Meta-Analysis of Clinical Trials Comparing Cefazolin to Cefuroxime, Ceftriaxone, and Cefamandole for Surgical Site Infection Prevention
Abstract
:1. Introduction
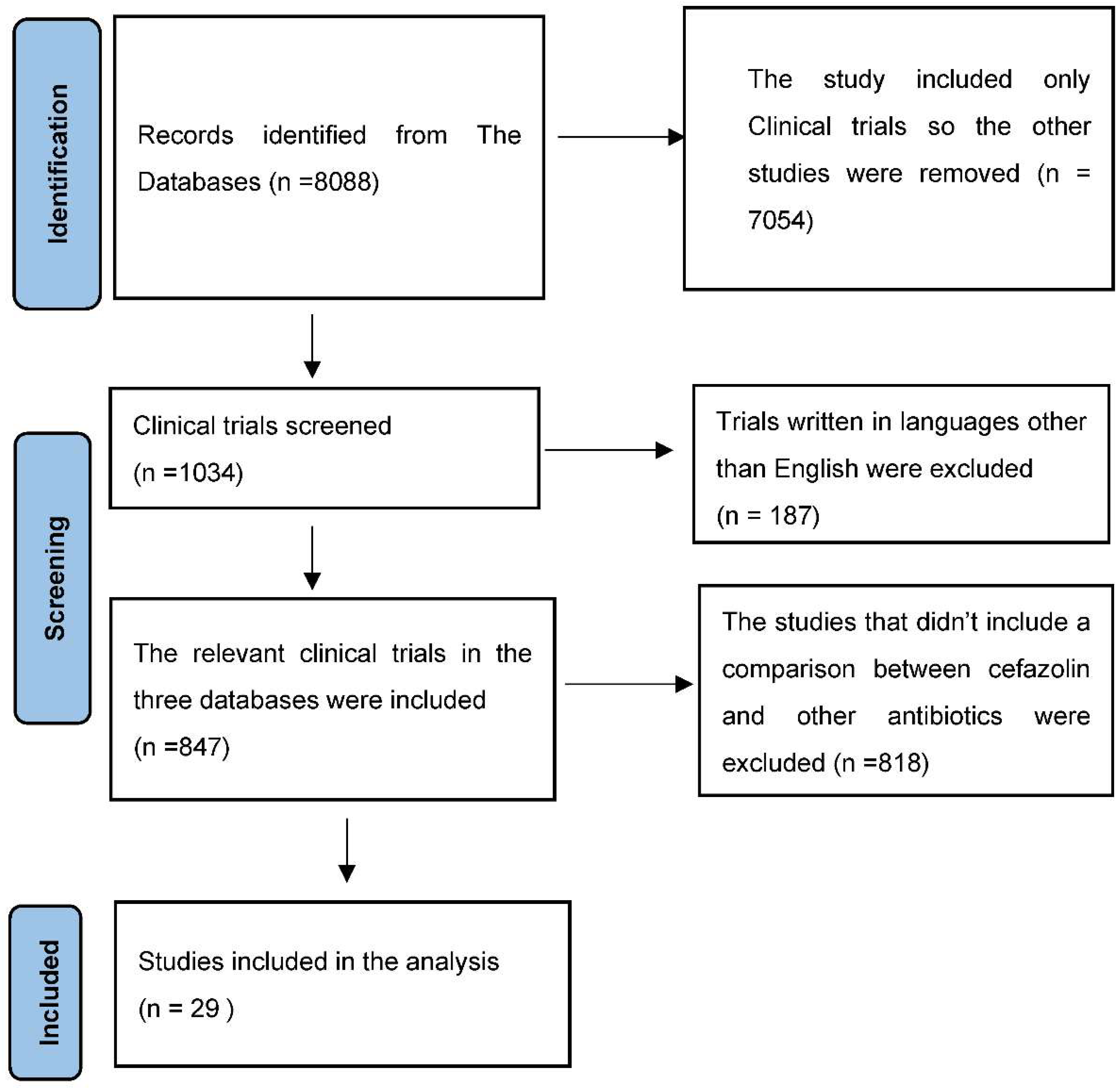
2. Materials and Methods
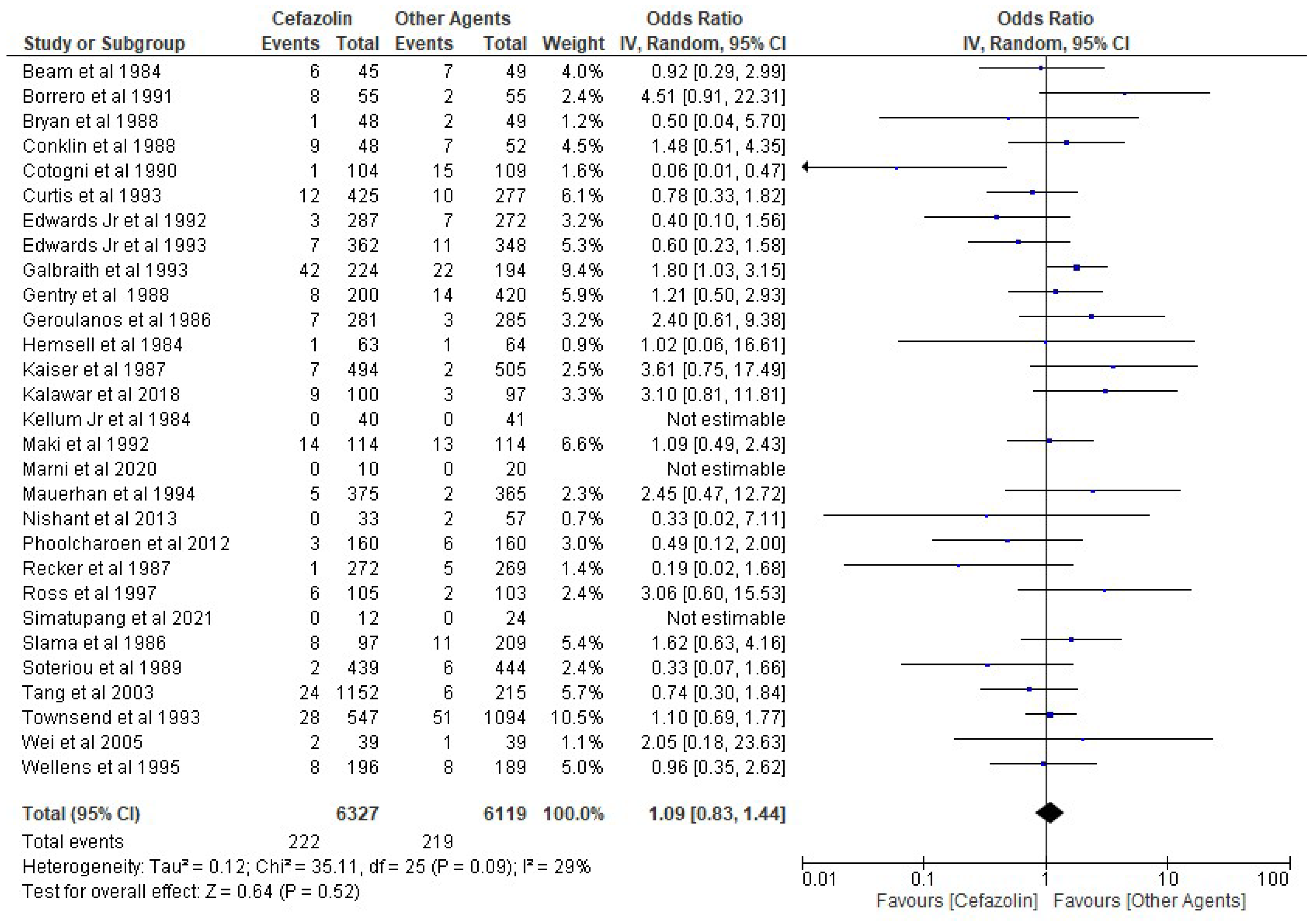
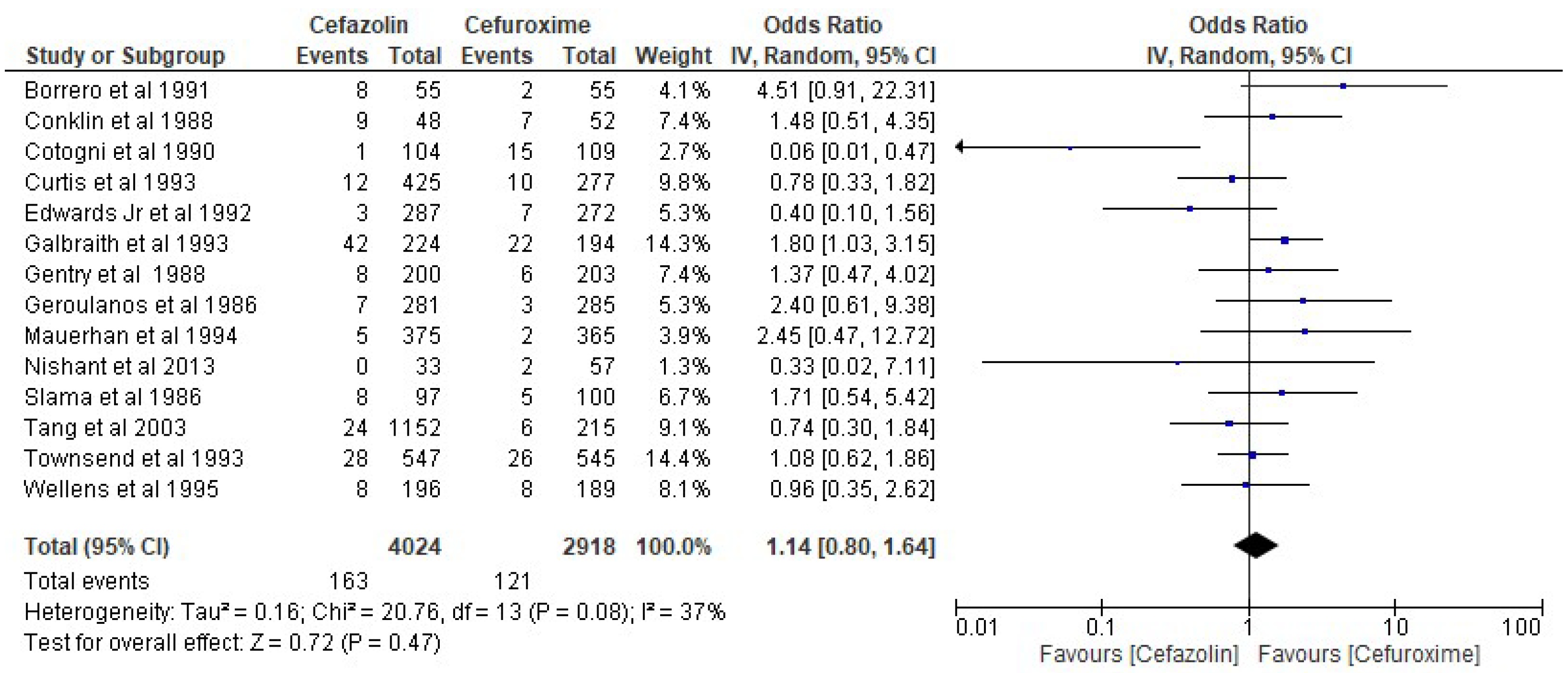
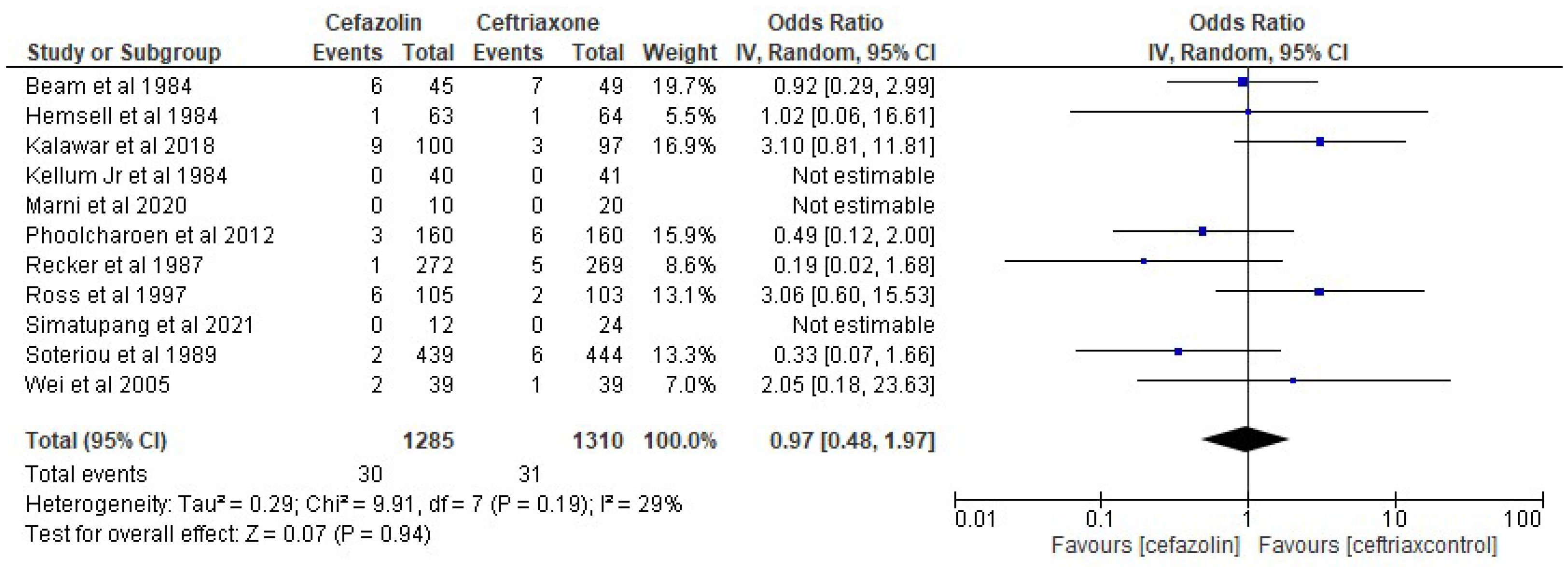
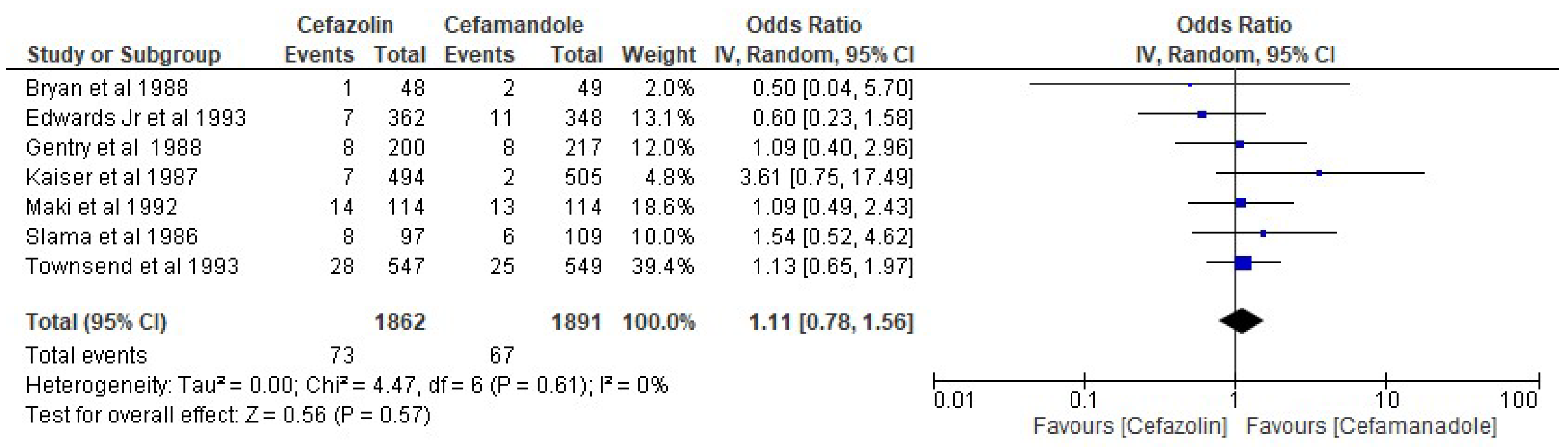
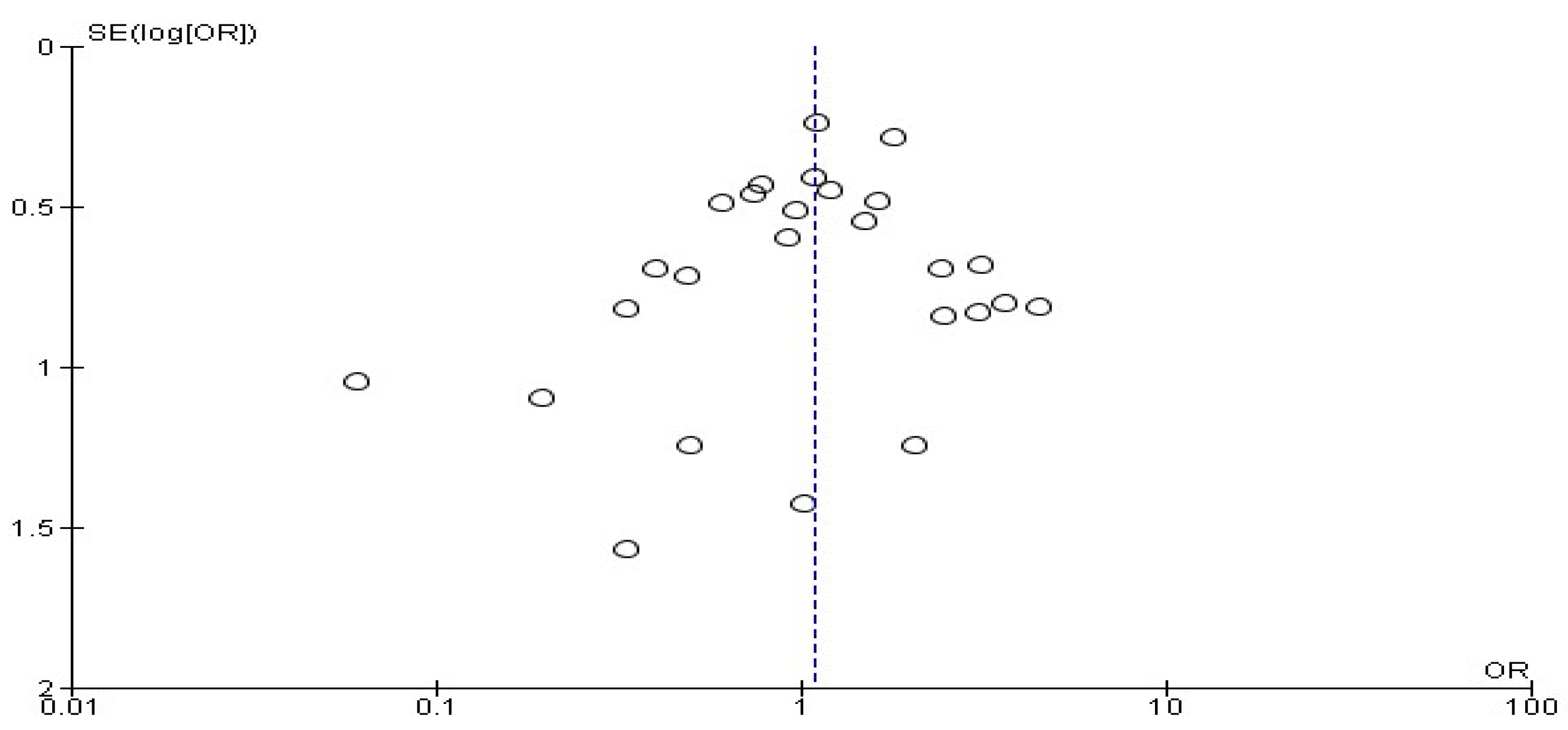
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, C.A.; Allen, J.; Barlow, G. Antibiotic prophylaxis. Surgery 2015, 33, 542–549. [Google Scholar]
- Borchardt, R.A.; Tzizik, D. Update on surgical site infections. JAAPA 2018, 31, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Idris, K.J.; Bamoosa, E.M.; Alsabbah, R.S.; Faidah, G.H.; Alharbi, S.J.; Bar, A.A.; Al-Ahdal, A.M. Awareness and level of knowledge of surgical site infection among surgical staff in King Abdullah Medical City during Hajj 2019: A cross-sectional study. IJMDC 2020, 4, 1873–1878. [Google Scholar] [CrossRef]
- Seidelman, J.; Anderson, D.J. Surgical Site Infections. Infect. Dis. Clin. North Am. 2021, 35, 901–929. [Google Scholar] [CrossRef] [PubMed]
- Surgical Treatment: Evidence-Based and Problem-Oriented. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6917 (accessed on 18 October 2022).
- Antibiotics for Surgical Prophylaxis. Available online: https://www.nps.org.au/australian-prescriber/articles/antibiotics-for-surgical-prophylaxis (accessed on 18 October 2022).
- Dehne, M.G.; Mühling, J.; Sablotzki, A.; Nopens, H.; Hempelmann, G. Pharmacokinetics of antibiotic prophylaxis in major orthopedic surgery and blood-saving techniques. Orthopedics 2001, 24, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Cephalosporins. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551517 (accessed on 18 October 2022).
- Peppard, W.J.; Eberle, D.G.; Kugler, N.W.; Mabrey, D.M.; Weigelt, J.A. Association between Pre-Operative Cefazolin Dose and Surgical Site Infection in Obese Patients. Surg. Infect. 2017, 18, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. American Society of Health-System Pharmacists (ASHP) Infectious Diseases Society of America (IDSA) Surgical Infection Society (SIS) Society for Healthcare Epidemiology of America (SHEA) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar] [CrossRef] [Green Version]
- Geroulanos, S.; Marathias, K.; Kriaras, J.; Kadas, B. Cephalosporins in surgical prophylaxis. J. Chemother. 2001, 1, 23–26. [Google Scholar] [CrossRef]
- Maki, D.G.; Bohn, M.J.; Stolz, S.M.; Kroncke, G.M.; Acher, C.W.; Myerowitz, D.P. Comparative study of cefazolin, cefaman-dole, and vancomycin for surgical prophylaxis in cardiac and vascular operations. A double-blind randomized trial. J. Thorac. Cardiovasc. Surg. 1992, 104, 1423–1434. [Google Scholar] [CrossRef]
- Surat, G.; Meyer-Sautter, P.; Rüsch, J.; Braun-Feldweg, J.; Markus, C.K.; Germer, C.T.; Lock, J.F. Cefazolin Might Be Adequate for Perioperative Antibiotic Prophylaxis in Intra-Abdominal Infections without Sepsis: A Quality Improvement Study. Antibiotics 2022, 11, 501. [Google Scholar] [CrossRef]
- Simatupang, M.D.; Dusak, I.W.S.; Suyasa, I.K.; Wiratnaya, I.G.E. Bacterial growth from the surgical wound base smear at the end of the operation and superficial surgical site infection in the administration of cefazolin single dose, ceftriaxone single dose, and ceftriaxone 3 days as prophylactic antibiotics in cases of. Intisari Sains Medis 2021, 12, 183–186. [Google Scholar]
- Marni, H.; Djanas, D.; Bachtiar, H. The Effect of Giving Prophylactic Antibiotic Ceftriaxone and Cefazolin and Giving Ceftriaxone Before and After Surgery to The Risk of Postoperative Wound Infection in Postoperative Patients. Andalas Obstet. Gynecol. J. 2020, 4, 77–86. [Google Scholar]
- Kalawar, R.P.S.; Shrestha, B.P.; Khanal, G.P.; Chaudhary, P.; Rijal, R.; Maharjan, R.; Paneru, S.R. Randomized controlled trial comparing cefazolin with ceftriaxone in perioperative prophylaxis in orthopaedic surgeries. JBPKIHS 2018, 1, 36–43. [Google Scholar] [CrossRef]
- Nishant; Kailash, K.K.; Vijayraghavan, P.V. Prospective randomized study for antibiotic prophylaxis in spine surgery: Choice of drug, dosage, and timing. Asian Spine J. 2013, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Phoolcharoen, N.; Nilgate, S.; Rattanapuntamanee, O.; Limpongsanurak, S.; Chaithongwongwatthana, S. A randomized controlled trial comparing ceftriaxone with cefazolin for antibiotic prophylaxis in abdominal hysterectomy. Int. J. Gynaecol. Obstet. 2012, 119, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, Z.P.; Zheng, X.H.; Chen, W.Y.; Guo, Q.Y.; Mao, H.P.; Ye, X.Q.; Yang, X.; Yu, X.Q. Prospective randomized controlled trial of antibiotic prophylaxis for newly placed peritoneal dialysis catheter to prevent postoperative peritonitis and wound infection. Chin. J. Nephrol. 2006, 22, 601–604. [Google Scholar]
- Tang, W.M.; Chiu, K.Y.; Ng, T.P.; Yau, W.P.; Ching, P.T.Y.; Seto, W.H. Efficacy of a single dose of cefazolin as a prophylactic antibiotic in primary arthroplasty. J. Arthroplast. 2003, 18, 714–718. [Google Scholar] [CrossRef]
- Ross, C.B.; Wheeler, W.G., 2nd; Jones, M.J.; Kerins, C.A.; Peek, T.E. Ceftriaxone versus cefazolin in peripheral arterial operations: A randomized, prospective trial. South. Med. J. 1997, 90, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wellens, F.; Pirlet, M.; Larbuisson, R.; De Meireleire, F.; De Somer, P. Prophylaxis in cardiac surgery. A controlled randomized comparison between cefazolin and cefuroxime. Eur. J. Cardiothorac. Surg. 1995, 9, 325–329. [Google Scholar] [CrossRef]
- Mauerhan, D.R.; Nelson, C.L.; Smith, D.L.; Fitzgerald, R.H., Jr.; Slama, T.G.; Petty, R.W.; Jones, R.E.; Evans, R.P. Prophylaxis against infection in total joint arthroplasty. One day of cefuroxime compared with three days of cefazolin. J. Bone Jt. Surg. Am. 1994, 76, 39–45. [Google Scholar] [CrossRef]
- Curtis, J.J.; Boley, T.M.; Walls, J.T.; Hamory, B.; Schmaltz, R.A. Randomized, prospective comparison of first- and second-generation cephalosporins as infection prophylaxis for cardiac surgery. Am. J. Surg. 1993, 166, 734–737. [Google Scholar] [CrossRef]
- Edwards, W.H., Jr.; Kaiser, A.B.; Tapper, S.; Edwards, W.H., Sr.; Martin, R.S., III; Mulherin, J.L., Jr.; Jenkins, J.M.; Roach, A.C. Cefamandole versus cefazolin in vascular surgical wound infection prophylaxis: Cost-effectiveness and risk factors. J. Vasc. Surg. 1993, 18, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Galbraith, U.; Schilling, J.; Von Segesser, L.K.; Carrel, T.; Turina, M.; Geroulanos, S. Antibiotic prophylaxis in cardiovascular surgery: A prospective randomized comparative trial of one day cefazolin versus single dose cefuroxime. Drugs Exp. Clin. Res. 1993, 19, 229–234. [Google Scholar] [PubMed]
- Townsend, T.R.; Reitz, B.A.; Bilker, W.B.; Bartlett, J.G. Clinical trial of cefamandole, cefazolin, and cefuroxime for antibiotic prophylaxis in cardiac operations. J. Thorac. Cardiovasc. Surg. 1993, 106, 664–670. [Google Scholar] [CrossRef]
- Edwards, W.H., Jr.; Kaiser, A.B.; Kernodle, D.S.; Appleby, T.C.; Edwards, W.H., Sr.; Martin, R.S., III; Mulherin, J.L., Jr.; Wood, C.A., Jr. Cefuroxime versus cefazolin as prophylaxis in vascular surgery. J. Vasc. Surg. 1992, 15, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Borrero, E.; Rosenthal, D. Comparison of cefuroxime and cefazolin: Prophylaxis against infection in arterial reconstructive surgery. Vasc. Surg. 1991, 25, 54–59. [Google Scholar] [CrossRef]
- Doebbeling, B.N.; Pfaller, M.A.; Kuhns, K.R.; Massanari, R.M.; Behrendt, D.M.; Wenzel, R.P. Cardiovascular surgery prophylaxis: A randomized, controlled comparison of cefazolin and cefuroxime. J. Thorac. Cardiovasc. Surg. 1990, 99, 981–989. [Google Scholar] [CrossRef]
- Soteriou, M.; Recker, F.; Geroulanos, S.; Turina, M. Perioperative antibiotic prophylaxis in cardiovascular surgery: A prospective randomized comparative trial of cefazolin versus ceftriaxone. World J. Surg. 1989, 13, 798–801. [Google Scholar] [CrossRef]
- Bryan, C.S.; Morgan, S.L.; Caton, R.J.; Lunceford, E.M., Jr. Cefazolin versus cefamandole for prophylaxis during total joint arthroplasty. Clin. Orthop. Relat. Res. 1988, 228, 117–122. [Google Scholar] [CrossRef]
- Conklin, C.M.; Gray, R.J.; Neilson, D.; Wong, P.; Tomita, D.K.; Matloff, J.M. Determinants of wound infection incidence after isolated coronary artery bypass surgery in patients randomized to receive prophylactic cefuroxime or cefazolin. Ann. Thorac. Surg. 1988, 46, 172–177. [Google Scholar] [CrossRef]
- Gentry, L.O.; Zeluff, B.J.; Cooley, D.A. Antibiotic prophylaxis in open-heart surgery: A comparison of cefamandole, cefuroxime, and cefazolin. Ann. Thorac. Surg. 1988, 46, 167–171. [Google Scholar] [CrossRef]
- Kaiser, A.B.; Petracek, M.R.; Lea, J.W.; Kernodle, D.S.; Roach, A.C.; Alford, W.C., Jr.; Burrus, G.R.; Glassford, D.M., Jr.; Thomas, C.S., Jr.; Stoney, W.S. Efficacy of cefazolin, cefamandole, and gentamicin as prophylactic agents in cardiac surgery. Results of a prospective, randomized, double-blind trial in 1030 patients. Ann. Surg. 1987, 206, 791. [Google Scholar] [CrossRef] [PubMed]
- Recker, F.; Geroulanos, S.; Turina, M. Perioperative antibiotic prophylaxis in heart and vascular surgery. A prospective randomized comparative study with cefazolin and ceftriaxone. Dtsch. Med. Wochenschr. 1987, 112, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Geroulanos, S.; Oxelbark, S.; Turina, M. Perioperative antimicrobial prophylaxis in cardiovascular surgery: A prospective randomized trial comparing two day cefuroxime prophylaxis with four day cefazolin prophylaxis. J. Cardiovasc. Surg. 1986, 27, 300–306. [Google Scholar]
- Slama, T.G.; Sklar, S.J.; Misinski, J.; Fess, S.W. Randomized comparison of cefamandole, cefazolin, and cefuroxime prophylaxis in open-heart surgery. Antimicrob. Agents Chemother. 1986, 29, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Beam, T.; Raab, T.; Spooner, J.; Balderman, S.; Aldridge, J.; Bhayana, J. Single-dose antimicrobial prophylaxis in open heart surgery. Eur. J. Clin. Microbiol. 1984, 3, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Hemsell, D.L.; Menon, M.O.; Friedman, A.J. Ceftriaxone or cefazolin prophylaxis for the prevention of infection after vaginal hysterectomy. Am. J. Surg. 1984, 148, 22–26. [Google Scholar]
- Kellum, J.M., Jr.; Gargano, S.; Gorbach, S.L.; Talcof, C.; Curtis, L.E.; Weiner, B.; McCoobery, M.; Tan, J.S.; Kelly, T.; Wagner, D. Antibiotic prophylaxis in high-risk biliary operations: Multicenter trial of single preoperative ceftriaxone versus multidose cefazolin. Am. J. Surg. 1984, 148, 15–18. [Google Scholar]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Umscheid, C.A.; Mitchell, M.D.; Doshi, J.A.; Agarwal, R.; Williams, K.; Brennan, P.J. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect. Control Hosp. Epidemiol. 2011, 32, 101–114. [Google Scholar] [CrossRef]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; van Rijen, M.; et al. WHO Guidelines Development Group. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e276–e287. [Google Scholar] [CrossRef]
- Crader, M.F.; Varacallo, M. Preoperative Antibiotic Prophylaxis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK442032 (accessed on 23 September 2022).
- Jocum, J. Surgical antibiotic prophylaxis: Are you doing it right. S. Afr. J. Anaesth. Analg. 2018, 24, S49–S53. [Google Scholar]
- Ahmed, N.J.; Haseeb, A.; Alfaifi, A.A.; Alahmari, A.K.; Alshehri, A.A.; Almalki, Z.S.; Almatrafi, B.H.; Alghamdi, F.M.; Khan, A.H. The Appropriateness of Using Cefazolin as a Surgical Prophylaxis Antibiotic. Lat. Am. J. Pharm. 2022, 41, 609–612. [Google Scholar]
- Wolfhagen, N.; Boldingh, Q.J.; de Lange, M.; Boermeester, M.A.; de Jonge, S.W. Intraoperative Redosing of Surgical Antibiotic Prophylaxis in Addition to Preoperative Prophylaxis Versus Single-dose Prophylaxis for the Prevention of Surgical Site Infection: A Meta-analysis and GRADE Recommendation. Ann. Surg. 2022, 275, 1050–1057. [Google Scholar] [CrossRef]
- Isserman, R.S.; Cheung, J.; Varallo, D.; Cafone, J.; Lee, J.; Chiotos, K.; Muhly, W.T.; Metjian, T.A.; Swami, S.; Baldwin, K.; et al. Increasing Cefazolin Use for Perioperative Antibiotic Prophylaxis in Penicillin-Allergic Children. Pediatrics 2022, 149, e2021050694. [Google Scholar] [CrossRef]
- Kusaba, T. Safety and efficacy of cefazolin sodium in the management of bacterial infection and in surgical prophylaxis. Clin. Med. Ther. 2009, 1, 1607–1615. [Google Scholar] [CrossRef] [Green Version]
- Alemkere, G. Antibiotic usage in surgical prophylaxis: A prospective observational study in the surgical ward of Nekemte referral hospital. PLoS ONE 2018, 13, e0203523. [Google Scholar] [CrossRef] [Green Version]
- Marano, L.; Carbone, L.; Poto, G.E.; Calomino, N.; Neri, A.; Piagnerelli, R.; Fontani, A.; Verre, L.; Savelli, V.; Roviello, F.; et al. Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review. Antibiotics 2022, 11, 230. [Google Scholar] [CrossRef]
- Ruol, A.; Bertiato, G.; Boscarino, S.; Cusinato, R.; Pascarella, M.; Tonin, E.A.; Santi, S.; Ancona, E. Short-term prophylaxis with ceftriaxone plus metronidazole in esophageal cancer surgery. J. Chemother. 2000, 12, 23–28. [Google Scholar] [CrossRef]
- Mohri, Y.; Tonouchi, H.; Kobayashi, M.; Nakai, K.; Kusunoki, M. Randomized clinical trial of single-versus multiple-dose antimicrobial prophylaxis in gastric cancer surgery. J. Br. Surg. 2007, 94, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Bastakoti, B. Therapeutic Guidelines: Antibiotic. Version 15. Aust. Prescr. 2015, 38, 137. [Google Scholar] [CrossRef] [Green Version]
- Ierano, C.; Thursky, K.; Peel, T.; Koning, S.; James, R.; Johnson, S.; Hall, L.; Worth, L.J.; Marshall, C. Factors associated with antimicrobial choice for surgical prophylaxis in Australia. JAC Antimicrob. Resist. 2020, 2, dlaa036. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Antibiotics |
|---|---|---|
| Simatupang et al. [14] | 2021 | Cefazolin vs. Ceftriaxone |
| Marni et al. [15] | 2020 | Cefazolin vs. Ceftriaxone |
| Kalawar et al. [16] | 2018 | Cefazolin vs. Ceftriaxone |
| Nishant et al. [17] | 2013 | Cefazolin vs. Cefuroxime |
| Phoolcharoen et al. [18] | 2012 | Cefazolin vs. Ceftriaxone |
| Wei et al. [19] | 2005 | Cefazolin vs. Ceftriaxone |
| Tang et al. [20] | 2003 | Cefazolin vs. Cefuroxime |
| Ross et al. [21] | 1997 | Cefazolin vs. Ceftriaxone |
| Wellens et al. [22] | 1995 | Cefazolin vs. Cefuroxime |
| Mauerhan et al. [23] | 1994 | Cefazolin vs. Cefuroxime |
| Curtis et al. [24] | 1993 | Cefazolin vs. Cefuroxime |
| Edwards Jr et al. [25] | 1993 | Cefazolin vs. Cefamandole |
| Galbraith et al. [26] | 1993 | Cefazolin vs. Cefuroxime |
| Townsend et al. [27] | 1993 | Cefazolin vs. Cefuroxime vs. Cefamandole |
| Edwards Jr et al. [28] | 1992 | Cefazolin vs. Cefuroxime |
| Maki et al. [12] | 1992 | Cefazolin vs. Cefamandole |
| Borrero et al. [29] | 1991 | Cefazolin vs. Cefuroxime |
| Cotogni et al. [30] | 1990 | Cefazolin vs. Cefuroxime |
| Soteriou et al. [31] | 1989 | Cefazolin vs. Ceftriaxone |
| Bryan et al. [32] | 1988 | Cefazolin vs. Cefamandole |
| Conklin et al. [33] | 1988 | Cefazolin vs. Cefuroxime |
| Gentry et al. [34] | 1988 | Cefazolin vs. Cefuroxime vs. Cefamandole |
| Kaiser et al. [35] | 1987 | Cefazolin vs. Cefamandole |
| Recker et al. [36] | 1987 | Cefazolin vs. Ceftriaxone |
| Geroulanos et al. [37] | 1986 | Cefazolin vs. Cefuroxime |
| Slama et al. [38] | 1986 | Cefazolin vs. Cefuroxime vs. Cefamandole |
| Beam et al. [39] | 1984 | Cefazolin vs. Ceftriaxone |
| Hemsell et al. [40] | 1984 | Cefazolin vs. Ceftriaxone |
| Kellum Jr et al. [41] | 1984 | Cefazolin vs. Ceftriaxone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, N.J.; Haseeb, A.; Alamer, A.; Almalki, Z.S.; Alahmari, A.K.; Khan, A.H. Meta-Analysis of Clinical Trials Comparing Cefazolin to Cefuroxime, Ceftriaxone, and Cefamandole for Surgical Site Infection Prevention. Antibiotics 2022, 11, 1543. https://doi.org/10.3390/antibiotics11111543
Ahmed NJ, Haseeb A, Alamer A, Almalki ZS, Alahmari AK, Khan AH. Meta-Analysis of Clinical Trials Comparing Cefazolin to Cefuroxime, Ceftriaxone, and Cefamandole for Surgical Site Infection Prevention. Antibiotics. 2022; 11(11):1543. https://doi.org/10.3390/antibiotics11111543
Chicago/Turabian StyleAhmed, Nehad J., Abdul Haseeb, Ahmad Alamer, Ziyad S. Almalki, Abdullah K. Alahmari, and Amer H. Khan. 2022. "Meta-Analysis of Clinical Trials Comparing Cefazolin to Cefuroxime, Ceftriaxone, and Cefamandole for Surgical Site Infection Prevention" Antibiotics 11, no. 11: 1543. https://doi.org/10.3390/antibiotics11111543
APA StyleAhmed, N. J., Haseeb, A., Alamer, A., Almalki, Z. S., Alahmari, A. K., & Khan, A. H. (2022). Meta-Analysis of Clinical Trials Comparing Cefazolin to Cefuroxime, Ceftriaxone, and Cefamandole for Surgical Site Infection Prevention. Antibiotics, 11(11), 1543. https://doi.org/10.3390/antibiotics11111543










