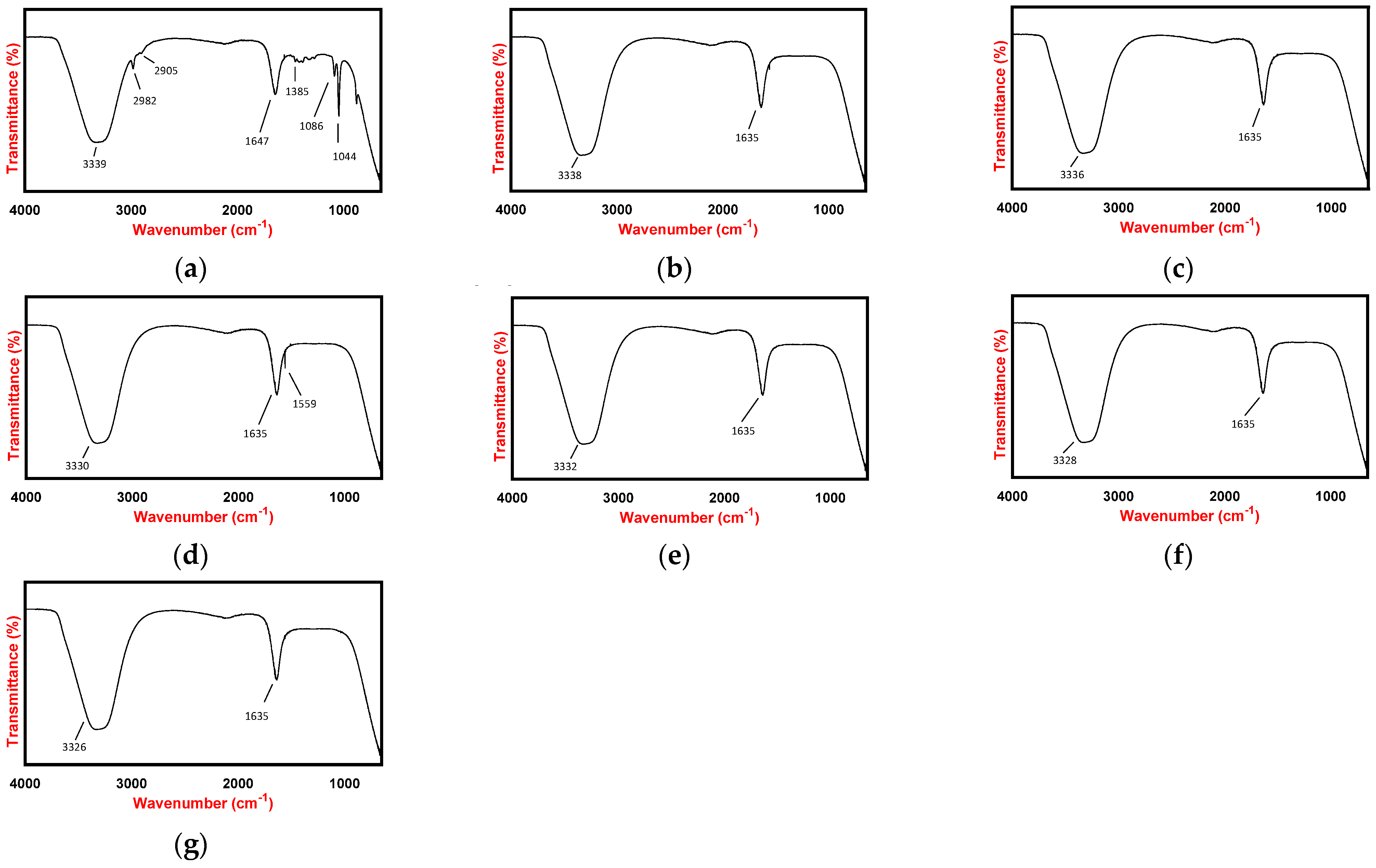
2.2.2. Fourier Transform Infrared Spectroscopy
2.2.3. Nanoparticle Morphology
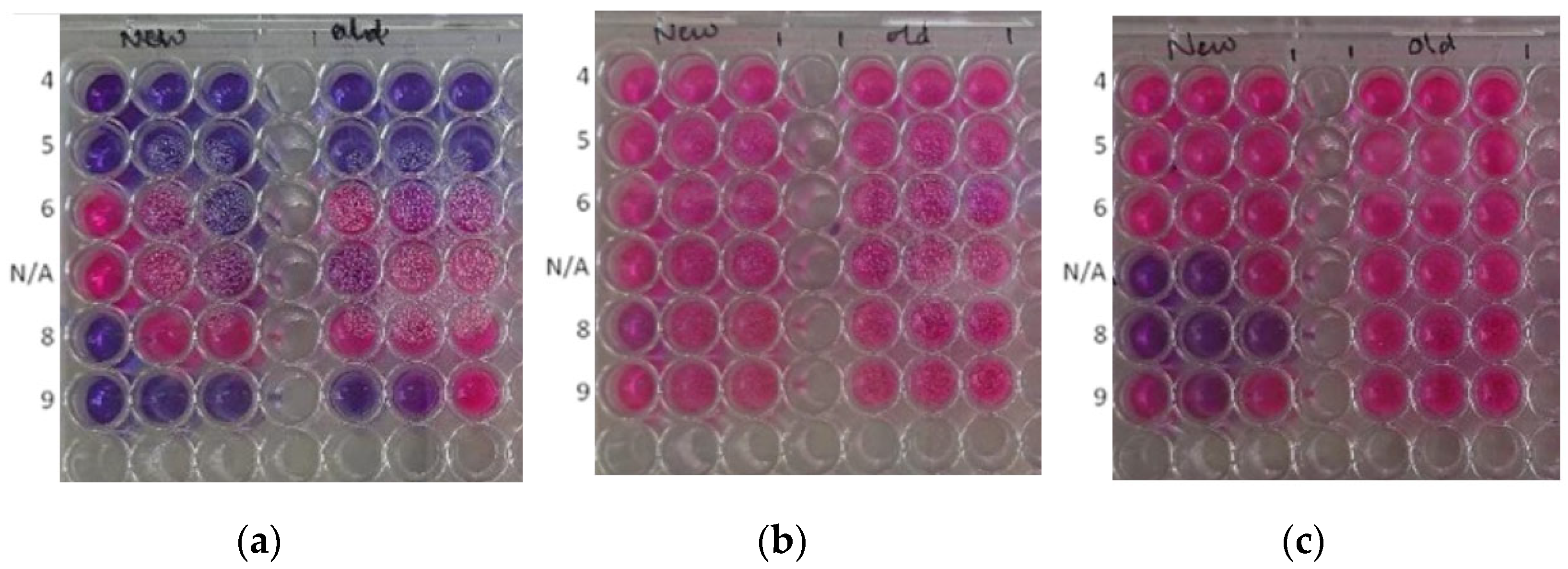
2.2.4. Antimicrobial Properties
3. Discussion
4. Materials and Methods
4.1. Materials
Materials and Chemicals
4.2. Spinacia oleracea Leaf Extraction
4.2.1. Maceration of Spinacia oleracea Leaf
4.2.2. Green Synthesis of AgNPs Using Spinacia oleracea Leaf Extract
4.3. Sample Characterization
4.3.1. Visual Observation
4.3.2. UV-Visible Spectroscopy
4.4. Nanoparticle Characterization
4.4.1. Dynamic Light Scattering
4.4.2. Fourier Transform Infrared Spectroscopy
4.4.3. Scanning Electron Microscopy
4.4.4. Transmission Electron Microscopy
4.4.5. Bacteria, Fungi Preparation and Antimicrobial Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.; Reis, I.G.; Bonatto, C.C. Green Synthesis of Metal Nanoparticles by Plants: Current Trends and Challenges. In Green Processes for Nanotechnology; Springer: New York, NY, USA, 2015; pp. 259–275. [Google Scholar]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Giordano, C.; Musetti, R. In Vivo synthesis of nanomaterials in plants—Location of silver nanoparticles and plant metabolism. Nanoscale Res. Lett. 2014, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effect of Organic and Conventional Cropping Systems on Ascorbic Acid, Vitamin C, Flavonoids, Nitrate, and Oxalate in 27 Varieties of Spinach (Spinacia oleracea L.). J. Agric. Food Chem. 2012, 60, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Edenharder, R.; Keller, G.; Platt, K.; Unger, K. Isolation and Characterization of Structurally Novel Antimutagenic Flavonoids from Spinach (Spinacia oleracea). J. Agric. Food Chem. 2001, 49, 2767–2773. [Google Scholar] [CrossRef]
- Roberts, J.L.; Moreau, R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 2014, 417305. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Arellano-Jimenez, M.J.; Lopez, F.D.; Lopez-Ribot, J.L. Protocol optimization for a fast, simple and economical chemical reduction synthesis of antimicrobial silver nanoparticles in non-specialized facilities. BMC Res. Notes 2019, 12, 773. [Google Scholar] [CrossRef]
- Devi, P.; Singh, S.; Sangwan, S.; Sihag, S.; Moond, M. Effect of pH on Phytochemical and Antioxidant Potential of Satawar Tubers (Asparagus Racemosus Willd.). J. Antioxi. Act. 2021, 2, 42–50. [Google Scholar] [CrossRef]
- Eddine, L.; Djamila, B.; Redha, O. Solvent pH extraction effect on phytochemical composition and antioxidant properties of Algerian Matricaria Pubescen. J. Pharm. Res. 2016, 10, 106–112. [Google Scholar]
- Velgosová, O.; Mražíková, A.; Marcinčáková, R. Influence of pH on green synthesis of Ag nanoparticles. Mater. Lett. 2016, 180, 336–339. [Google Scholar] [CrossRef]
- Reyes, D.F.; Cabrera, G.F.S.; Mata, S.M.V.; San Pedro, J.P.D.; Palioc, A.C.C.; Tandingan, G.S. Effect of pH on Size and Concentration of Silver Nanoparticles Synthesized using Ixora coccinea Linn. Leaf Extracts. Orient. J. Chem. 2020, 36, 1103–1106. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Mostafa, A.; Oudadesse, H.; Legal, Y.; Foad, E.; Cathelineau, G. Characteristics of Silver-Hydroxyapatite/PVP Nanocomposite. Bioceram. Dev. Appl. 2010, 1, D101128. [Google Scholar] [CrossRef]
- Rajan, R.; Chandran, K.; Harper, S.L.; Yun, S.-I.; Kalaichelvan, P.T. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crops Prod. 2015, 70, 356–373. [Google Scholar] [CrossRef]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2021.
- Anigol, L.B.; Charantimath, J.S.; Gurubasavaraj, P.M. Effect of Concentration and pH on the size of silver nanoparticles synthesized by green chemistry. Org. Med. Chem. Int. J. 2017, 3, 555622. [Google Scholar] [CrossRef]
- Niaz, S.; Forbes, B.; Raimi-Abraham, B.T. Exploiting Endocytosis for Non-Spherical Nanoparticle Cellular Uptake. Nanomanufacturing 2022, 2, 1. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: Berlin, Germany, 1995. [Google Scholar]
- Bohren, C.; Huffman, D. Absorption and Scattering of Light by Small Particles; John Wiley and Sons: New York, NY, USA, 1998. [Google Scholar]
- Fatimah, I.; Aftrid, Z.H.V.I. Characteristics and antibacterial activity of green synthesized silver nanoparticles using red spinach (Amaranthus Tricolor L.) leaf extract. Green Chem. Lett. Rev. 2019, 12, 25–30. [Google Scholar] [CrossRef]
- Sastry, M.; Patil, V.; Sainkar, S.R. Electrostatically Controlled Diffusion of Carboxylic Acid Derivatized Silver Colloidal Particles in Thermally Evaporated Fatty Amine Films. J. Phys. Chem. B 1998, 102, 1404–1410. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Alim-Al-Razy, M.; Bayazid, G.; Rahman, R.; Bosu, R.; Shamma, S. Silver nanoparticle synthesis, UV-Vis spectroscopy to find particle size and measure resistance of colloidal solution. J. Physics Conf. Ser. 2020, 1706, 012020. [Google Scholar] [CrossRef]
- Fleger, Y.; Rosenbluh, M. Surface Plasmons and Surface Enhanced Raman Spectra of Aggregated and Alloyed Gold-Silver Nanoparticles. Res. Lett. Opt. 2009, 2009, 475941. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef]
- Garibo, D.; Borbón-Nuñez, H.A.; De León, J.N.D.; Mendoza, E.G.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Travnickova, E.; Mikula, P.; Oprsal, J.; Bohacova, M.; Kubac, L.; Kimmer, D.; Soukupova, J.; Bittner, M. Resazurin assay for assessment of antimicrobial properties of electrospun nanofiber filtration membranes. AMB Express 2019, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2015, 6, 755–766. [Google Scholar] [CrossRef]
- Manosalva, N.; Tortella, G.; Diez, M.C.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green synthesis of silver nanoparticles: Effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Abu-Ghazaleh, A.; Lightfoot, D.A. Evaluation of the antimicrobial activities of ultrasonicated spinach leaf extracts using RAPD markers and electron microscopy. Arch. Microbiol. 2017, 199, 1417–1429. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- McQuillan, J.S.; Infante, H.G.; Stokes, E.; Shaw, A.M. Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology 2012, 6, 857–866. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of Silver(I) Ions with the Respiratory Chain of Escherichia coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- Gontijo, L.A.P.; Raphael, E.; Ferrari, D.P.S.; Ferrari, J.L.; Lyon, J.P.; Schiavon, M.A. pH effect on the synthesis of different size silver nanoparticles evaluated by DLS and their size-dependent antimicrobial activity. Matéria Rio Jan. 2020, 25, e-12845. [Google Scholar] [CrossRef]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, e1901556. [Google Scholar] [CrossRef]
- Ramesh, P.; Kokila, T.; Geetha, D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, S.; Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, J.; Chen, G.; Cui, F.; Kim, T.; Kim, J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Verma, V.C.; Kharwar, R.N.; Gange, A.C. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 2010, 5, 33–40. [Google Scholar] [CrossRef]
- Ray, S.; Sarkar, S.; Kundu, S. Extracellular biosynthesis of silver nanoparticles using the mycorrhizal mushroom Tricholoma crassum (Berk.) SACC: Its antimicrobial activity against pathogenic bacteria and fungus, including multidrug resistant plant and human bacteria. Dig. J. Nanomater. Biostructures 2011, 6, 1289–1299. [Google Scholar]
- Perween, N.; Khan, H.M.; Fatima, N. Silver nanoparticles: An upcoming therapeutic agent for the resistant Candida infections. J. Microbiol. Exp. 2019, 7, 49–54. [Google Scholar] [CrossRef]
- Pascu, B.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Nemeş, N.S.; Seiman, C.; Marian, E.; Micle, O. A Green, Simple and Facile Way to Synthesize Silver Nanoparticles Using Soluble Starch. pH Studies and Antimicrobial Applications. Materials 2021, 14, 4765. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chung, E.; Johnston, I.; Ren, G.; Cheong, Y.-K. Exploitation of Antimicrobial Nanoparticles and Their Applications in Biomedical Engineering. Appl. Sci. 2021, 11, 4520. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
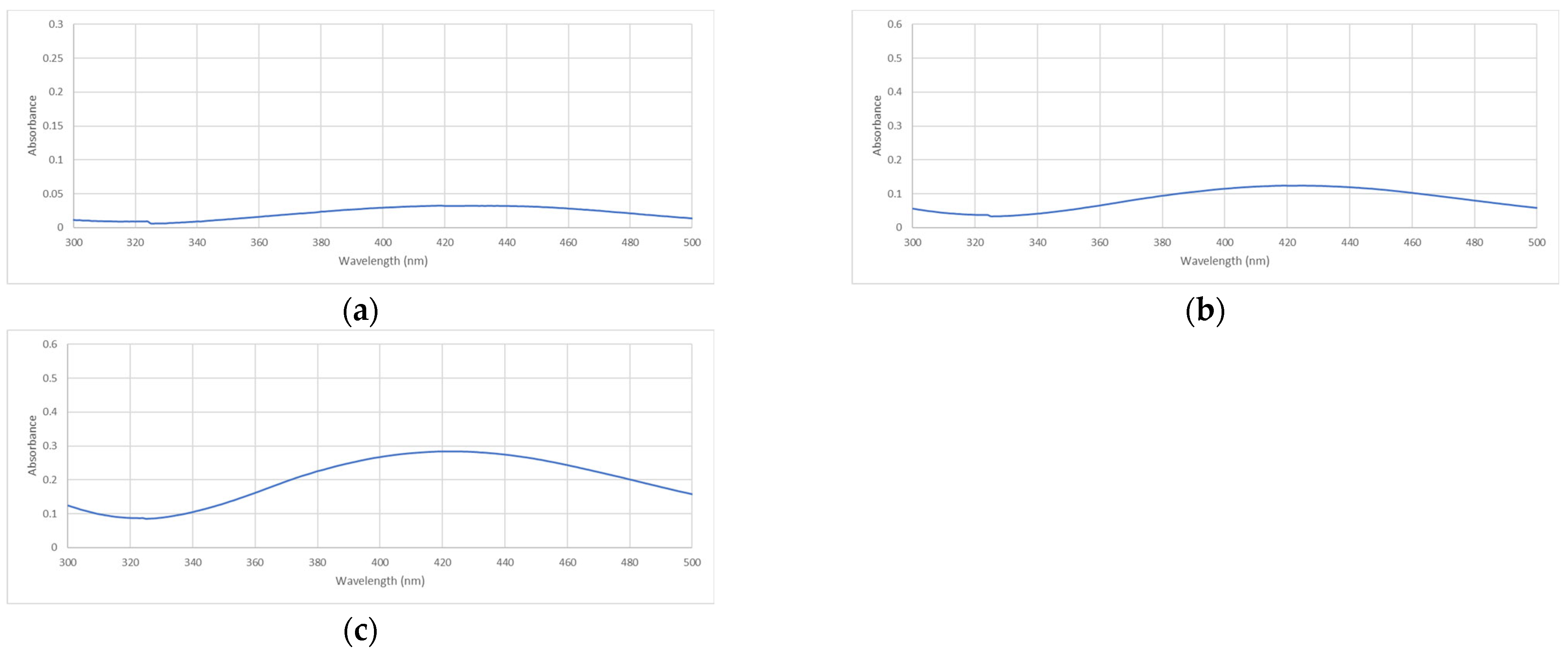

| pH | λmax | Absorbance |
|---|---|---|
| AgNP-6 | 418–419 nm | 0.0326 |
| AgNP-8 | 419 nm | 0.1244 |
| AgNP-9 | 422–424 nm | 0.2845 |
| Parameter | Samples | |||||
|---|---|---|---|---|---|---|
| AgNP-4 | AgNP-5 | AgNP-6 | AgNP-N/A | AgNP-8 | AgNP-9 | |
| Z-average | 1234 d.nm ± 663.30 | 193.9 d.nm ± 30.98 | 184.2 d.nm ± 18.55 | 405.3 d.nm ± 161.6 | 91.75 d.nm ± 8.31 | 263.6 d.nm ± 42.27 |
| pDI | 0.781 ± 0.241 | 0.494 ± 0.234 | 0.249 ± 0.029 | 0.418 ± 0.180 | 0.213 ± 0.023 | 0.332 ± 0.074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, A.; Akpobolokemi, T.; Chung, E.; Ren, G.; Raimi-Abraham, B.T. pH Alteration in Plant-Mediated Green Synthesis and Its Resultant Impact on Antimicrobial Properties of Silver Nanoparticles (AgNPs). Antibiotics 2022, 11, 1592. https://doi.org/10.3390/antibiotics11111592
Miranda A, Akpobolokemi T, Chung E, Ren G, Raimi-Abraham BT. pH Alteration in Plant-Mediated Green Synthesis and Its Resultant Impact on Antimicrobial Properties of Silver Nanoparticles (AgNPs). Antibiotics. 2022; 11(11):1592. https://doi.org/10.3390/antibiotics11111592
Chicago/Turabian StyleMiranda, Amalia, Tamara Akpobolokemi, Etelka Chung, Guogang Ren, and Bahijja Tolulope Raimi-Abraham. 2022. "pH Alteration in Plant-Mediated Green Synthesis and Its Resultant Impact on Antimicrobial Properties of Silver Nanoparticles (AgNPs)" Antibiotics 11, no. 11: 1592. https://doi.org/10.3390/antibiotics11111592
APA StyleMiranda, A., Akpobolokemi, T., Chung, E., Ren, G., & Raimi-Abraham, B. T. (2022). pH Alteration in Plant-Mediated Green Synthesis and Its Resultant Impact on Antimicrobial Properties of Silver Nanoparticles (AgNPs). Antibiotics, 11(11), 1592. https://doi.org/10.3390/antibiotics11111592