Prompt and Appropriate Antimicrobial Therapy Improves Outcomes of NDM-Producing and KPC-Producing Klebsiella pneumoniae Bloodstream Infections in Patients Hospitalized for COVID-19: A Comparative Retrospective Case-Series
Abstract
1. Introduction
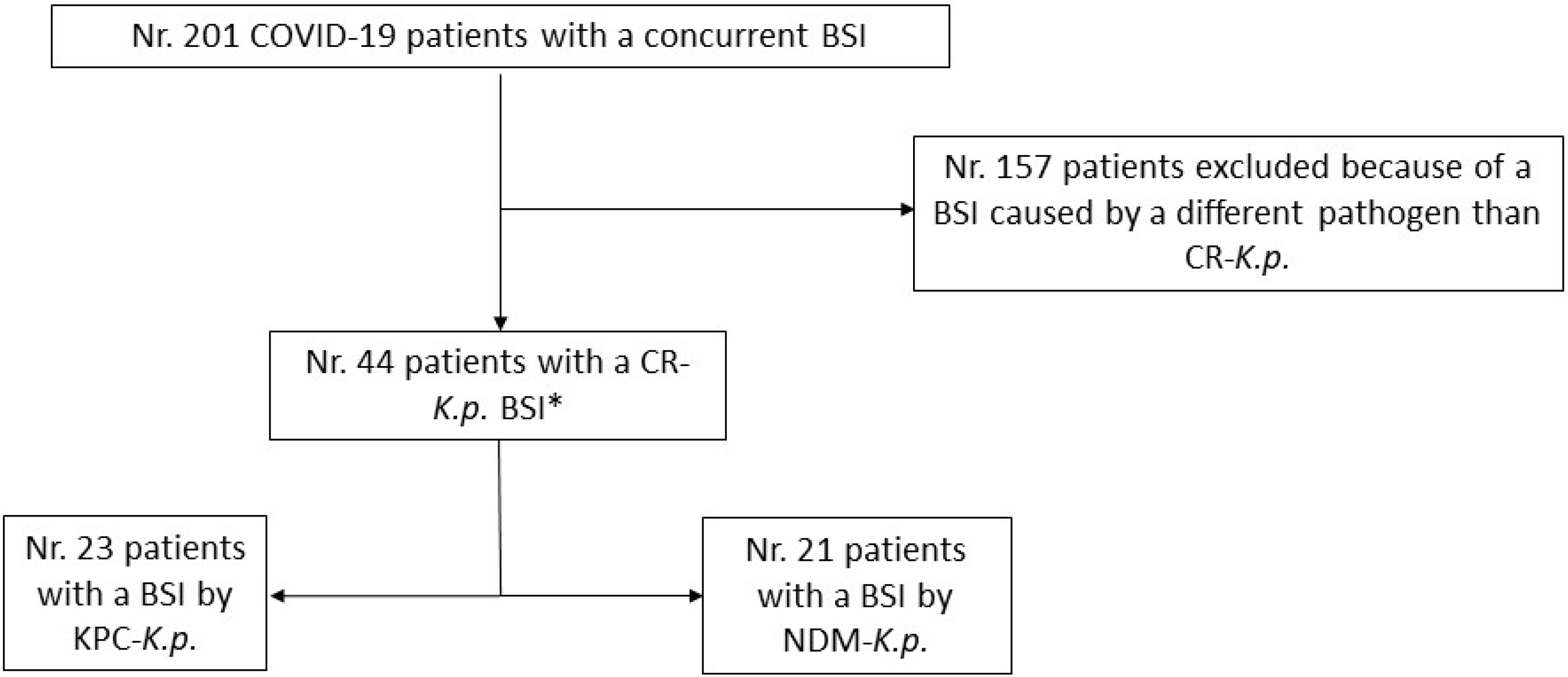
2. Materials and Methods
2.1. Study Design
2.2. Study Outcome Variables
2.3. Sampling Process
2.4. Antibiotic Therapy
2.4.1. Empirical and Targeted Therapy
2.4.2. Combination Therapy
2.5. Data Analysis
3. Results
3.1. General Characteristics of the Study Population
3.2. Characteristics of BSIs
3.3. Antibiotic Therapy
3.4. Risk of Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammerlaan, H.S.; Harbarth, S.; Buiting, A.G.; Crook, D.W.; Fitzpatrick, F.; Hanberger, H.; Herwaldt, L.A.; van Keulen, P.H.J.; Kluytmans, J.A.J.W.; Kola, A.; et al. Secular trends in nosocomial bloodstream infections: Antibiotic-resistant bacteria increase the total burden of infection. Clin. Infect. Dis. 2013, 56, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M. A multicentre analysis of epidemiology of the nosocomial bloodstream infections in Japanese university hospitals. Clin. Microbiol. Infect. 2013, 19, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Al-Hasan, M.N. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin. Microbiol. Infect. 2013, 19, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, D.F.; Pizzutilo, P.; Catino, A.; Signorile, F.; Pesola, F.; Di Gennaro, F.; Cassiano, S.; Marech, I.; Lamorgese, V.; Angarano, G.; et al. Incidence of Infections and Predictors of Mortality During Checkpoint Inhibitor Immunotherapy in Patients with Advanced Lung Cancer: A Retrospective Cohort Study. Open Forum Infect. Dis. 2021, 8, ofab187. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, M.; Simone, B.; Filisina, C.; Catalanotto, F.R.; Catalisano, G.; Marino, C.; Misseri, G.; Giarratano, A.; Cortegiani, A. Bloodstream Infections in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Microorganisms 2021, 9, 2016. [Google Scholar] [CrossRef]
- Segala, F.V.; Bavaro, D.F.; Di Gennaro, F.; Salvati, F.; Marotta, C.; Saracino, A.; Murri, R.; Fantoni, M. Impact of SARS-CoV-2 Epidemic on Antimicrobial Resistance: A Literature Review. Viruses 2021, 13, 2110. [Google Scholar] [CrossRef]
- Khatri, A.; Malhotra, P.; Izard, S.; Kim, A.; Oppenheim, M.; Gautam-Goyal, P.; Chen, T.; Doan, T.L.; Berlinrut, I.; Niknam, N.; et al. Hospital-Acquired Bloodstream Infections in Patients Hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (Coronavirus Disease 2019): Association with Immunosuppressive Therapies. Open Forum Infect. Dis. 2021, 8, ofab339. [Google Scholar] [CrossRef]
- Balena, F.; Bavaro, D.F.; Fabrizio, C.; Bottalico, I.F.; Calamo, A.; Santoro, C.R.; Brindicci, G.; Bruno, G.; Mastroianni, A.; Greco, S.; et al. Tocilizumab and corticosteroids for COVID-19 treatment in elderly patients. Gerontol. Geriatr. 2020, 68, 197–203. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Pattabiraman, V.; Konnor, R.Y.; Patel, P.R.; Wong, E.; Xu, S.Y.; Smith, B.; Edwards, J.R.; Dudeck, M.A. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2022, 43, 12–25. [Google Scholar] [CrossRef]
- Karruli, A.; Bocciam, F.; Gagliardi, M.; Patauner, F.; Ursi, M.P.; Sommese, P.; De Rosa, R.; Murino, P.; Ruocco, G.; Corcione, A.; et al. Multidrug-Resistant Infections and Outcome of Critically Ill Patients with Coronavirus Disease 2019: A Single Center Experience. Microb. Drug Resist. 2021, 27, 1167–1175. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Bartzavali, C.; Lambropoulou, A.; Solomou, A.; Tsiata, E.; Anastassiou, E.D.; Fligou, F.; Marangos, M.; Spiliopoulou, I.; Christofidou, M. Reversal of carbapenemase-producing Klebsiella pneumoniae epidemiology from blaKPC- to blaVIM-harbouring isolates in a Greek ICU after introduction of ceftazidime/avibactam. J. Antimicrob. Chemother. 2019, 74, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Nori, P.; Szymczak, W.; Puius, Y.; Sharma, A.; Cowman, K.; Gialanella, P.; Fleischner, Z.; Corpuz, M.; Torres-Isasiga, J.; Bartash, R.; et al. Emerging Co-Pathogens: New Delhi Metallo-beta-lactamase producing Enterobacterales Infections in New York City COVID-19 Patients. Int. J. Antimicrob. Agents 2020, 56, 106179. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Suardi, L.R.; Tiseo, G.; Galfo, V.; Occhineri, S.; Verdenelli, S.; Ceccarelli, G.; Poli, M.; Merli, M.; Bavaro, D.F.; et al. Superinfections caused by carbapenem-resistant Enterobacterales in hospitalized patients with COVID-19: A multicentre observational study from Italy (CREVID Study). JAC Antimicrob. Resist. 2022, 4, dlac064. [Google Scholar] [CrossRef]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-avibactam use for KPC-K.p. infections: A retrospective observational multicenter study. Clin. Infect. Dis. 2021, 73, ciab176. [Google Scholar] [CrossRef]
- Falcone, M.; Daikos, G.L.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of Ceftazidime-avibactam Plus Aztreonam in Patients with Bloodstream Infections Caused by Metallo-β-lactamase-Producing Enterobacterales. Clin. Infect. Dis. 2021, 72, 1871–1878. [Google Scholar] [CrossRef]
- Belati, A.; Bavaro, D.F.; Diella, L.; De Gennaro, N.; Di Gennaro, F.; Saracino, A. Meropenem/Vaborbactam Plus Aztreonam as a Possible Treatment Strategy for Bloodstream Infections Caused by Ceftazidime/Avibactam-Resistant Klebsiella pneumoniae: A Retrospective Case Series and Literature Review. Antibiotics 2022, 11, 373. [Google Scholar] [CrossRef]
- Behzadi, P.; García-Perdomo, H.A.; Karpiński, T.M.; Issakhanian, L. Metallo-ß-lactamases: A review. Mol. Biol. Rep. 2020, 47, 6281–6294. [Google Scholar] [CrossRef]
- Pintado, V.; Ruiz-Garbajosa, P.; Escudero-Sanchez, R.; Gioia, F.; Herrera, S.; Vizcarra, P.; Fortún, J.; Cobo, J.; Martín-Dávila, P.; Morosini, M.I.; et al. Carbapenemase-producing Enterobacterales infections in COVID-19 patients. Infect. Dis. 2022, 54, 36–45. [Google Scholar] [CrossRef]
- Onorato, L.; Sarnelli, B.; D’Agostino, F.; Signoriello, G.; Trama, U.; D’Argenzio, A.; Montemurro, M.V.; Coppola, N. Epidemiological, Clinical and Microbiological Characteristics of Patients with Bloodstream Infections Due to Carbapenem-Resistant, K. pneumoniae in Southern Italy: A Multicentre Study. Antibiotics 2022, 11, 633. [Google Scholar] [CrossRef]
- Falcone, M.; Bassetti, M.; Tiseo, G.; Giordano, C.; Nencini, E.; Russo, A.; Graziano, E.; Tagliaferri, E.; Leonildi, A.; Barnini, S.; et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit. Care 2020, 24, 29. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Galfo, V.; Giordano, C.; Leonildi, A.; Marciano, E.; De Simone, P.; Biancofiore, G.; Boggi, U.; Barnini, S.; et al. Bloodstream infections in patients with rectal colonization by Klebsiella pneumoniae producing different type of carbapenemases: A prospective, cohort study (CHIMERA study). Clin. Microbiol. Infect. 2021, 28, 298.e1–298.e7. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Ranjbar, R.; Behzadi, P.; Mohammadian, T. Virulence factors, antibiotic resistance patterns, and molecular types of clinical isolates of Klebsiella pneumoniae. Expert Rev. Anti Infect. Ther. 2022, 20, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.; Ramalho, J.F.; Duarte, A.; Pedrosa, A.; Silva, A.C.; Méndez, L.; Caneiras, C. First Outbreak of NDM-1-Producing Klebsiella pneumoniae ST11 in a Portuguese Hospital Centre during the COVID-19 Pandemic. Microorganisms 2022, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect 2021, 27, 451–457. [Google Scholar] [CrossRef]
- Seo, H.; Kim, H.J.; Kim, M.J.; Chong, Y.P.; Kim, S.H.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Jung, J. Comparison of clinical outcomes of patients infected with KPC- and NDM-producing Enterobacterales: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 1167.e1–1167.e8. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Belati, A.; Diella, L.; Stufano, M.; Romanelli, F.; Scalone, L.; Stolfa, S.; Ronga, L.; Maurmo, L.; Dell’Aera, M.; et al. Cefiderocol-Based Combination Therapy for “Difficult-to-Treat” Gram-Negative Severe Infections: Real-Life Case Series and Future Perspectives. Antibiotics 2021, 10, 652. [Google Scholar] [CrossRef]
- Karaiskos, I.; Lagou, S.; Pontikis, K.; Rapti, V.; Poulakou, G. The “Old” and the “New” Antibiotics for MDR Gram-Negative Pathogens: For Whom, When, and How. Front. Public Health 2019, 7, 151. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Romanelli, F.; Stolfa, S.; Belati, A.; Diella, L.; Ronga, L.; Fico, C.; Monno, L.; Mosca, A.; Saracino, A. Recurrent neurosurgical site infection by extensively drug-resistant P. aeruginosa treated with cefiderocol: A case report and literature review. Infect. Dis. 2021, 53, 206–211. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef]
- Delattre, I.K.; Hites, M.; Laterre, P.F.; Dugernier, T.; Spapen, H.; Wallemacq, P.E.; Jacobs, F.; Taccone, F.S. What is the optimal loading dose of broad-spectrum β-lactam antibiotics in septic patients? Results from pharmacokinetic simulation modelling. Int. J. Antimicrob. Agents 2020, 56, 106113. [Google Scholar] [CrossRef]
- Rhodes, N.J.; MacVane, S.H.; Kuti, J.L.; Scheetz, M.H. Impact of loading doses on the time to adequate predicted beta-lactam concentrations in prolonged and continuous infusion dosing schemes. Clin. Infect. Dis. 2014, 59, 905–907. [Google Scholar] [CrossRef] [PubMed]


| Characteristic * | Overall (n. 44) | BSIs Caused by KPC-K.p. (n. 23) | BSIs Caused by NDM-K.p. (n. 21) | p Value |
|---|---|---|---|---|
| Age (years), median (q1–q3) | 67 (57–75) | 62 (52–75) | 70 (65–79) | 0.176 |
| Male Sex, n. (%) | 32 (73) | 15 (65) | 17 (81) | 0.318 |
| Female Sex, n. (%) | 12 (27) | 8 (35) | 4 (19) | |
| Charlson Comorbidity Index, median (q1–q3) | 4 (2–7) | 4 (2–9) | 3 (2–6) | 0.306 |
| Comorbidity, n (%) | ||||
| Hypertension | 11 (25) | 7 (30) | 4 (19) | 0.494 |
| Chronic Obstructive Pulmonary Disease | 10 (23) | 6 (26) | 4 (19) | 0.724 |
| Type II Diabetes | 14 (32) | 8 (35) | 6 (29) | 0.752 |
| Chronic Kidney Diseases | 14 (32) | 8 (35) | 6 (29) | 0.752 |
| Dialysis | 5 (11) | 5 (22) | 0 | 0.050 |
| Obesity | 14 (32) | 7 (30) | 7 (33) | 0.999 |
| Malignancies | 5 (11) | 4 (17) | 1 (5) | 0.348 |
| COVID-19-related Acute Respiratory Failure at the time of infection, n (%) | ||||
| Low flux oxygen therapy | 11 (25) | 5 (22) | 6 (29) | |
| Non-invasive Ventilation | 19 (43) | 11 (48) | 8 (38) | 0.800 |
| Mechanical Ventilation | 14 (32) | 8 (35) | 7 (33) | |
| Hospital ward of evaluation, n. (%) | ||||
| Infectious Diseases/General Medicine | 16 (36) | 3 (13) | 13 (62) | 0.001 § |
| Sub-Intensive/Intermediate Care Units | 13 (30) | 11 (48) | 2 (10) | |
| Intensive Care Units | 15 (34) | 9 (39) | 6 (29) | |
| CR-K.p. colonization before infection, n. (%) | 29 (66) | 12 (52) | 17 (81) | 0.044 |
| Duration of Hospitalization before diagnosis of infection (days), median (q1–q3) | 21 (11–38) | 17 (11–38) | 23 (11–38) | 0.769 |
| Characteristic * | Overall (n. 44) | BSIs Caused by KPC-K.p. (n. 23) | BSIs Caused by NDM-K.p. (n.21) | p Value |
|---|---|---|---|---|
| Source of bloodstream infection, n (%) | ||||
| Urinary Tract | 13 (30) | 3 (13) | 10 (48) | |
| Primary Bacteriemia/Unknown Source | 15 (34) | 7 (30) | 8 (38) | 0.013 § |
| CVC-related bacteriemia | 14 (32) | 11 (48) | 3 (14) | |
| Ventilator-associated pneumonia | 2 (5) | 2 (9) | 0 | |
| Septic Shock at presentation, n (%) | 18 (41) | 10 (43) | 8 (38) | 0.707 |
| Polymicrobial infections, n (%) | 10 (23) | 5 (22) | 5 (24) | 0.999 |
| Other pathogen(s) than K.p., n (%): | ||||
| CR-A. baumannii | 6 (60) | 4 (80) | 2 (40) | n.e. |
| CR-A. baumannii (only) | 3 (50) | 1 (25) | 2 (40) | |
| CR-A. baumannii + Enterococcus spp. | 1 (16) | 1 (25) | 0 | |
| CR-A. baumannii + S. marcescens | 1 (16) | 1 (25) | 0 | |
| CR-A. baumannii + C. glabrata | 1 (16) | 1 (25) | 0 | |
| Enterococcus spp. | 3 (30) | 1 (20) | 2 (40) | |
| Enterococcus spp. + P. aeruginosa | 1 (10) | 0 | 1 (20) | |
| Time to appropriate antimicrobial therapy, n (%) | ||||
| within 24 h | 20 (45) | 12 (52) | 8 (38) | |
| between 24 and 72 h | 14 (32) | 7 (30) | 7 (33) | 0.587 |
| >72 h | 10 (23) | 4 (17) | 6 (29) | |
| Beta-lactams loading dose, n (%) | 18 (41) | 13 (57) | 5 (24) | 0.036 |
| Antibiotic therapy for K. pneumoniae BSI, n (%) | ||||
| Ceftazidime-Avibactam | 23 (52) | 23 (100) | 0 | <0.001 |
| Ceftazidime-Avibactam + Aztreonam | 21 (48) | 0 | 21 (100) | |
| Companion antibiotics added to therapy for BSI, n (%) | 19 (43) | 16 (70) | 3 (14) | <0.001 |
| Colistin | 3 (16) | 1 (6) | 2 (67) | n.e. |
| Aminoglycosides | 6 (32) | 6 (38) | 0 | |
| Fosfomycin | 3 (16) | 3 (19) | 0 | |
| Colistin + Tigecycline | 2 (11) | 1 (6) | 1 (33) | |
| Meropenem | 5 (26) | 5 (31) | 0 | |
| 14-day Mortality, n (%) | 14 (32) | 6 (26) | 8 (38) | 0.521 |
| 28-day Mortality, n (%) | 18 (41) | 8 (35) | 10 (48) | 0.541 |
| Variable * | Overall (n. 44) | 14-Day Mortality | p Value | |
|---|---|---|---|---|
| Deaths (n.14) | Survivors (n.30) | |||
| Age (years), median (q1–q3) | 67 (57–75) | 72 (62–75) | 65 (56–75) | 0.284 |
| Male Sex, n (%) | 32 (73) | 9 (64) | 23 (77) | 0.475 |
| Female Sex, n (%) | 12 (27) | 5 (36) | 7 (23) | |
| Charlson Comorbidity Index, median (q1–q3) | 4 (2–7) | 4 (3–7) | 3 (2–6) | 0.397 |
| Degree of Acute Respiratory Failure at the time of infection | ||||
| Low flux oxygen therapy | 11 (25) | 3 (21) | 8 (27) | 0.055 § |
| Non-invasive Ventilation | 19 (43) | 3 (21) | 16 (53) | |
| Mechanical Ventilation | 14 (32) | 8 (57) | 6 (20) | |
| Septic Shock at presentation, n (%) | 18 (41) | 9 (64) | 9 (30) | 0.049 |
| Source of bloodstream infection, n (%) | ||||
| Urinary Tract | 13 (30) | 1 (7) | 12 (40) | 0.093 §§ |
| Primary Bacteriemia/Unknown Source | 15 (34) | 7 (50) | 8 (27) | |
| CVC-related bacteriemia | 14 (32) | 5 (36) | 9 (30) | |
| Ventilator-associated pneumonia | 2 (5) | 1 (7) | 1 (3) | |
| Type of carbapenemases produced by K. pneumoniae | ||||
| KPC | 23 (52) | 6 (43) | 17 (57) | |
| NDM | 21 (48) | 8 (57) | 13 (43) | 0.521 |
| Time to appropriate antimicrobial therapy, n (%) | ||||
| within 24 h | 20 (45) | 1 (7) | 19 (63) | |
| between 24 and 72 h | 14 (32) | 4 (29) | 10 (33) | <0.001 # |
| >72 h | 10 (23) | 9 (64) | 1 (3) | |
| Loading dose of targeted antimicrobial therapy, n (%) | 18 (41) | 3 (21) | 15 (50) | 0.104 |
| Combination therapy, n (%) | 19 (43) | 7 (50) | 12 (40) | 0.745 |
| Polymicrobial BSI, n (%) | 10 (23) | 4 (29) | 6 (20) | 0.701 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | aHR | 95% CI | p Value | |
| Age, per 1 year increase | 1.01 | 0.97–1.06 | 0.431 | 1.03 | 0.93–1.13 | 0.520 |
| Male Sex | 0.70 | 0.23–2.12 | 0.538 | 0.39 | 0.64–2.37 | 0.308 |
| Charlson Comorbidity Index, per 1 point increase | 1.04 | 0.87–1.24 | 0.624 | 0.97 | 0.67–1.40 | 0.909 |
| Degree of Acute Respiratory Failure at the time of infection | ||||||
| Low flux oxygen therapy | 1 | \ | ||||
| Non-invasive Ventilation | 0.67 | 0.13–3.37 | 0.636 | \ | ||
| Mechanical Ventilation | 2.39 | 0.63–9.02 | 0.198 | \ | ||
| Septic Shock at presentation, n (%) | 2.59 | 0.86–7.77 | 0.088 | 1.38 | 0.33–5.72 | 0.656 |
| Source of bloodstream infection, n (%) | ||||||
| Urinary Tract | 1 | 1 | ||||
| Source other than Urinary Tract | 4.66 | 0.60–35.8 | 0.139 | 4.81 | 0.32–71.4 | 0.254 |
| Carbapenemases produced by K. pneumoniae | ||||||
| KPC | 1 | 1 | ||||
| NDM | 1.89 | 0.64–5.55 | 0.242 | 0.92 | 0.13–6.53 | 0.939 |
| Time to appropriate antimicrobial therapy, n (%) | ||||||
| within 24 h | 1 | 1 | ||||
| between 24 and 72 h | 8.35 | 0.92–75 | 0.058 | 12.03 | 1.10–130 | 0.041 |
| >72 h | 26.83 | 3.37–213 | 0.002 | 36.9 | 3.22–424 | 0.004 |
| Loading dose of Beta-lactams, n (%) | 0.35 | 0.09–1.26 | 0.110 | 0.16 | 0.02–1.10 | 0.064 |
| Combination therapy, n (%) | 1.22 | 0.42–3.49 | 0.707 | \ | ||
| Polymicrobial/ multiple infections, n (%) | 1.34 | 0.41–4.29 | 0.622 | 3.73 | 0.87–15.8 | 0.074 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bavaro, D.F.; Belati, A.; Diella, L.; Poli, M.A.; Calamo, A.; De Candia, G.; Altamura, M.; Spadavecchia, F.A.; Brindicci, G.; De Gennaro, N.; et al. Prompt and Appropriate Antimicrobial Therapy Improves Outcomes of NDM-Producing and KPC-Producing Klebsiella pneumoniae Bloodstream Infections in Patients Hospitalized for COVID-19: A Comparative Retrospective Case-Series. Antibiotics 2022, 11, 1519. https://doi.org/10.3390/antibiotics11111519
Bavaro DF, Belati A, Diella L, Poli MA, Calamo A, De Candia G, Altamura M, Spadavecchia FA, Brindicci G, De Gennaro N, et al. Prompt and Appropriate Antimicrobial Therapy Improves Outcomes of NDM-Producing and KPC-Producing Klebsiella pneumoniae Bloodstream Infections in Patients Hospitalized for COVID-19: A Comparative Retrospective Case-Series. Antibiotics. 2022; 11(11):1519. https://doi.org/10.3390/antibiotics11111519
Chicago/Turabian StyleBavaro, Davide Fiore, Alessandra Belati, Lucia Diella, Melita Anna Poli, Angela Calamo, Giovanna De Candia, Maurantonio Altamura, Felicia Anna Spadavecchia, Gaetano Brindicci, Nicolò De Gennaro, and et al. 2022. "Prompt and Appropriate Antimicrobial Therapy Improves Outcomes of NDM-Producing and KPC-Producing Klebsiella pneumoniae Bloodstream Infections in Patients Hospitalized for COVID-19: A Comparative Retrospective Case-Series" Antibiotics 11, no. 11: 1519. https://doi.org/10.3390/antibiotics11111519
APA StyleBavaro, D. F., Belati, A., Diella, L., Poli, M. A., Calamo, A., De Candia, G., Altamura, M., Spadavecchia, F. A., Brindicci, G., De Gennaro, N., Di Gennaro, F., Saracino, A., & Carbonara, S. (2022). Prompt and Appropriate Antimicrobial Therapy Improves Outcomes of NDM-Producing and KPC-Producing Klebsiella pneumoniae Bloodstream Infections in Patients Hospitalized for COVID-19: A Comparative Retrospective Case-Series. Antibiotics, 11(11), 1519. https://doi.org/10.3390/antibiotics11111519








